Abstract
Human activities have fundamental impacts on the distribution of species through altered land use, but also directly by dispersal of propagules. Rare long-distance dispersal events have a disproportionate importance for the spread of species including invasions. While it is widely accepted that humans may act as vectors of long-distance dispersal, there are few studies that quantify this process. We studied in detail a mechanism of human-mediated dispersal (HMD). For two plant species we measured, over a wide range of distances, how many seeds are carried by humans on shoes. While over half of the seeds fell off within 5 m, seeds were regularly still attached to shoes after 5 km. Semi-mechanistic models were fitted, and these suggested that long-distance dispersal on shoes is facilitated by decreasing seed detachment probability with distance. Mechanistic modelling showed that the primary vector, wind, was less important as an agent of long-distance dispersal, dispersing seeds less than 250 m. Full dispersal kernels were derived by combining the models for primary dispersal by wind and secondary dispersal by humans. These suggest that walking humans can disperse seeds to very long distances, up to at least 10 km, and provide some of the first quantified dispersal kernels for HMD.
Keywords: human impacts, human-mediated dispersal, long-distance dispersal, mechanistic models, shoe dispersal, wind dispersal
1. Introduction
Humans affect the behaviour, evolution and extinction of biological species. In particular, humans influence the distribution of species (e.g. Sykora 1990; Thompson & Jones 1999; Mayfield et al. 2006) in two major ways. First, it is well established how human land use can alter habitat availability and fragmentation (Andrén 1994; Ries et al. 2004; Blaum & Wichmann 2007). Second, species distribution is driven by dispersal, i.e. the movement of individuals or their dispersal units (Clobert et al. 2001; Bullock et al. 2002). In this context, humans have been suggested to be important vectors of dispersal (Ridley 1930; Suarez et al. 2001; von der Lippe & Kowarik 2007).
Dispersal information is critical in assessing the ability of species to support fragmented populations, to colonize new habitat and to spread spatially (Rees 1993; Clark et al. 2003). In such processes, dispersal over long distances has been recognized as having disproportionate importance (Cain et al. 2000; Higgins et al. 2003). Even extremely small numbers of individuals in the long-distance tail of the dispersal kernel can drive large-scale ecological patterns (Nathan 2006). Processes leading to long-distance dispersal include the dispersal of seeds in a population by a range of vectors (Jordano et al. 2007; Spiegel & Nathan 2007), and multiple dispersal, whereby a seed is dispersed in a sequence of two or more dispersal events (Bullock et al. 2006). Higgins et al. (2003) suggested that ‘non-standard’ vectors could be the major cause of long-distance dispersal and include humans in this category.
We define human-mediated dispersal (HMD) as dispersal directly by humans, on their clothes or by human-associated vectors, including all means of human transport, pets and livestock, human equipment and food. One may distinguish between intentional HMD (deliberate translocation) and unintentional HMD (humans having no control over hitch-hiking species; Bonn & Poschlod 1998). Because HMD includes a wide variety of mechanisms, dispersal units may include many types of propagules (plants: mostly seeds but also bulbs or ramets; smaller animals: eggs or pupae) but can be adult individuals.
Human-mediated dispersal has been recognized for at least two centuries (Humboldt & Bonpland 1807; Woodruffe-Peacock 1918). Ridley (1930) states: ‘it is highly probable that … herbs … owe much of their distribution to their [seeds] becoming attached in mud to the feet of men … but, the amount of actual proof of this is not great’ (Ridley 1930, p. 533). Since Ridley's book, more proof has accumulated showing that seeds attach to humans (Healy 1943; Clifford 1956; Bullock & Primack 1977; Kirby 2008) or their cars (Clifford 1959; Lonsdale & Lane 1994; von der Lippe & Kowarik 2007) and are dispersed by these means, with remarkable detail about seed quantities and species. These studies, however, can only speculate about dispersal distances. A different approach correlates human movement with species distribution to postulate HMD (Buchan & Padilla 1999; Suarez et al. 2001; Gilbert et al. 2004; Cushman & Meentemeyer 2008), but such studies cannot demonstrate the dispersal mechanisms. In summary, we still lack detailed knowledge about the distances and frequencies achieved by HMD (Hodkinson & Thompson 1997; Bonn & Poschlod 1998). Therefore, in order to bridge the knowledge gap, empirical studies are needed to quantify the dispersal kernel for well-defined mechanisms of HMD.
Seed dispersal mechanisms have already been quantified for non-human vectors. Among these, wind is a particularly well-studied vector for which detailed data (e.g. Bullock & Clarke 2000; Tackenberg et al. 2003; Soons et al. 2004a; Jongejans et al. 2007) and a selection of mechanistic and semi-mechanistic models are available (review by Kuparinen 2006). Currently, researchers are developing a more detailed understanding of seed dispersal by animals (Römermann et al. 2005; Jordano et al. 2007; Will & Tackenberg 2008), while quantification of dispersal by water is still in its infancy (Boedeltje et al. 2003; Vogt et al. 2004).
In this study, we provide a quantification of a dispersal mechanism by human vectors. We explore dispersal of seeds of two Brassica species on human shoes. Both species regularly occur along the South West Coast Path (SWCP) in Dorset, southern England, which attracts many walkers who have the potential to disperse seeds and affect species' distributions. We also use the data on wind, environmental and seed characteristics to model the potential of the dispersal by wind and compare this to HMD in our study system. In particular, we ask the following three questions:
Over what distances are seeds of our study species dispersed by humans on their shoes?
How does HMD of Brassica compare with wind as a vector for long-distance dispersal?
What are the potential dispersal kernels of the Brassica species in the cliff system as a combination of wind and human dispersal?
2. Material and methods
(a) Study system
We studied seed dispersal in two species of Brassica. In Britain, the perennial Brassica oleracea ssp. oleracea (wild cabbage) occurs exclusively on coastal cliffs. The annual Brassica nigra (black mustard) is also found along the coast, but also occurs along the sides of rivers or farm tracks (Stace 1997). In Dorset, a southern English county, both species occupy a narrow strip along the coast. This strip comprises the area occupied by unfarmed vegetation on the cliff top, the cliff face and the hinterland of the sandy beaches, with its inland boundary marked by the fenced-off farmland which is usually pasture. The average width of this strip is approximately 10 m, varying from 5 to 20 m, but up to 50 m in few cases. Both species show patchy distributions of populations along this strip which is explained not only by variation in the abiotic and biotic environment but also by historical distributions (Wichmann et al. in press).
The English SWCP generally occupies this strip as well, with both Brassica species growing either side of the path as it follows the Dorset coastline. The SWCP is 1014 km in length, starting at South Haven Point at the eastern edge of the Dorset coast, running west along the southern coast to Lands' End and continuing along the northern coasts of Cornwall and Devon to Minehead in Somerset. The coast and the SWCP attract many walkers, both casual holiday makers in large numbers as well as long-distance walkers. The usage of the SWCP is estimated at approximately 23 million walks per year with 45 per cent of these walks lasting 1–2 hours and 35 per cent lasting longer (Coles et al. 2003). Every year approximately 200 walkers complete the entire length of the SWCP (E. Wallis, SWCP Association 2008, personal communication).
(b) HMD of seeds
(i) Measuring HMD
For our experiments of HMD on shoes, ripe seeds were collected from several populations of each Brassica species along the Dorset coast in autumn 2005 and stored in dark and dry conditions. Seeds of both species are roughly spherical and have a smooth surface with no appendages. Seed size varied between 1.5 and 2.6 mm maximum diameter (n=100; mean=1.98; s.d.=0.27) for B. oleracea and 1.0 and 1.9 mm (mean=1.38; s.d.=0.19) for B. nigra. Prior to experiments, seeds were coloured with bright pink paint (‘mark-it’ by LANDMARK, Wolfurt, Austria) to aid their detection. A pilot study found no difference for coloured and non-coloured seeds in our target measures (t-test; rate seeds picked up pup, d.f.=77, t=−0.84, p=0.40; proportion of seeds left on vector lov, d.f.=37, at 5 m t=1.66, p=0.11 and at 20 m t=−0.20, p=0.84). Soil (a sandy silty loam) was collected from sites on the Dorset cliffs and oven dried at 30°C.
A standardized procedure was used to reduce variation among replicates. Dried soil (500 g) was spread evenly in a tray (0.4×0.25 m2), wetted with 50 ml of water using a plant mister and stirred. A person (the ‘walker’) then placed both shoe-clad feet in this tray and took 20 steps on the spot to pick up soil. The walker then stepped into a second tray containing 100 evenly spread seeds, again taking 20 steps on the spot. The number of seeds picked up was calculated by subtracting the number left in the tray from 100, yielding the pick-up rate (pup). The walker walked a given distance at which point any soil matter and seeds held in the shoe soles were carefully transferred into a third tray. These seeds were counted giving the proportion of seeds left on the shoe vector (lov) after walking a certain distance. In contrast to Will & Tackenberg (2008), our methodology considers dispersal events at single, not multiple, distances, making the data points independent.
This protocol was used in two experiments. In experiment 1, to quantify the dispersal kernel, the footwear (heavy walking boots) was kept constant but distances were varied from 5 to 5000 m for both species (B. nigra: 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 150, 200, 300, 400, 500, 1000, 3000 and 5000 m; B. oleracea: 5, 10, 25, 50, 75, 100, 200, 400, 600, 800, 1000, 2000, 3000 and 5000 m) using only one walker per species. (N.B.: Choice of distances for the second species was informed by results for the first species.) In experiment 2, only three distances (10, 50 and 200 m) and only one species (B. oleracea) were used, but variation among human vectors was assessed using 10 different walkers. Given personal availability, the footwear varied with seven walkers wearing walking boots and the three others wearing Wellington boots (‘rubber boots’). For both experiments, each described parameter combination was repeated 10 times. The order of walks carried out by a walker was randomized with respect to distance. Experiments were carried out only during dry weather conditions from April 2006 to March 2007 at the Winfrith Technology Centre, Dorset, UK on a flat, regularly mown lawn spanning an area of 250×200 m, and walking routes were chosen to minimize repeated coverage of the same area.
(ii) Fitting models to HMD
The exponential function is commonly used to describe the pattern of seed deposition over distance (the dispersal kernel) for a variety of taxa (Willson 1993; Bullock et al. 2002, 2006). Here, we employ the exponential function to describe how seeds attached to the vector change over distance walked. The assumption behind this simple function is that seeds fall from the dispersal vector at a constant rate. Assuming the vector moves at a constant speed in one direction, this gives an exponential function where the proportion of seeds left on the vector (lov) at distance d,
| (2.1) |
Here a is the proportion of attached seeds at d=0, i.e. lov(0)=a, which should be approximately 1, and seeds fall at a constant dropping rate (which in equation 2.1=b). This approach could be given increased flexibility by allowing dropping rate to change with distance. If we use a more general form of the exponential function for lov,
| (2.2) |
where we can examine different forms of the change in dropping rate with distance, f(d). The most straightforward forms of f(d) assume either an exponential or a power function. Ensuring that the functions give a consistent change of lov with distance, that lov cannot become negative and only two parameters a and b are fitted (a and b have different values in each version of f(d)), the exponential relationship gives
| (2.3a) |
or
| (2.3b) |
and the power relationship gives
| (2.4) |
while the original model (equation 2.1) assumes
| (2.5) |
The two versions of the exponential relationship assume either a decrease (equation 2.3a) or an increase (equation 2.3b) in dropping rate f(d), while the power function (equation 2.4) is more flexible with the direction of change depending on the value of b (table 1).
Table 1.
Overview of the alternative models to describe seed dispersal by humans, their general features and fits to our empirical data (Bn, B. nigra; Bo, B. oleracea).
| model (number) equation | lov at d=0 | dropping rate | fitted parameters | residual sum of squares | |
|---|---|---|---|---|---|
| a | b | ||||
| simple exponential (2.5)ae(−bd) | a | b constant | Bn: 0.295 Bo: 0.190 | Bn: 0.00682 Bo: 0.00254 | Bn: 2.26 Bo: 1.52 |
| double exponential (2.3a)ae(e−bd) | ae Bn: 0.290 Bo: 0.196 | be−bd decreasing | Bn: 0.107 Bo: 0.0721 | Bn: 0.0124 Bo: 0.00736 | Bn: 2.44 Bo: 1.55 |
| double exponential (2.3b)ae(−ebd) | a/e Bn: 0.264 Bo: 0.176 | bebd increasing | Bn: 0.717 Bo: 0.479 | Bn: 0.00386 Bo: 0.00134 | Bn: 2.38 Bo: 1.55 |
| power exponential (2.4)ae(−db) | a | bdb−1b>1: increasing; b<1: decreasing | Bn: 1.480 Bo: 0.947 | Bn: 0.191 Bo: 0.165 | Bn: 1.99 Bo: 1.37 |
We used least-squares nonlinear regression to fit these models to the raw data of experiment 1 (walker constant, large range of distances) for both species separately using the R package (R Development Core Team 2008). We can directly compare models as they all have the same number of parameters (i.e. two), and hence we use the residual sum of squares as a measure of goodness of fit.
(c) Simulating dispersal kernels
Our next aim is to use our models to quantify patterns of seed dispersal and to compare primary dispersal by wind only with dispersal by wind followed by secondary dispersal by humans. We therefore aim to derive dispersal kernels that combine these processes for a single plant.
(i) Simulating HMD
We used the model among equations 2.3–2.5 that best fitted the experimental data to create a probability density function. For seeds deposited on the path, this function describes the proportion of seeds dispersed by humans at each of 10 000 intervals of 1 m length up to 10 km covering the spatial scale of many 1 day walks at the SWCP (Coles et al. 2003). This was then used to simulate dispersal distances for individual seeds.
In our experiment, we focused on the detachment of seeds from the shoe vector lov, but the measured attachment rate pup may not match the real situation (due to the high density of seeds and repeated steps). Detailed determination of attachment pup in the ‘natural’ system may require large effort and a separate experimental set-up (e.g. Römermann et al. 2005). Therefore, in our simulations, a wide range of pup was explored using three alternative values: 0.5, 0.1 and 0.01.
(ii) Simulating primary dispersal by wind
The primary dispersal vector for Brassica seeds is wind, when ripe seeds fall to the ground and are transported by wind during their fall (Mitchell & Richards 1979). We quantified natural Brassica wind dispersal kernels by applying a mechanistic model that calculates dispersal trajectories of individual seeds based on seed, plant, vegetation and wind characteristics, the Markov chain Synthetic Turbulence Generation model (Soons et al. 2004a). This model has been shown to be highly accurate in quantifying wind dispersal kernels of species with terminal velocities comparable to the two Brassica species (Soons et al. 2004a,b). Seed and plant characteristics used for simulations with this stochastic model were species-specific seed terminal velocity (average and s.d., measured following Askew et al. 1997) and release height (modal heights of seed pods on plants). Vegetation characteristics were average vegetation height and leaf area index for habitats occupied by each species. To simulate horizontal wind velocities, we used the distribution of hourly averaged horizontal wind velocities measured during the dispersal season of the two species (August–November) in 2000–2006 as data input (weather station Isle of Portland, Dorset, UK grid reference SY677692). Our Brassica species grow close to the cliff edge, and here prolonged upward wind velocities may occur during landward winds, which may uplift seeds and contribute to long-distance dispersal (Nathan et al. 2002; Tackenberg et al. 2003; Soons et al. 2004a). We therefore measured vertical wind velocities at the National Coastwatch Institution station at St. Aldhelms Head (UK grid reference SY961755), directly at the cliff edge, using a 3D sonic anemometer (Windmaster, Gill Instruments Ltd. and CR 3000 data-logger, Campbell Scientific Ltd.), logging vertical wind velocities at 10 Hz during the Brassica dispersal season in August–December 2007. We then ran simulations for three wind scenarios: (scenario 1) flat terrain conditions, using simulated hourly averaged vertical wind velocities equal to zero; (scenario 2) average conditions at the cliffs, using the mean and standard deviation of measured hourly averaged vertical wind velocities during the dispersal season; and (scenario 3) extreme landward wind conditions, using the maximum of upward hourly averaged vertical wind velocities during the dispersal season. In all scenarios, naturally autocorrelated fluctuations in vertical wind velocity around the average value were simulated. All model input data are given in table 2. As the model is stochastic, we simulated dispersal of 50 000 seeds for each species and each scenario.
Table 2.
Input parameters for the mechanistic wind dispersal model.
| terminal velocity (ms−1) | B. nigra: 3.7±0.10 | B. oleracea: 3.9±0.11 |
| plant height (m) | B. nigra: 0.64–1.22 | B. oleracea: 0.37–0.64 |
| vegetation height (m) | B. nigra: 1.1 | B. oleracea: 0.27 |
| leaf area index | B. nigra: 2.57 | B. oleracea: 4.56 |
| hourly averaged horizontal wind velocity (ms−1) | frequency distribution, ranging from 0 to 25 | |
| hourly averaged vertical wind velocity (ms−1) | scenario 1, flat terrain | 0 |
| scenario 2, average conditions at cliff edge | 0.07±0.28 | |
| scenario 3, extreme conditions at cliff edge | 1 |
(iii) How many seeds land on the path?
The next aim is to calculate how many seeds are available for secondary dispersal by humans, i.e. how many seeds land on the path. From our observations, we assumed a straight path of width 1 m, and the plant is at a distance of z metres away from the path. First, the wind dispersal probability density function f(x) was derived from the simulations of wind-dispersed distances using scenario 2 (average conditions at the cliffs). This was converted to a radially symmetric two-dimensional probability density function with appropriate scaling. Finally, the following integral was numerically approximated for chosen values of z (using two examples of 0 and 3 m) using Matlab. The y integral, with infinite limits, was suitably truncated.
| (2.6) |
where
| (2.7) |
and
| (2.8) |
The above calculations were applied using the average number of seeds dispersing from an individual plant. For B. nigra, this was calculated by counting the number of seeds in 100 pods and estimating the number of pods on 100 plants at each of three sites in each of 3 years (2005–2007). For the perennial B. oleracea, seed production was calculated for approximately 150 reproductive plants in each year (2002–2007) at each of the same three sites by estimating pod number per plant and counting seeds in a sample of pods from each plant.
(iv) Simulating the combined dispersal kernel
Bringing together our calculated predictions of seeds available for HMD (reaching the path) and using the model best fitting the experimental data, we can now calculate the combined dispersal kernels for wind and human dispersal under four scenarios of two species and two locations of the source plant in relation to the path. The number of seeds dispersed onto the path by wind in accordance with each of the four scenarios yielded the number of seeds for one simulation replicate. One thousand simulation replicates were performed (using R package) and the average number of seeds per distance was calculated in binned categories.
3. Results
(a) Experiment 1
In experiment 1 for both species, seeds were regularly found still attached after 5000 m, although more than 50 per cent of seeds fell off within 5 m (figure 1). Both species showed a high level of variation in the proportion of seeds remaining attached (lov), with the coefficient of variation ranging from 0.22 to 2.6, and this proportion did not differ between species (natural log transformation: ANOVA, d.f.=1169, p=0.235) or show an interaction effect of species with distance (p=0.212). Pick-up rates (pup) varied greatly from 0.04 to 0.93 (mean 0.52 and 0.42 for B. oleracea and B. nigra, respectively).
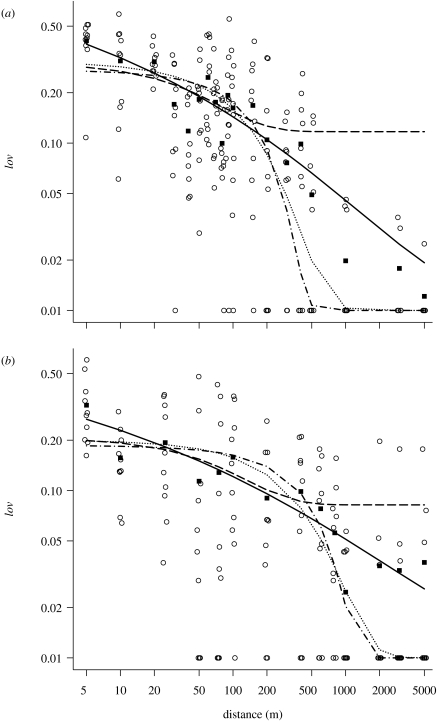
Figure 1.
The proportion of seeds left on the shoe, lov, per distance walked, d. Ten replicates (circles) were performed at each distance by one single walker each for (a) B. nigra and (b) B. oleracea. Means (squares) and model fits (lines, compare table 1) are shown. A small amount of random variation j (log j=0.3) was applied to the x-axis values of the data (‘jitter’) in order to visually separate replicates with identical results. Also, 0.01 was added to each value of lov, including averages and model plots in order to visualize lov=0 on this log–log plot. Dotted line, ae(−bd) (equation 2.5); solid line, ae(−db) (equation 2.4); dashed line, ae(e−bd) (equation 2.3a); dot-dashed line, ae(−ebd) (equation 2.3b).
(b) Model fitting
All models (equations 2.3–2.5) explained a significant amount of variation in our data (p<0.05). The simple exponential model (equation 2.5) represented lov poorly, both at the shorter and longer distances; in both the regions the predicted lov was too low (figure 1). In addition, the fitted a was much less than 1 (table 1) in contrast to the expected value of 1. Comparing the more flexible models which allowed a change of dropping rate with distance, the double exponential model is less satisfactory because both versions predicted lov(0)≪1 and the fitted flat tail was either fat and seriously over-predicted lov at long distances (equation 2.3a) or was rather thin and under-predicted lov (equation 2.3b). The residual sum of squares indicate that the model fits for the double exponential (equations 2.3a,b) were worse than for the simple exponential (equation 2.5). For the power exponential model the fitted a gives the expected lov(0) approximately 1, and both parameters result in a tail which fitted the data well. Overall, the power exponential model showed the best fit for both species, in terms of residual sum of squares (table 1, figure 1).
(c) Simulating dispersal kernels
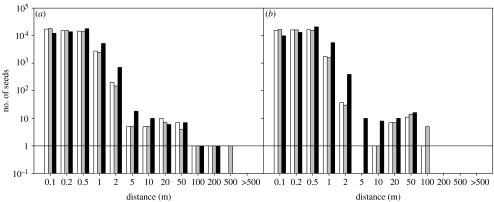
Simulated distances for wind dispersal (figure 2) were shorter than distances measured and modelled for HMD (compare figure 1). By wind, the vast majority of seeds are dispersed within 2 m of the parent plant due to the relatively high terminal velocities of seeds, but small numbers of seeds (of the order of 1 seed per 50 000) travel over distances up to 70 m (B. oleracea) and 221 m (B. nigra). Owing to the relatively high terminal velocities (table 2), there were only small differences between the three wind scenarios. Use of maximum vertical wind speeds in the simulations led to a small increase in the proportion of seeds dispersed beyond short distances (i.e. more than 0.5 m), but did not increase the maximum distances dispersed (figure 2).
Figure 2.
Predicted dispersal kernels from the mechanistic wind dispersal model using local data and vertical wind velocities of zero average (table 2, scenario 1, white bars), measured average (scenario 2, grey bars) and measured hourly extreme (scenario 3, black bars) conditions for (a) B. nigra and (b) B. oleracea. For each of the three scenarios, 50 000 seeds were simulated for both species. x-axis values represent upper limits of binned results. A solid line indicates y=1.
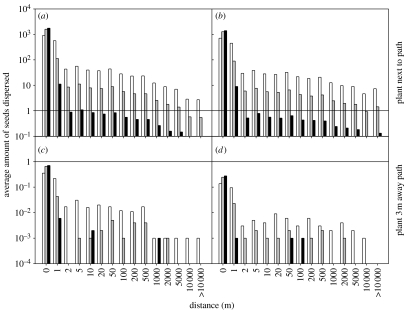
The data indicated an average of 3584 and 2832 seeds per reproductive plant for B. nigra and B. oleracea, respectively. The number of seeds landing on the path varied strongly with the distance of the mother plant from the path. For a plant located at the path edge, the probability of each seed to land on the path was 0.496 and 0.499 for B. nigra and B. oleracea, respectively, resulting in an average of 1778 and 1413 seeds per plant deposited on the path. For a plant located 3 m away from the path edge the respective probabilities were 0.0002 and 0.0001, resulting in 0.72 and 0.28 seeds per plant landing on the path.
The dispersal kernels combining wind and human dispersal demonstrated the potential for seed dispersal over many kilometres, up to at least 10 km. The exact pattern was affected by greater proximity to the path and higher pick-up rates, both of which led to longer dispersal distances (figure 3).
Figure 3.
Combined dispersal kernel for wind dispersal and subsequent shoe dispersal. Average vertical winds (table 2, scenario 2) and the fitted power–exponential model (table 1, equation 2.4) were used. (a–d) correspond to four scenarios: (a,b) plant growing next to the path or (c,d) 3 m away for (a,c) B. nigra and (b,d) B. oleracea. Hence, the average amount of seeds on the path that were potentially available to shoe dispersal varied between subfigure settings (a, 1778; b, 1413; c, 0.72; d, 0.28). The probability of a seed on the path to be picked up by the walker's shoes was also varied (white bars, 0.5; grey bars, 0.1; black bars, 0.01). Results were averaged over 1000 replicated simulations. x-axis values represent upper limits of binned results. A solid line indicates y=1.
(d) Experiment 2
In experiment 2, pup differed greatly among individual walkers (0.26–0.52; ANOVA, d.f.=9288; p<0.001), but not among walked distances as expected (d.f.=2288; p=0.773). The proportion of seeds remaining on shoes (lov) differed among walkers (0.037–0.14; ANOVA on arcsine transformed data, d.f.=9288, p<0.01) and distances (d.f.=2288, p<0.001). Tukey's pairwise comparisons suggested that the cause of lov differences among walkers was due to the effects of footwear (probably through differences in the material of the sole and the pattern of tread). Two individuals wearing Wellington boots had significantly lower lov values than all wearing walking boots and a third wearer of Wellingtons. On removing those two individuals from the ANOVA the distance effect remained (d.f.=2230, p<0.001) but the walker effect disappeared (d.f.=7230, p=0.350). Using shoe size as an ANOVA co-variate did not show any effects on pup or lov.
4. Discussion
(a) Long-distance dispersal through human vectors
This study gives empirical evidence for the dispersal of seeds on shoes over very long distances. These long distances (more than 5 km) were achieved by means of secondary dispersal on human vectors and were frequently at least one order of magnitude higher than maximum distances predicted for primary dispersal by wind. This represents a step forward in the studies of HMD by describing a detailed dispersal kernel which can be used to understand the dynamics of plants at large scales. Interestingly, using molecular markers, Raybould et al. (1999) concluded that there is ‘restricted but significant gene flow among the Dorset populations’ of B. oleracea, a pattern which may be driven by the dispersal mechanism evidenced in our study.
Very few published studies have considered seed dispersal by walking humans (Praeger 1915; Woodruffe-Peacock 1918; Ridley 1930; Healy 1943 and references therein; Clifford 1956; Higashino et al. 1983; Kirby 2008). Occasionally, a single dispersal distance has been reported for this mechanism (Carey & Watkinson 1993: 0.19 m). Bullock & Primack (1977) measured seed retention of three plant species on shirts and trousers over distances of up to 250 m. Beyond this, no study appears to have quantified a detailed kernel for dispersal directly by humans. Moreover, our study provides one of the first kernels for any form of HMD. Considering human-associated vectors, kernels have been reported by Bullock et al. (2003, 2008) for dispersal by agricultural machinery and by Manzano & Malo (2006) for dispersal in the fleece of domestic sheep.
Our study introduces a new feature for seed dispersal models by allowing the rate at which seeds fall off their vector to change with distance. For the very flexible exponential power model, the parameter b converged to values indicating a decrease in this dropping rate with distance. This implies a process by which seeds are attached to the vector with varying strengths of adhesion. The experiments suggest that many seeds attach loosely and detach rapidly, while a small proportion of seeds attach more strongly and are carried for much longer distances. This finding is in contrast to many studies on animal-mediated dispersal, which assume detachment rates to be independent of distance and time (e.g. Wehncke et al. 2003; Will & Tackenberg 2008).
The exponential power model (equation 2.4) best fits the data and also has several advantages over other models. First, it can be seen as semi-mechanistic as its mathematical notation is inspired by an ecological process, i.e. the rate at which seeds fall off their vector. Second, this is a relatively simple model as it is restricted to two parameters. Third, each of the two parameters is used only once and, unlike some dispersal functions, has a clear biological meaning (a yields seeds on vector at distance 0, and b yields the dropping rate) (c.f. Bullock et al. 2006; Jongejans et al. 2008). The above points also hold for all other models used here. Fourth, the exponential power model is more flexible than the double and simple exponential models, and allows the direction of change in dropping rate to emerge from fitting the model to the data.
To predict primary dispersal by wind, we used local environmental and species-specific data informing a well-validated model (Nathan et al. 2002; Soons et al. 2004b; Soons & Bullock 2008). In coastal systems, landward winds may be of disproportionate importance for dispersal of seeds (Greene et al. 2008). In our system, strong landward winds are the likely cause of the measured prolonged upward movement of air at the cliff-top, and our simulation results showed its potential to increase wind dispersal distances under extreme conditions. However, for both Brassica species, wind dispersal distances were generally low as seeds are spherical and have relatively high terminal velocities.
Our results for secondary dispersal by humans, both predicted (more than 10 km) and measured (more than 5 km), evidence much longer distances than those predicted for wind dispersal (<500 m). This matches an observation already made for Vulpia ciliata by Carey & Watkinson (1993), albeit at a much smaller spatial scale (<0.2 m). While this comparison remains to be tested for species with clear morphological adaptations for wind dispersal, ‘wind-dispersed’ herbs and shrubs can show even lower modelled and measured wind dispersal distances than both of our Brassica species (Skarpaas & Shea 2007; Soons & Bullock 2008). Whether or not primary wind dispersal is followed by secondary dispersal on human shoes for an individual seed is likely to be highly stochastic and would depend strongly on the distance of the mother plant from the path and on pick-up probabilities.
Other potential Brassica dispersal vectors in this system are unlikely to lead to effective long-distance dispersal. While seeds may be transported long distances by sea, they are highly unlikely to travel from the sea to the cliff-tops, which reach 100 m above sea level. Livestock are excluded, and small rodents that may gather seeds have relatively small foraging ranges (e.g. <300 m diameter for Apodemus sylvaticus in a coastal area; Corp et al. 1997).
When comparing different walkers, the reasons for the variation in pick-up rate remains unclear as the most obvious co-variate, shoe size, did not show any effects. The lack of an among-walker difference in detachment rate may be explained by the great variation among the replicate walks by a single walker in experiment 1. The exact causes of this within-walker variation are unclear, but it suggests that this dispersal mechanism is a highly stochastic process. Stochasticity is thought to be integral to the dispersal process (Clark et al. 2003), which suggests that dispersal studies should more often include replication (see Bullock et al. 2006).
One may argue that seeds still attached to the vector after the maximum distance measured may never be released, i.e. they may be ‘stuck’ (e.g. Will & Tackenberg 2008) and thus may not be successfully dispersed to new sites. Indeed, Manzano & Malo (2006) found that a large proportion of those seeds remaining in the fleece of sheep after long-distance movements were still attached when the sheep were shorn. We assessed the propensity for seeds to remain attached to shoes for very long periods by using our protocol and two walkers spending a day (roughly 8 hours) walking and working on the Dorset cliffs on five separate days. In none of the 10 replicates were any seeds left on the shoe after a day of walking. We conclude that seeds may remain attached to the shoe for a long distance walked but that they eventually fall off and are thus potentially able to colonize new sites.
It must also be considered whether seeds dispersed on shoes arrive at conditions that are suitable for germination and establishment. For example, Bullock et al. (2008) showed that hay cutting by humans benefited both seed dispersal (attachment to machinery) and establishment (creation of gaps) of an annual herb. It is possible that the mud in which seeds are transported on the shoe aids germination and establishment. Another possibility is that the shoe-dispersed seeds may have a good chance to arrive into disturbed habitat near the coast path. Sowing experiments have shown that both Brassica species establish better in disturbed conditions (M. C. Wichmann 2005–2007, unpublished data), so HMD on shoes may be followed by high establishment rates. Alternatively, because walkers leave the cliffs after a walk, seeds may leave the shoe vector at urban and other unsuitable sites for establishment.
(b) Simulation uncertainties
Our simulations show the potential of secondary dispersal of seeds on human shoes, both in terms of numbers and distances covered. However, we consider our simulation results as an indication of the potential of these combined modes of dispersal as they remain subject to several factors causing uncertainty, which we discuss below.
Our measured pick-up rates (pup) for seeds on shoes were probably artificially high and also underlie inherent variation. In particular, experiment 2 comparing among walkers emphasizes variation in pup but much less so in the rate of seeds dispersed, lov. Accordingly, our simulation scenarios explore a wide range of pup, including the magnitude of our average measured (0.5), as well as two considerably lower values (0.1 and 0.01). Attachment rates are generally not well known in animal (Wehncke et al. 2003), human (Manzano & Malo 2006; von der Lippe & Kowarik 2007) or even wind-mediated dispersal (i.e. abscission; but see Jongejans et al. 2007; Soons & Bullock 2008), and a detailed quantification may be amenable to further studies (e.g. Römermann et al. 2005). Our results emphasize the impact of attachment rates on the dispersal kernel.
Even if one single seed may hardly be sufficient to found a new population, our simulations suggest that tens to hundreds of seeds can be dispersed over hundreds to thousands of metres. While these are the results for one single mother plant, in reality a population consists of hundreds of plants and hence a much larger number of seeds may be moved over long distances. Moreover, our results consider only one single walker while several million people walk on the SWCP every year (Coles et al. 2003). Therefore, a seed could be subject to multiple HMD events by attachment to a sequence of walkers. Interestingly, in our study area, we observe low frequencies of B. oleracea in areas poorly frequented by walkers (e.g. White Nothe) but higher frequencies in areas heavily frequented by holidaymakers (e.g. Durdle Door). From our data we cannot conclude on a general pattern, and a more detailed study including the whole SWCP may confirm or reject this hypothesis. Many walkers may finish their coastal walks before seeds fall from their shoes and thus reduce the number of seeds dispersed long distances. Nevertheless, 8 per cent of all walks on the SWCP last for more than 4 hours (Coles et al. 2003) and thus potentially cover distances of more than 10 km.
(c) The potential of HMD
Here we have studied a mechanism of HMD on shoes. However, there are indications, and in some cases evidence, for other forms of HMD of seeds and propagules of small animals. These include dispersal by cars (von der Lippe & Kowarik 2007), by boat (Buchan & Padilla 1999), in packaging material (Ridley 1930) or by soil transport (Hodkinson & Thompson 1997). Dispersal on shoes may be a basic form of HMD as the walker may not simply disperse seeds by walking but may use other means of transport such as cars, boats or aeroplanes and thus potentially carry seeds on his or her shoes over much longer distances than suggested here.
Acknowledgments
We are thankful to Carsten Eichberg and Tom Harwood for their advice, Ken Thompson for measuring terminal velocities and Chris Quarrie, Graham Leven and all members of the St Aldhelm's station of the National Coastwatch Institution for having our anemometer on their roof. The British Atmospheric Data Centre provided access to the Portland wind data. European Union funding supported M.B.S. under Euro-limpacs (GOCE-CT-2003-505540) and M.N. under Evoltree. All other authors were funded by the NERC grant NE/B503141/1.
References
- Andrén H. Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos. 1994;71:355–366. doi:10.2307/3545823 [Google Scholar]
- Askew A.P., Corker D., Hodkinson D.J., Thompson K. A new apparatus to measure the rate of fall of seeds. Funct. Ecol. 1997;11:121–125. doi:10.1046/j.1365-2435.1997.00049.x [Google Scholar]
- Blaum N., Wichmann M.C. Short-term transformation of matrix into hospitable habitat facilitates gene flow and mitigates fragmentation. J. Anim. Ecol. 2007;76:1116–1127. doi: 10.1111/j.1365-2656.2007.01283.x. doi:10.1111/j.1365-2656.2007.01283.x [DOI] [PubMed] [Google Scholar]
- Boedeltje G., Bakker J.P., Bekker R.M., Van Groenendael J.M., Soesbergen M. Plant dispersal in a lowland stream in relation to occurrence and three specific life-history traits of the species in the species pool. J. Ecol. 2003;91:855–866. doi:10.1046/j.1365-2745.2003.00820.x [Google Scholar]
- Bonn S., Poschlod P. Quelle & Meyer; Wiesbaden, Germany: 1998. Ausbreitungsbiologie der Pflanzen Mitteleuropas: Grundlagen und historische Aspekte. [Google Scholar]
- Buchan L.A.J., Padilla D.K. Estimating the probability of long-distance overland dispersal of invading aquatic species. Ecol. Appl. 1999;9:254–265. doi:10.1890/1051-0761(1999)009[0254:ETPOLD]2.0.CO;2 [Google Scholar]
- Bullock J.M., Clarke R.T. Long distance seed dispersal by wind: measuring and modelling the tail of the curve. Oecologia. 2000;124:506–521. doi: 10.1007/PL00008876. doi:10.1007/PL00008876 [DOI] [PubMed] [Google Scholar]
- Bullock J.M., Kenward R.E., Hails R.S. Blackwell Science; Oxford, UK: 2002. Dispersal ecology. [Google Scholar]
- Bullock J.M., Moy I.L., Coulson S.J., Clarke R.T. Habitat-specific dispersal: environmental effects on the mechanisms and patterns of seed movement in a grassland herb Rhinanthus minor. Ecography. 2003;26:692–704. doi:10.1034/j.1600-0587.2003.03525.x [Google Scholar]
- Bullock J.M., Shea K., Skarpaas O. Measuring plant dispersal: an introduction to field methods and experimental design. Plant Ecol. 2006;186:217–234. doi:10.1007/s11258-006-9124-5 [Google Scholar]
- Bullock J.M., Pywell R.F., Coulson-Phillips S.J. Managing plant population spread: prediction and analysis using a simple model. Ecol. Appl. 2008;18:945–953. doi: 10.1890/07-1128.1. doi:10.1890/07-1128.1 [DOI] [PubMed] [Google Scholar]
- Bullock S.H., Primack R.B. Comparative experimental study of seed dispersal on animals. Ecology. 1977;58:681–686. doi:10.2307/1939019 [Google Scholar]
- Cain M.L., Milligan B.G., Strand A.E. Long-distance seed dispersal in plant populations. Am. J. Bot. 2000;87:1217–1227. doi:10.2307/2656714 [PubMed] [Google Scholar]
- Carey P.D., Watkinson A.R. The dispersal and fates of seeds of the winter annual grass Vulpia ciliata. J. Ecol. 1993;81:759–767. doi:10.2307/2261673 [Google Scholar]
- Clark J.S., Lewis M., McLachlan J.S., HilleRisLambers J. Estimating population spread: what can we forecast and how well? Ecology. 2003;84:1979–1988. doi:10.1890/01-0618 [Google Scholar]
- Clifford H.T. Seed dispersal on footwear. Proc. Bot. Soc. Brit. Isles. 1956;2:129–131. [Google Scholar]
- Clifford H.T. Seed dispersal by motor vehicles. J. Ecol. 1959;47:311–315. doi:10.2307/2257368 [Google Scholar]
- Clobert J., Danchin E., Dhondt A.A., Nichols J.D. Oxford University Press; Oxford, UK: 2001. Dispersal. [Google Scholar]
- Coles T., Hudson P., Stevens E. 2003. The economic value of the South West Coast Path. Exeter, UK: Tourism Associates & South West Tourism. [Google Scholar]
- Corp N., Gorman M.L., Speakman J.R. Ranging behaviour and time budgets of male wood mice Apodemus sylvaticus in different habitats and seasons. Oecologia. 1997;109:242–250. doi: 10.1007/s004420050079. doi:10.1007/s004420050079 [DOI] [PubMed] [Google Scholar]
- Cushman J.H., Meentemeyer R.K. Multi-scale patterns of human activity and the incidence of an exotic forest pathogen. J. Ecol. 2008;96:766–776. doi:10.1111/j.1365-2745.2008.01376.x [Google Scholar]
- Gilbert M., Gregoire J.C., Freise J.F., Heitland W. Long-distance dispersal and human population density allow the prediction of invasive patterns in the horse chestnut leafminer Cameraria ohridella. J. Ecol. 2004;73:459–468. doi:10.1111/j.0021-8790.2004.00820.x [Google Scholar]
- Greene D.F., Quesada M., Calogeropoulos C. Dispersal of seeds by the tropical sea breeze. Ecology. 2008;89:118–125. doi: 10.1890/06-0781.1. doi:10.1890/06-0781.1 [DOI] [PubMed] [Google Scholar]
- Healy A.J. Seed dispersal by human activity. Nature. 1943;151:140. doi:10.1038/151140b0 [Google Scholar]
- Higashino P.K., Guyer W., Stone C.P. The Kilauea Wilderness Marathon and Crater Rim Runs: sole searching experiences. Newslett. Hawaii. Bot. Soc. 1983;22:25–28. [Google Scholar]
- Higgins S.I., Nathan R., Cain M.L. Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal? Ecology. 2003;84:1945–1956. doi:10.1890/01-0616 [Google Scholar]
- Hodkinson D.J., Thompson K. Plant dispersal: the role of man. J. Appl. Ecol. 1997;34:1484–1496. doi:10.2307/2405264 [Google Scholar]
- Humboldt A.v., Bonpland A. Wissenschaftliche Buchgesellschaft, Nachdruck; Darmstadt, Germany: 1807. Ideen zu einer Geographie der Pflanzen, 1963. [Google Scholar]
- Jongejans E., Pedatella N.M., Shea K., Skarpaas O., Auhl R. Seed release by invasive thistles: the impact of plant and environmental factors. Proc. R. Soc. B. 2007;274:2457–2464. doi: 10.1098/rspb.2007.0190. doi:10.1098/rspb.2007.0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejans E., Skarpaas O., Shea K. Dispersal, demography and spatial population models for conservation and control management. Perspect. Plant Ecol. 2008;9:153–170. doi:10.1016/j.ppees.2007.09.005 [Google Scholar]
- Jordano P., Garcia C., Godoy J.A., Garcia-Castano J.L. Differential contribution of frugivores to complex seed dispersal patterns. Proc. Natl Acad. Sci. USA. 2007;104:3278–3282. doi: 10.1073/pnas.0606793104. doi:10.1073/pnas.0606793104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K.J. Now wash your boots! BES Bull. 2008;39:41. [Google Scholar]
- Kuparinen A. Mechanistic models for wind dispersal. Trends Plant Sci. 2006;11:296–301. doi: 10.1016/j.tplants.2006.04.006. doi:10.1016/j.tplants.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Lonsdale W.M., Lane A.M. Tourist vehicles as vectors of weed seeds in Kakadu National Park, Northern Australia. Biol. Conserv. 1994;69:277–283. doi:10.1016/0006-3207(94)90427-8 [Google Scholar]
- Manzano P., Malo J.E. Extreme long-distance seed dispersal via sheep. Front. Ecol. Environ. 2006;4:244–248. doi:10.1890/1540-9295(2006)004[0244:ELSDVS]2.0.CO;2 [Google Scholar]
- Mayfield M.M., Ackerly D., Daily G.C. The diversity and conservation of plant reproductive and dispersal functional traits in human-dominated tropical landscapes. J. Ecol. 2006;94:522–536. doi:10.1111/j.1365-2745.2006.01108.x [Google Scholar]
- Mitchell N.D., Richards A.J. Biological flora of the British Isles: Brassica oleracea L. ssp. oleracea. J. Ecol. 1979;67:1087–1096. doi:10.2307/2259229 [Google Scholar]
- Nathan R. Long-distance dispersal of plants. Science. 2006;313:786–788. doi: 10.1126/science.1124975. doi:10.1126/science.1124975 [DOI] [PubMed] [Google Scholar]
- Nathan R., Katul G.G., Thomas S.M., Oren R., Avissar R., Pacala S.W., Levin S.A. Mechanisms of long-distance dispersal of seeds by wind. Nature. 2002;418:409–413. doi: 10.1038/nature00844. doi:10.1038/nature00844 [DOI] [PubMed] [Google Scholar]
- Praeger R.L. A biological survey of Clare Island in the county of Mayo, Ireland. Proc. R. Irish Acad. 1915;31:47–54. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. See http://www.R-project.org. [Google Scholar]
- Raybould A.F., Mogg R.J., Clarke R.T., Gliddon C.J., Gray A.J. Variation and population structure at microsatellite and isozyme loci in wild cabbage (Brassica oleracea L.) in Dorset (UK) Genet. Res. Crop Evol. 1999;46:351–360. doi:10.1023/A:1008658630440 [Google Scholar]
- Rees M. Trade-offs among dispersal strategies in British plants. Nature. 1993;366:150–152. doi:10.1038/366150a0 [Google Scholar]
- Ridley H.N. Reeve; Ashford, UK: 1930. The dispersal of plants throughout the world. [Google Scholar]
- Ries L., Fletcher R.J., Battin J., Sisk T.D. Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu. Rev. Ecol. Evol. Syst. 2004;35:491–522. doi:10.1146/annurev.ecolsys.35.112202.130148 [Google Scholar]
- Römermann C., Tackenberg O., Poschlod P. How to predict attachment potential of seeds to sheep and cattle coat from simple morphological seed traits. Oikos. 2005;110:219–230. doi:10.1111/j.0030-1299.2005.13911.x [Google Scholar]
- Skarpaas O., Shea K. Dispersal patterns, dispersal mechanisms, and invasion wave speeds for invasive thistles. Am. Nat. 2007;170:421–430. doi: 10.1086/519854. doi:10.1086/519854 [DOI] [PubMed] [Google Scholar]
- Soons M.B., Bullock J.M. Non-random seed abscission, long-distance wind dispersal and plant migration rates. J. Ecol. 2008;96:581–590. doi:10.1111/j.1365-2745.2008.01370.x [Google Scholar]
- Soons M.B., Heil G.W., Nathan R., Katul G.G. Determinants of long-distance seed dispersal by wind in grasslands. Ecology. 2004a;85:3056–3068. doi:10.1890/03-0522 [Google Scholar]
- Soons M.B., Nathan R., Katul G.G. Human effects on long-distance wind dispersal and colonization by grassland plants. Ecology. 2004b;85:3069–3079. doi:10.1890/03-0398 [Google Scholar]
- Spiegel O., Nathan R. Incorporating dispersal distance into the disperser effectiveness framework: frugivorous birds provide complementary dispersal to plants in a patchy environment. Ecol. Lett. 2007;10:718–728. doi: 10.1111/j.1461-0248.2007.01062.x. doi:10.1111/j.1461-0248.2007.01062.x [DOI] [PubMed] [Google Scholar]
- Stace C. 2nd edn. Cambridge University Press; Cambridge, UK: 1997. New flora of the British Isles. [Google Scholar]
- Suarez A.V., Holway D.A., Case T.J. Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proc. Natl Acad. Sci. USA. 2001;98:1095–1100. doi: 10.1073/pnas.98.3.1095. doi:10.1073/pnas.98.3.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora K.V. History of the impact of man on the distribution of plant species. In: Di Castri F., Hansen A.J., Debuschke M., editors. Biological invasions in Europe and the Mediterranean basin. Kluwer; Dordrecht, The Netherlands: 1990. pp. 37–50. [Google Scholar]
- Tackenberg O., Poschlod P., Bonn S. Assessment of wind dispersal potential in plant species. Ecol. Monogr. 2003;73:191–205. doi:10.1890/0012-9615(2003)073[0191:AOWDPI]2.0.CO;2 [Google Scholar]
- Thompson K., Jones A. Human population density and prediction of local plant extinction in Britain. Conserv. Biol. 1999;13:185–189. doi:10.1046/j.1523-1739.1999.97353.x [Google Scholar]
- Vogt K., Rasran L., Jensen K. Water-borne seed transport and seed deposition during flooding in a small river-valley in Northern Germany. Flora. 2004;199:377–388. doi:10.1078/0367-2530-00166 [Google Scholar]
- von der Lippe M., Kowarik I. Long-distance dispersal of plants by vehicles as a driver of plant invasions. Conserv. Biol. 2007;21:986–996. doi: 10.1111/j.1523-1739.2007.00722.x. doi:10.1111/j.1523-1739.2007.00722.x [DOI] [PubMed] [Google Scholar]
- Wehncke E.V., Hubbell S.P., Foster R.B., Dalling J.W. Seed dispersal patterns produced by white-faced monkeys: implications for the dispersal limitation of neotropical tree species. J. Ecol. 2003;91:677–685. doi:10.1046/j.1365-2745.2003.00798.x [Google Scholar]
- Wichmann, M. C., Alexander, M. J., Hails, R. S. & Bullock, J. M. In press. Historical distribution and regional dynamics of two Brassica species. Ecography31 (doi:10.1111/j.0906-7590.2008.05564.x)
- Will H., Tackenberg O. A mechanistic simulation model of seed dispersal by animals. J. Ecol. 2008;96:1011–1022. doi:10.1111/j.1365-2745.2007.01341.x [Google Scholar]
- Willson M.F. Dispersal mode, seed shadows, and colonization patterns. Vegetation. 1993;108:261–280. [Google Scholar]
- Woodruffe-Peacock E.A. A fox-covert study. J. Ecol. 1918;6:110–125. doi:10.2307/2255362 [Google Scholar]