Abstract
The endemic Hawaiian lobeliads are exceptionally species rich and exhibit striking diversity in habitat, growth form, pollination biology and seed dispersal, but their origins and pattern of diversification remain shrouded in mystery. Up to five independent colonizations have been proposed based on morphological differences among extant taxa. We present a molecular phylogeny showing that the Hawaiian lobeliads are the product of one immigration event; that they are the largest plant clade on any single oceanic island or archipelago; that their ancestor arrived roughly 13 Myr ago; and that this ancestor was most likely woody, wind-dispersed, bird-pollinated, and adapted to open habitats at mid-elevations. Invasion of closed tropical forests is associated with evolution of fleshy fruits. Limited dispersal of such fruits in wet-forest understoreys appears to have accelerated speciation and led to a series of parallel adaptive radiations in Cyanea, with most species restricted to single islands. Consistency of Cyanea diversity across all tall islands except Hawai `i suggests that diversification of Cyanea saturates in less than 1.5 Myr. Lobeliad diversity appears to reflect a hierarchical adaptive radiation in habitat, then elevation and flower-tube length, and provides important insights into the pattern and tempo of diversification in a species-rich clade of tropical plants.
Keywords: community assembly, ecological saturation, island radiation, species richness
1. Introduction
The endemic Hawaiian lobeliads (6 genera, 126 spp.) have long been viewed as one of the most spectacular examples of adaptive radiation in plants (Rock 1919; Carlquist 1970, 1974; Mabberley 1974, 1975; Givnish et al. 1995, 2004; Givnish 1998, 1999a; Lammers 1999). They represent the largest family of Hawaiian angiosperms, comprising one-eighth of the native flora of the most isolated archipelago on the Earth (Wagner 1999). They include high-elevation bog rosettes, cliff succulents, forest and bog shrubs and trees, and even a few epiphytes and vine-like species, and vary strikingly in floral form and leaf shape (figure 1). The origins of this group have been hotly debated, with the morphological data suggesting three to five independent colonization events (table A1 in the electronic supplementary material), even though Hawai `i is more than 3600 km away from the nearest continent and most other tall islands in the central Pacific lack woody lobeliads entirely.
Figure 1.
Representative habit and habitat of the endemic genera/sections of Hawaiian lobeliads: (a) Lobelia gloriamontis (sect. Galeatella), montane bog atop west Maui, (b) Trematolobelia kauaiensis, wet subalpine opening, Kaua `i, (c) Brighamia rockii, Kapailoa cliffs, Moloka `i, (d) C. hamatiflora (tree with long leaves), streamside cloud forest, east Maui, (e) Cyanea floribunda, cloud-forest understorey, Hawai `i, (f) Clermontia kakeana, cloud-forest gap, west Maui, and (g) Delissea rhytidosperma, outplanting from mesic forest, Waimea Canyon, Kaua `i. Photo credits: (a,c) K.R.W., (b,d) K.J.S., (e,f) T.J.G., (g) Vickie Caraway, Hawai `i Department of Land and Natural Resources.
To investigate the origin(s) and patterns of diversification of the Hawaiian lobeliads, we obtained a molecular phylogeny based on seven rapidly evolving segments of the plastid genome of representatives of each of the endemic Hawaiian genera/sections and all proposed groups of relatives or ancestors from Africa, Asia, Australia, South America, the Greater Antilles and the Pacific Basin (table A1). We overlaid morphological, ecological and biogeographic character states on this phylogeny to evaluate their evolution. We inferred the time of origin of the Hawaiian taxa by calibrating the molecular phylogeny against the ages of fossil Asterales, and against the ages of islands to which individual Hawaiian species are restricted. Finally, we compiled data on the geographical distribution of all currently recognized species of Hawaiian lobeliads, and used these to assess whether they have evolved a predictable number of species per island, and if so, how rapidly such diversity reaches saturation.
2. Material and methods
We sequenced the rpl16 intron, the 5′ end of rbcL and five rapidly evolving intergenic spacers (psbA-trnH, trnL-trnF, trnT-trnL, tnrV-tnrk and atpB-rbcL) from the plastid genome of 23 species representing all endemic Hawaiian genera, both Hawaiian sections of Lobelia and 15 species representing all groups previously proposed as close relatives or potential ancestors (table A1). Previous RFLP studies encompassing most extant species indicated that the endemic Hawaiian genera and sections of Lobelia are each monophyletic (Givnish 1998, 1999a). Methods for amplification, sequencing and alignment are detailed in the electronic supplementary material. We evaluated evolutionary relationships using maximum parsimony (MP), maximum likelihood (ML) and Bayesian analysis (BA), employing Lobelia cardinalis and Lobelia vivaldii as out-groups. MP analysis was conducted in PAUP* v. 4.0b8 (Swofford 2002), giving nucleotide and indel characters equal weights. One thousand MP replicate searches were begun with random starting trees, and run to completion using tree–bisection–reconnection branch swapping while retaining multiple trees. Using the same search strategy and 500 replicate resamplings of the data, jackknife support for each node was evaluated and a parsimony jackknife tree was constructed following Farris et al. (1996). Procedures for ML and BA, using only nucleotide characters, are described in the electronic supplementary material. Out-groups were identified based on an extensive analysis of rbcL sequences across campanulid asterids, using Arabidopsis as the out-group (see the electronic supplementary material). This study indicated that rbcL has little phylogenetic signal for resolving relationships among the very closely related in-group taxa, and that Pratia borneensis (one proposed relative; table 1) was highly divergent from all other in-group taxa. Consequently, only a relatively variable portion of rbcL (approx. 350 nt near the 5′ end) was included in the in-group analysis, and Pratia was excluded.
We overlaid morphological, ecological and biogeographic character states using MacClade v. 4.0 (Maddison & Maddison 2001) on the parsimony jackknife tree. This tree is identical in topology to one of the two MP trees (figure 2) and preserves the monophyly of Cyanea, consistent with previous analyses (Givnish et al. 1995). It is also identical to the ML and BA trees, except for Isotoma's placement, which has no effect on character-state overlays. We inserted Lobelia erinus and P. borneensis at the bottom of the overlay tree, based on their position in the rbcL tree (see the electronic supplementary material). Accelerated transformation was used to minimize the number of convergent gains of character states. The data sources and atomizations are provided in the electronic supplementary material. Character-state overlays were used to test whether Hawaiian lobeliads had undergone divergent or convergent evolution, thereby confounding analyses of their origin based on morphology.
Figure 2.
Relationships among Hawaiian lobeliads and allies (psbA-trnH, trnL-trnF, rpl16, trnT-trnL, trnV-trnK, atpB-rbcL, rbcL partial cds). Phylogram is one of two shortest trees arising from MP analysis of sequence and indel variation, using L. cardinalis and L. vivaldii as out-groups; branch lengths are proportional to the inferred amounts of genetic change. This tree is identical in topology to the parsimony jackknife tree and (except for the position of Isotoma) to the ML and BA trees based on nucleotide characters only (see text); the open arrow indicates the node that collapses in the strict consensus of the two MP trees. Tree length=1393 steps under parsimony; consistency index CI=0.86; CI′=0.76; excluding autapomorphies. Jackknife support is shown above each node; posterior probability is shown below (the single node that collapses in the BA tree has no such probability shown). The Hawaiian lobeliads (green box) are monophyletic with 100% posterior probability.
We calibrated the in-group phylogeny against time using ‘bottom-up’ and ‘top-down’ approaches. In the bottom-up approach, five asterid fossils 70–89 Myr old were used to calibrate the out-group phylogeny and calculate the ages of the Hawaiian stem group and of L. cardinalis–L. vivaldii, using cross-verified penalized likelihood (CVPL) as implemented in r8s (Sanderson 2002). The latter were then used to calibrate the in-group phylogeny using CVPL. In the top-down approach, we used the ages of islands (0.6–5.2 Myr) to which individual Hawaiian species were restricted to calibrate the in-group phylogeny directly, again using CVPL. Details of both calibrations and bootstrapping of age estimates (involving both the out-group and in-group phylogenies) are provided in the electronic supplementary material.
We compiled data on the geographical distribution and elevational range of all currently recognized species of native Hawaiian lobeliads from Lammers (1996, 1998, 1999, 2004) and Lammers & Proctor (2004); additional data on certain rare species were provided by D. Lorence, H. Oppenheimer, F. Duvall and C. Imada. We compiled data on the numbers of species from each genus on each of the eight large islands (N `ihau to Hawai `i), as well as island age, elevation and area (Walker 1990; Clague 1996; Price & Wagner 2004). We related species number per island and the number of islands occupied to the mode of seed dispersal, to test the hypothesis that decreasing dispersal ability—via wind, birds in gaps or forest edges, and birds in forest understoreys, respectively—should result in increasing levels of speciation, single-island endemism and net diversification (Givnish et al. 1995, 1998; Price & Wagner 2004).
We tallied mean elevation and flower-tube length for each member of the largest genus Cyanea (76 spp.) to evaluate whether patterns of diversification in these traits were broadly similar across each of the largest four islands ( Kaua `i, O `ahu, Maui and Hawai `i). Parallel adaptive radiations are expected on different islands among poorly dispersing taxa such as Cyanea (Givnish 1998). To assess the ecological and evolutionary determinants of species richness in Cyanea on each island, we conducted backward-elimination stepwise regressions of ln (1+ species number) against ln island age, ln island elevation and ln island area, including all islands or the four largest islands, and including or excluding the youngest island of Hawai `i (details are provided in the electronic supplementary material). We used these data to test the hypothesis that limited dispersal should interact with selection for ecological divergence to produce parallel adaptive radiations on different islands (Givnish 1998). To facilitate comparisons with other island floras, we compiled the data on the sizes of the largest known plant clades restricted to individual oceanic (i.e. non-raft, non-land bridge) islands or archipelagos around the world (see the electronic supplementary material).
3. Results
From a total of 4287 aligned bases and 120 indels we detected 1069 variable characters, of which 504 (11.7% of 4407) were phylogenetically informative. The in-group analysis produced two shortest trees; in one, the monophyly of Cyanea is preserved (figure 2). This MP tree is identical in topology to the parsimony jackknife tree, and to the ML tree and the BA tree for all taxa except for Isotoma at the base of the phylogeny. In the ML and BA trees, Isotoma is sister to the tropical American taxa, forming a clade sister to all taxa except the out-groups (see the electronic supplementary material). Eight principal conclusions regarding the evolution of the Hawaiian lobeliads emerge from our phylogenetic analyses and the accompanying data.
(a) Monophyly
Contrary to morphology-based studies, the Hawaiian lobeliads are monophyletic, in both MP trees (86% bootstrap support) and in the ML and Bayesian trees (100% posterior support) that exclude the indel data (figure 2). Our data also strongly support (≥99% BS, 100% PS) four Hawaiian subclades: Clermontia–Cyanea, Brighamia–Delissea, Trematolobelia–Lobelia sect. Galeatella and Lobelia sect. Revolutella (figure 2).
(b) Diversity
Traditionally, the fleshy-fruited clade Cyanea–Clermontia–Delissea (108 spp.) has been viewed as the largest group of Hawaiian plants derived from a single ancestor (Carlquist 1970, 1974; Lammers 1999; Wagner 1999). Our results imply that the lobeliad radiation is even more dramatic, involving four more genera/sections, 19 more species and a much wider range of growth forms and habitats. The Hawaiian lobeliads (126 species) are the most species-rich radiation of plants derived from a single colonist to be resolved on any single oceanic island or archipelago—greatly exceeding the Aeonium alliance (63 spp.) in Macaronesia and Cyrtandra (58 spp.) and the Hawaiian mints (57 spp.) (see the electronic supplementary material).
(c) Origin
The closest relatives of the Hawaiian lobeliads are one clade centred in Africa (with apparent long-distance dispersal across the Atlantic generating Lobelia organensis in Brazil (as shown by Knox & Palmer 1998)), and another including Apetahia and Sclerotheca from Polynesia and Lobelia boninensis from the Bonin Islands, south of Japan (figure 2). While the African clade is more closely related to the Hawaiian clade than the Pacific clade, this relationship is weakly corroborated (44% jackknife support under MP; 96% posterior probability under BA (figure 2; figure A1 in the electronic supplementary material). However, the lineage comprising these three clades plus Lobelia nicotianaefolia has 100 per cent jackknife support under MP and 100 per cent posterior probability under BA. The region that served as a source for the Hawaiian lobeliads is unresolved; the current data make Polynesia, Africa and southern Asia equally plausible (but see §4).
(d) Convergence and divergence
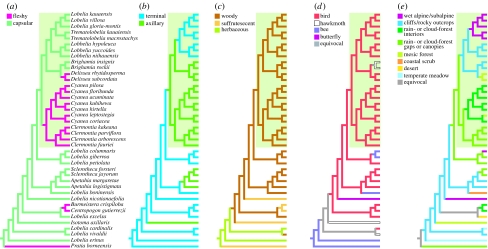
Fleshy fruits arose independently four times in the woody lobeliads—in Pratia, Centropogon–Burmeistera, Clermontia–Cyanea and Delissea—in moist tropical forests in each case (figure 3a). Axillary inflorescences evolved at least four times, each time in moist or wet tropical forests (figure 3b). The woody habit evolved long before lobeliads colonized Hawai `i or other Pacific islands (figure 3c), so the Hawaiian lobeliads cannot be considered an example of the evolution of woodiness on oceanic islands. Similarly, the origin of avian pollination appears to have long preceded the arrival of lobeliads in the Hawaiian chain (figure 3d). Based on the convergence and function of these four traits, each appears adapted to forest understoreys and highly subject to environmentally determined selection pressures rather than phylogenetic constraints (see §4).
Figure 3.
Evolution of morphological and ecological characters among the Hawaiian lobeliads and close relatives inferred using parsimony, and with L. erinus and Pratia grafted to the bottom of the in-group tree (see the electronic supplementary material). Overlays of inferred ancestral traits illustrate the evolution of (a) fruit type, (b) inflorescence position, (c) habit, (d) pollination syndrome and (e) habitat.
(e) Dates of colonization
Based on a ‘bottom-up’ calibration of the in-group tree using the rbcL asterid phylogeny, lobeliads arrived in the Hawaiian chain 13.6±3.11 Myr ago (mean±s.d. based on bootstrapping of in-group tree and variance in calibration ages across out-group trees); a ‘top-down’ calibration of the in-group tree against the ages of islands to which extant Hawaiian taxa are restricted produces a remarkably similar estimate of 13.0±1.00 Myr ago; across nodes, top-down age =0.921·bottom-up age +1.34 (r2=0.966, p<0.0001 for 33 d.f.). This places the origin of the Hawaiian lobeliads on a former tall island near French Frigate Shoals and Gardner Pinnacles (figure 4a,b).
Figure 4.
Chronograms illustrating timing of lobeliad evolution based on (a) bottom-up calibration of the in-group molecular phylogeny against asterid fossils and (b) top-down calibration against ages of islands to which individual Hawaiian species are restricted; arrows indicate inferred times and places of initial colonization in the Hawaiian chain. Map shows extant tall islands (green) and submarine contours (blue) associated with original shorelines of islands that are now reduced to pinnacles or reefs in the northwest Hawaiian Islands. Current/former volcanoes are highlighted in red; dated volcanoes are highlighted by triangles. Bars represent ±1 s.d. for age estimates, based on bootstrapping of the in-group data (SDb=grey) and variation across out-group trees in calibration set point(s) (SDc=magenta). Total s.d.=(SDb2+SDc2)0.5. SDc falls monotonically towards the present, while SDb first rises and then falls (see the electronic supplementary material).
(f) Ancestral habitats and cladogensis
The ancestral habitats of Hawaiian lobeliads appear to have been openings in montane forests, swamps or grasslands, such as those occupied by Apetahia and Sclerotheca in southwestern Polynesia, and by Lobelia giberroa, Lobelia columnaris and Lobelia petiolata in Africa (figure 3e). Cliffs and alpine bogs, by contrast, are habitats that develop late in the lifetimes of individual islands (Carlquist 1970; Clague 1996) and thus unlikely to be ancestral habitats. The distinctive ecologies of each of the Hawaiian genera and endemic sections of Lobelia appear to have arisen early, at the origin of each major lineage. Based on top-down calibration, the initial split between forest and non-forest lineages occurred only 0.30 Myr after initial colonization; the split between forest gap and forest-interior groups, 2.57±0.48 Myr later (figures 3e and 4b). All genera had diverged within 3.39±0.48 Myr of initial colonization.
(g) Ecological determinants of diversification across genera
Coincident with the invasion of closed forest understoreys, the Hawaiian lobeliads showed a striking acceleration in net species generation, with 76 modern species in Cyanea versus 22 in its sister Clermontia and 29 in all remaining lineages. This variation in diversity is associated, as predicted, with differences among taxa in dispersability, with the lowest overall diversity and per cent single-island endemism in lineages with wind-dispersed seeds (i.e. Lobelia sect. Galeatella, Trematolobelia, Lobelia sect. Revolutella, Brighamia), and the highest diversity and per cent single-island endemism in Cyanea, with fruits poorly dispersed by forest-interior birds (see §4).
(h) Ecological determinants of diversification and diversity saturation in Cyanea
As we predicted, limited dispersal in Cyanea (which includes 60% of all Hawaiian lobeliad species) appears to have interacted synergistically with selection for ecological divergence to produce parallel adaptive radiations on different islands, generating both species richness and ecological diversity. When Cyanea species are plotted by their average flower length and elevation, they show a strikingly similar partitioning of pollinators and habitats on each of the four major islands, involving members of two or more clades in each instance (figure 5). Furthermore, the three oldest such islands harbour similar numbers of Cyanea species—21 on Kaua `i (4.7 Myr old), 16 on O `ahu (3.0 Myr) and 23 on Maui (1.5 Myr)—and almost all of these taxa are endemic to individual islands. Most interisland dispersal events appear to have been from one island to the next younger island in the chain (figure 5).
Figure 5.
Parallel adaptive radiations in mean elevation and flower-tube length in Cyanea on the four major Hawaiian Islands. Width of the box reflects elevation of island up to the maximum (approx. 2000 m) invaded by most Cyanea species. Dots are colour coded to reflect phylogeny and species membership in the six major clades (inset at right) recognized by Givnish et al. (1995) using plastid restriction site variation; grey dots indicate species (many extinct) not included in that analysis. The six species groups are the Acuminata clade (green); the Hirtella clade (blue); the Solanaceae clade (gold); the Aculeatiflora clade (gold); the Hardyi clade (purple); and the Leptostegia clade (slate blue). The first four clades bear orange fruits and the latter two, purple fruits.
Species richness peaks on Maui (1.5 Myr old), then drops abruptly to 12 spp. on the youngest island of Hawai `i (0.6 Myr). The higher diversity in Maui versus Kaua `i and O `ahu may simply reflect the much greater elevational range available for ecological partitioning among Cyanea species on Maui. With all islands included, only elevation remained as a factor significantly influencing the number of Cyanea species: species number=6.39×10−4·(elevational range between 200 and 2000 m)1.38−1; r2=0.89, p<0.001 (see the electronic supplementary material). The smaller number of species on Hawai `i (65% of that expected; see the electronic supplementary material), and the fact that one-quarter of them (Cyanea copelandii, Cyanea hamatiflora and the dubious segregate Cyanea cylindrocalyx) also occur on Maui or earlier islands, is consistent with Cyanea having colonized this youngest island recently, and with it still undergoing differentiation there and not having speciated sufficiently to reach ecological saturation within 0.6 Myr. Based on the remarkable convergence in the number of Cyanea species across all eight tall islands except Hawai `i when elevation and area are taken into account (r2=0.994, p<5×10−5 for backward-elimination multiple regression including island age, area and elevation; see the electronic supplementary material), as well as Hawai `i's much lower than expected species richness (12 observed versus 95.6 predicted; see the electronic supplementary material), we conclude that the time required for Cyanea to reach ecological saturation is more than 0.6 Myr and less than 1.5 Myr.
4. Discussion
(a) Monophyly and convergence/divergence
The Hawaiian lobeliads appear to be the product of a single colonization, the second oldest plant radiation in Hawai `i (see below), and the largest on any single oceanic island or archipelago, and are thus crucial to any comparative study of constraints on plant speciation. This result is consistent with the earlier RFLP analyses (Givnish et al. 1995; Givnish 1998), which incorporated most living Hawaiian species but were inconclusive regarding their monophyly because they included only five non-Hawaiian taxa. The strongly supported Hawaiian subclades (i.e. Clermontia–Cyanea, Brighamia–Delissea, Trematolobelia–Lobelia sect. Galeatella and Lobelia sect. Revolutella) are identical in the sequence and restriction site analyses, but the restriction site studies placed Clermontia–Cyanea in a slightly different but weakly supported position, sister to Brighamia–Delissea.
Striking patterns of morphological convergence and divergence appear to have misled early conclusions that this group represented up to five independent colonizations. The unusual combination of fleshy fruits, woody habit and axillary inflorescences led most previous researchers to conclude that Cyanea, Clermontia and Delissea formed a clade and were closely related to groups in tropical America (Burmeistera and Centropogon) or Borneo (Pratia sect. Colensoa) which share all or most of these traits (Rock 1919; Brown 1921; Stone 1967; Carlquist 1970, 1974; Lammers 1999). The exclusion of Centropogon, Burmeistera and Pratia as close relatives of Hawaiian Clermontia, Cyanea and Delissea—despite their shared possession of fleshy fruits, woody habit and (in Pratia) axillary inflorescences—graphically illustrates the problems of assessing phylogeny based on characters subject to strong selection pressure, especially in insular groups that have undergone extensive radiation and diverged sharply from mainland ancestors (Givnish & Sytsma 1997; Baldwin et al. 1998; Givnish 1998; Lindqvist & Albert 2002; Mort et al. 2002; Nepokroeff et al. 2003; Schneider et al. 2005).
Based on the convergence and function, each of these traits—fleshy fruits, woody habit, and axillary inflorescences—appears to be an adaptation to forest understoreys. Among Neotropical trees, the fraction of species with fleshy fruits increases sharply with rainfall, with up to 95 per cent of woody species in the wet forest understoreys having fleshy fruits (Gentry 1982a,b). Among the taxa we studied, fleshy fruits appear to have evolved at least four times. Fleshy fruits arose at least three more times in Campanulaceae more broadly, based on the phylogeny presented by Eddie et al. (2005)—always in taxa from rainforest understoreys. Across monocots, fleshy fruits evolved at least 20 times, almost always associated with the invasion of forest understoreys (Givnish et al. 2005). Closed-forest understoreys militate against wind dispersal, and may also foster the evolution of fleshy fruits by favouring leaf-eating insects that provide a vital protein subsidy to frugivorous birds (Givnish 1998).
Among lobeliads, woodiness is associated with growth in scrub, forests and tropical alpine zones (Rock 1919; Carlquist 1970; Mabberley 1975; Givnish et al. 1995; Knox & Palmer 1998). The tallest forms (Cyanea (up to 18 m tall), Clermontia, Delissea, Apetahia and Sclerotheca) are restricted to moist tropical forests and adjacent habitats, where the advantages of growth in height under crowded conditions should favour them (Givnish 1998). Axillary inflorescences are very common in rainforest understorey trees (Hallé et al. 1978), and—in contrast to terminal inflorescences—may facilitate iteroparity and continued growth and reproduction under predictable conditions. All Hawaiian lobeliads with terminal inflorescences are semelparous and occur in open habitats; the possible advantages of big-bang reproduction in such sites (e.g. visual conspicuousness of large inflorescences) may not be testable, however, given the mass extinctions suffered by honeycreepers and other pollinators over the past century. All Hawaiian lobeliads except low-elevation, cliff-dwelling Brighamia are ornithophilous. The habitats of bird-pollinated lobeliads in Hawai `i and elsewhere in tropical montane Africa, Borneo and America are mostly wet and/or cold, in which a thermoregulating pollinator would be well adapted (Mabberley 1975).
(b) Time and source of origin
Calibrating a molecular phylogeny using both ‘bottom-up’ and ‘top-down’ approaches to obtain nearly the same time of origin of a lineage is, to our knowledge, unique. An origin of 13.0–13.6 Myr ago is consistent with the Hawaiian lobeliads having played a crucial role as ‘keystone mutualists’ that helped trigger diversification in other major elements of the Hawaiian biota (Givnish et al. 1995), given the estimated origins of the Hawaiian honeycreepers (their major pollinators) ca 5 Myr ago (Fleischer et al. 1998), and of the extraordinarily diverse Hawaiian Drosophila (many of which use lobeliads for mating or oviposition (Kambysellis & Craddock 1997)) ca 30 Myr ago (Tamura et al. 2004). Among the Hawaiian plant clades whose origins have been dated (Price & Clague 2002), the lobeliads are younger than only the small fern genus Diellia, which arose 23 Myr ago (Schneider et al. 2005); the silversword alliance, with approximately 30 spp., is the next youngest, having arisen 5 Myr ago (Baldwin & Sanderson 1998).
The origin of the Hawaiian lobeliads near Gardner Pinnacles is notable. Gardner was unusually large and tall, and—with younger volcanoes near present-day French Frigate Shoals—formed the only islands more than 2000 m tall (with a full range of wet habitats) in the chain before the present-day tall islands began to emerge; some of these were still 1000–2000 m tall when Kaua `i emerged 4.7 Myr ago (Price & Clague 2002). Maximum island elevations only briefly exceeded 1000 m—and were mostly less than 500 m—32 Myr ago until the emergence of Gardner (Price & Clague 2002).
Our phylogeny does not identify the source of the Hawaiian clade. However, we suspect that additional study may show that the evolution of the three major clades of tropical woody lobeliads—involving very short branches in all phylogenies—reflects a nearly simultaneous divergence of the Hawaiian, Pacific and African clades from ancestors drawn from the L. nicotianaefolia complex. The latter ranges from western India (4500 km from the cradle of the African woody lobeliads in Tanzania (Knox & Palmer 1998)) to the Philippines (2400 km from the Bonin Islands, 8500 km from Hawai `i and 9500 km from Rarotonga; Hawai `i lies more than 2400 km away from southwestern Polynesia). These distances are large but not insuperable, given the minute seeds of the taxa in question and their occurrence in open, windswept habitats at mid to high elevations. The paradox of a single origin for the Hawaiian lobeliads—while their relatives have repeatedly dispersed across vast stretches of ocean—probably reflects the small target area of the Hawaiian chain, and the far greater proximity of the tall Hawaiian Islands to each other than to other moist, mountainous areas that could support woody lobeliads in the tropics.
(c) Tempo and pattern of evolution
The high diversity of Hawaiian lobeliads appears to reflect a hierarchical adaptive radiation in habitat at the generic level, then by elevation and flower-tube length within Cyanea. The initial radiation happened very quickly, with a split between forest and non-forest lineages only 0.3 Myr after colonization, and a split between forest-gap and forest-interior groups 2.57 Myr later, based on top-down calibration (figures 3 and 4). All genera and both endemic sections of Lobelia had diverged by 9.76 Myr ago (6.97 Myr for bottom-up calibration), with Trematolobelia and Lobelia sect. Galeatella diverging last. Each group except Cyanea apparently arrived on the modern islands via a single dispersal event to Kaua `i. Cyanea colonized Kaua `i twice, involving the progenitors of today's orange- and purple-fruited clades (see also Givnish et al. 1995). Given the substantial rates of diversification subsequently, these facts imply high rates of extinction in all lineages before they colonized Kaua `i. Such high rates of extinction would be consistent with the known history of erosion and subsidence of the northwestern Hawaiian Islands (Clague 1996; Price & Clague 2002), and the near absence of native lobeliads below 200 m in the Hawaiian chain.
Differences among genera in species richness are associated, as predicted (Givnish et al. 1995; Givnish 1998) with differences in the ecology of seed dispersal. Groups with minute, wind-dispersed seeds and inhabiting open, windswept, high-elevation habitats should show low rates of net species generation, based on their dispersing well over the relatively small distances among the tall islands of the Hawaiian archipelago. High dispersal rates should thus tend to reduce genetic divergence of populations in Lobelia sect. Galeatella, Trematolobelia, Lobelia sect. Revolutella and Brighamia, slowing geographical isolation and speciation, thereby reducing species richness and local endemism; indeed, these groups have only 4, 4, 9 and 2 present-day species, respectively, and each species occupies 1.84 islands on average. Cyanea—with fruits dispersed by forest-interior birds, which are loathe to cross water or habitat barriers (Givnish et al. 1995; Givnish 1998)—should disperse less well, on average, within the Hawaiian archipelago. Low rates of dispersal associated with endozoochory in the wet forest interiors should increase divergence, accelerate isolation and speciation and favour local endemism. Indeed, Cyanea has 76 species, with each occupying only 1.11 islands on average and 93 per cent endemic to single islands. We note that 7 of the 11 largest Hawaiian plant clades (Price & Wagner 2004) are bird-dispersed elements of the wet-forest understoreys, consistent with our hypothesis; avian dispersal (including ectozoochory) is the strongest correlate of species richness across 28 Hawaiian plant clades (Price & Wagner 2004). Lobeliads that depend on more vagile, dry forest or forest-edge birds to disperse their fruits should be intermediate in rates of dispersal, genetic divergence, speciation and within-island endemism. So they appear to be: Clermontia and Delissea have 22 and 10 species, respectively, with each occupying an average of 1.50 islands.
Species richness within Cyanea is highly predictable and appears to reflect, in part, parallel radiations in elevation and flower-tube length on each of the largest islands. This pattern of repeated parallel adaptive radiations is similar to that documented in a number of groups, including Anolis lizards in the Greater Antilles (Losos et al. 1998) and Tetragnatha spiders in Hawai `i (Gillespie 2004). Based on interisland comparisons, community assembly in Cyanea appears to saturate in less than 1.5 Myr. This compares with estimates of 4 Myr for ecological saturation in equids in North America (MacFadden & Hulbert 1988), and 39 Myr for half saturation of adaptive shifts to sun/shade in monocots across continents (Givnish et al. 2005). The net rate of species diversification in Cyanea on individual islands—ln (final no. of species/no. of colonists)/age of island—is 1.36 species species−1 Myr−1 for Maui if we assume it took the full 1.5 Myr to achieve current diversity (23 species, approx. three initial surviving colonizations), and 2.04 species species−1 Myr−1 if we instead assume that saturation occurred in 1 Myr. These rates approach those in Andean lupines (1.93–3.72 species species−1 Myr−1), which have the highest known rates of diversification in plants and among the highest in the global Tree of Life (Hughes & Eastwood 2006). Andean lupines, however, diversified over a far greater spatial area than ever existed at any one time in the Hawaiian archipelago, with a much greater diversity of habitats based on latitude, elevation and substrate, and a much greater number of low-elevation barriers to gene flow, making the high rate of diversification in Hawaiian lobeliads all the more remarkable.
Species richness of Cyanea peaks on Maui (1.5 Myr old), then drops abruptly to 12 spp. on the youngest island of Hawai `i (0.6 Myr). Several mechanisms have been advanced to explain a similar pattern in Tetragnatha spiders—including moderate rates of initial speciation and a time lag between speciation and competitive exclusion (Gillespie 2004), and an intermediate rate of ‘disturbance’ based on time since island formation (Kassen et al. 2004). However, we believe that the drop in diversity in moving from Maui to O `ahu and Kaua `i simply reflects the much greater elevational range available for ecological partitioning among Cyanea species on Maui (and possibly the effects of greater island area; see Results), while the drop in moving from Maui to Hawai `i reflects slow speciation rates relative to the time scales involved, as argued for Tetragnatha by Gillespie (2004).
Our analyses of diversity saturation in Cyanea are based on current island areas and elevations. These have varied over time, and so should be viewed partly as proxies for areas and elevations in the recent past as well. Maui was part of a substantially larger land mass—Maui Nui, including Kaho `olawe, Lana `i and Moloka `i—during the last glacial era (Price & Clague 2002). The fact that analyses based on current areas and elevations suggest equilibration under current conditions points towards extinction on all components of Maui Nui during the past 12 ky; the slow build-up of diversity over a much longer time frame (0.6–1.2 Myr) suggests that speciation rates may have been substantially lower than rates of natural extinction since the Pleistocene.
Similar to Cyanea, many large angiosperm genera (e.g. Chamaedorea, Piper, Psychotria, Solanum) are composed of tropical forest understorey trees or shrubs with fleshy fruits (Givnish 1998, 1999a,b; Smith 2001; Price & Wagner 2004). Consistent with our hypothesis (Givnish et al. 1995; Givnish 1998, 1999a), 7 of the 11 largest Hawaiian plant clades (Price & Wagner 2004) are bird-dispersed elements of the wet forest understoreys; Cyanea may thus provide a model for understanding the pattern and tempo of speciation in such groups. Its rapid saturation in local diversity demonstrates that community assembly within such groups can proceed and culminate in high levels of standing diversity within one or a few million years in the wet tropical areas that favour them (Gentry 1982a; Givnish 1999a,b; Phillips & Miller 2005), facilitating the rise of high levels of tree diversity even in geologically young areas such as the Colombian Chocó (Gentry 1982b).
The high diversity of the Hawaiian lobeliads might reflect, in part, their early arrival in the Hawaiian chain via their pre-emption of niches available to other lineages. The negative impacts of such niche pre-emption has been argued, within rather than between lineages, by Carine et al. (2004), Silvertown (2004) and Silvertown et al. (2005), for congeners in the Macaronesian flora (see contrary views by Herben et al. 2005 and Saunders & Gibson 2005). However, niche pre-emption seems likely to be less important than the effects of dispersal and habitat partitioning in Cyanea and the Hawaiian lobeliads generally, given the restriction of most lobeliad species to individual islands, the rapid demise of older islands and the species on them, and the much smaller sizes of other lineages (e.g. Hawaiian mints, silver-sword alliance) that invaded the current islands as they were formed, at the same time they were being invaded by lobeliads.
Acknowledgments
This research was supported by grants from the National Geographic Society (T.J.G. and K.J.S.), National Science Foundation (BSR-9007293 and BSR-9020055 to T.J.G. and K.J.S., DEB-0444705 to T.J.G.), and Hertel gift fund (T.J.G.). Added logistical support was furnished by the National Tropical Botanical Garden, Hawai `i Volcanoes National Park and Kokeìe Natural History Museum. D. Lorence, T. Flynn, S. Perlman, K. Reinard, J. Obata, D. Palmer, R. Hobdy, A. Medeiros, E. Misaki, H. Oppenheimer, L. Stemmermann and L. Pratt helped us locate many rare Hawaiian species; J. Y. Meyer, E. Knox, T. Lammers and C. Imada assisted in locating other sample material. W. Souza, E. Pettys, D. Foote, L. Pratt, B. Gagné, V. Caraway, R. Kennedy, W. Stormont and J. Giffen provided research permits. J. Price and two anonymous reviewers provided helpful comments on the manuscript.
Supplementary Material
Summary of methods, materials, and ancillary data
References
- Baldwin B.G., Sanderson M.J. Age and rate of diversification of the Hawaiian silversword alliance (Compositae) Proc. Natl Acad. Sci. USA. 1998;95:9402–9406. doi: 10.1073/pnas.95.16.9402. doi:10.1073/pnas.95.16.9402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin B.G., Crawford D.J., Francisco-Ortega F., Kim S.-C., Sang T., Stuessy T.F. Molecular phylogenetic insights on the origin and evolution of island plants. In: Soltis D.E., Soltis P.S., Doyle J.J., editors. Molecular systematics of plants II. Kluwer; Boston, MA: 1998. pp. 410–441. [Google Scholar]
- Brown F.B.H. Origin of the Hawaiian flora. Special Publ. Bernice Pauahi Bishop Mus. 1921;7:131–142. [Google Scholar]
- Carine M.A., Russell S.J., Santos-Guerra A., Francisco-Ortega J. Relationships of the Macaronesian and Mediterranean floras: molecular evidence for multiple colonizations into Macaronesia and back-colonization of the continent in Convolvulus (Convolvulaceae) Am. J. Bot. 2004;91:1070–1085. doi: 10.3732/ajb.91.7.1070. doi:10.3732/ajb.91.7.1070 [DOI] [PubMed] [Google Scholar]
- Carlquist S. Natural History Press; New York, NY: 1970. Hawaii: a natural history. [Google Scholar]
- Carlquist S. Columbia University Press; New York, NY: 1974. Island biology. [Google Scholar]
- Clague D.A. The growth and subsidence of the Hawaiian-Emperor volcanic chain. In: Keast A., Miller S.E., editors. The origin and evolution of Pacific biotas, New Guinea to eastern Polynesia: patterns and processes. SPB Academic Publishing; Amsterdam, UK: 1996. pp. 35–50. [Google Scholar]
- Eddie W.M.M., Shulkina T., Gaskin J., Haberle R.C., Jansen R.K. Phylogeny of Campanulaceae s. str. inferred from ITS sequences of nuclear ribosomal DNA. Ann. Mo. Bot. Gard. 2005;90:554–575. doi:10.2307/3298542 [Google Scholar]
- Farris J.S., Albert V.A., Ka¨llersjo¨ M., Lipscomb D., Kluge A.G. Parsimony jackknifing outperforms neighbor-joining. Cladistics. 1996;12:99–124. doi: 10.1111/j.1096-0031.1996.tb00196.x. doi:10.1111/j.1096-0031.1996.tb00196.x [DOI] [PubMed] [Google Scholar]
- Fleischer R.C., McIntosh C.E., Tarr C.L. Evolution on a volcanic conveyor belt: using phylogeographic reconstructions and K-Ar-based ages of the Hawaiian Islands to estimate molecular evolutionary rates. Mol. Ecol. 1998;7:533–545. doi: 10.1046/j.1365-294x.1998.00364.x. doi:10.1046/j.1365-294x.1998.00364.x [DOI] [PubMed] [Google Scholar]
- Gentry A.H. Patterns of neotropical plant species diversity. Evol. Biol. 1982;15:1–84. [Google Scholar]
- Gentry A.H. Phytogeographic patterns as evidence for a Choco refuge. In: Prance G.T., editor. Biological diversification in the tropics. Columbia University Press; New York, NY: 1982. pp. 112–136. [Google Scholar]
- Gillespie R. Community assembly through adaptive radiation in Hawaiian spiders. Science. 2004;303:356–359. doi: 10.1126/science.1091875. doi:10.1126/science.1091875 [DOI] [PubMed] [Google Scholar]
- Givnish T.J. Adaptive radiation of plants on oceanic islands: classical patterns, molecular data, new insights. In: Grant P., editor. Evolution on islands. Oxford University Press; Oxford, UK: 1998. pp. 281–304. [Google Scholar]
- Givnish T.J. Adaptive radiation, dispersal, and diversification of the Hawaiian lobeliads. In: Kato M., editor. The biology of biodiversity. Springer; Tokyo, Japan: 1999. pp. 67–90. [Google Scholar]
- Givnish T.J. On the causes of gradients in tropical tree diversity. J. Ecol. 1999;87:193–210. doi:10.1046/j.1365-2745.1999.00333.x [Google Scholar]
- Givnish T.J., Sytsma K.J. Homoplasy in molecular vs. morphological data: the likelihood of correct phylogenetic inference. In: Givnish T.J., Sytsma K.J., editors. Molecular evolution and adaptive radiation. Cambridge University Press; New York, NY: 1997. pp. 55–100. [Google Scholar]
- Givnish T.J., Sytsma K.J., Smith J.F., Hahn W.S. Molecular evolution, adaptive radiation, and geographic speciation in Cyanea (Campanulaceae, Lobelioideae) In: Wagner W.L., Funk V., editors. Hawaiian biogeography: evolution on a hot spot archipelago. Smithsonian Institution; Washington, DC: 1995. pp. 288–337. [Google Scholar]
- Givnish T.J., Montgomery R.A., Goldstein G. Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses, and whole-plant compensation points. Am. J. Bot. 2004;91:228–246. doi: 10.3732/ajb.91.2.228. doi:10.3732/ajb.91.2.228 [DOI] [PubMed] [Google Scholar]
- Givnish T.J., et al. Repeated evolution of net venation and fleshy fruits among monocots in shaded habitats confirms a priori predictions: evidence from an ndhF phylogeny. Proc. R. Soc. B. 2005;272:1481–1490. doi: 10.1098/rspb.2005.3067. doi:10.1098/rspb.2005.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallé F., Oldeman R.A.A., Tomlinson P.B. Springer; Berlin, Germany: 1978. Tropical trees and forests. [Google Scholar]
- Herben T., Suda J., Munclinger P. The ghost of hybridization past: niche pre-emption is not the only explanation of apparently monophyly in island endemics. J. Ecol. 2005;93:572–575. doi:10.1111/j.1365-2745.2005.01005.x [Google Scholar]
- Hughes C., Eastwood R. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc. Natl Acad. Sci. USA. 2006;103:10 334–10 339. doi: 10.1073/pnas.0601928103. doi:10.1073/pnas.0601928103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambysellis M.P., Craddock E.M. Ecological and reproductive shifts in the diversification of the endemic Hawaiian Drosophila. In: Givnish T.J., Sytsma K.J., editors. Molecular evolution and adaptive radiation. Cambridge University Press; New York, NY: 1997. pp. 475–509. [Google Scholar]
- Kassen R., Llewellyn M., Rainey P.B. Ecological constraints on diversification in a model adaptive radiation. Nature. 2004;431:984–988. doi: 10.1038/nature02923. doi:10.1038/nature02923 [DOI] [PubMed] [Google Scholar]
- Knox E.B., Palmer J.D. Chloroplast evidence on the origin and radiation of the giant Lobelias in eastern Africa. Syst. Bot. 1998;23:109–149. doi:10.2307/2419583 [Google Scholar]
- Lammers T.G. A new linear-leaved Cyanea (Campanulaceae: Lobelioideae) from Kaua `i, and the “rediscovery” of Cyanea linearifolia. Brittonia. 1996;48:237–240. doi:10.2307/2807820 [Google Scholar]
- Lammers T.G. New names and new combinations in Campanulaceae. Novon. 1998;8:31–35. doi:10.2307/3391887 [Google Scholar]
- Lammers T.G. Campanulaceae. In: Wagner W.L., Herbst D.R., Sohmer S.H., editors. Manual of the flowering plants of Hawai `i. Bishop Museum; Honolulu, HI: 1999. pp. 420–489. [Google Scholar]
- Lammers T.G. Five new species of the endemic Hawaiian genus Cyanea (Campanulaceae: Lobelioideae) Novon. 2004;14:84–101. [Google Scholar]
- Lammers T.G., Proctor G.R. Lobelia vivaldii (Campanulaceae, Lobelioideae), a remark-able new species of sect Tylomium from Isla de Mona, Puerto Rico. Brittonia. 2004;46:273–278. doi:10.2307/2806909 [Google Scholar]
- Lindqvist C., Albert V.A. Origin of the Hawaiian endemic mints within North American Stachys. Am. J. Bot. 2002;89:1709–1724. doi: 10.3732/ajb.89.10.1709. doi:10.3732/ajb.89.10.1709 [DOI] [PubMed] [Google Scholar]
- Losos J.B., Jackman T.R., Larson A., de Queiroz K., Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. doi:10.1126/science.279.5359.2115 [DOI] [PubMed] [Google Scholar]
- Mabberley D.J. The pachycaul lobelias of Africa and St. Helena. Kew Bull. 1974;29:535–584. doi:10.2307/4108000 [Google Scholar]
- Mabberley D.J. The giant lobelias: pachycauly, biogeography, ornithophily, and continental drift. New Phytol. 1975;74:365–374. doi:10.1111/j.1469-8137.1975.tb02623.x [Google Scholar]
- MacFadden B.J., Hulbert R.C., Jr Explosive speciation at the base of the adaptive radiation of Miocene grazing horses. Nature. 1988;326:466–468. doi:10.1038/336466a0 [Google Scholar]
- Maddison W.P., Maddison D.R. Sinauer Press; Sunderland, MA: 2001. MacClade vers. 4.0. [Google Scholar]
- Mort M.E., Soltis D.E., Soltis P.S., Francisco-Ortega F., Santos-Guerra A. Phylogenetics and evolution of the Macaronesian clade of Crassulaceae inferred from nuclear and chloroplast sequence data. Syst. Bot. 2002;27:271–288. [Google Scholar]
- Nepokroeff M., Sytsma K.J., Wagner W.L., Zimmer E.A. Reconstructing ancestral patterns of colonization and dispersal in the Hawaiian understory tree genus Psychotria (Rubiaceae): a comparison of parsimony and likelihood approaches. Syst. Biol. 2003;52:820–838. doi:10.1080/10635150390251072 [PubMed] [Google Scholar]
- Phillips O.L., Miller J.S. Missouri Botanical Garden Press; St. Louis, MO: 2005. Global patterns of plant diversity. [Google Scholar]
- Price J.P., Clague D.A. How old is the Hawaiian biota? Geology and phylogeny suggest recent divergence. Proc. R. Soc. B. 2002;269:2429–2435. doi: 10.1098/rspb.2002.2175. doi:10.1098/rspb.2002.2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.P., Wagner W.L. Speciation in Hawaiian angiosperm lineages: cause, consequence, and mode. Evolution. 2004;58:2185–2200. doi: 10.1111/j.0014-3820.2004.tb01597.x. doi:10.1554/03-498 [DOI] [PubMed] [Google Scholar]
- Rock J.F. Bishop Museum; Honolulu, HI: 1919. A monographic study of the Hawaiian species of the tribe Lobelioideae, family Campanulaceae. [Google Scholar]
- Sanderson M.J. Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 2002;19:101–109. doi: 10.1093/oxfordjournals.molbev.a003974. [DOI] [PubMed] [Google Scholar]
- Saunders N.E., Gibson D.J. Breeding system, branching processes, hybrid swarm theory, and the hump-back diversity relationship as additional explanations for apparent monophyly in the Macaronesian island flora. J. Ecol. 2005;93:649–652. doi:10.1111/j.1365-2745.2005.01027.x [Google Scholar]
- Schneider H., et al. Origin of the endemic fern genus Diellia coincides with the renewal of Hawaiian terrestrial life in the Miocene. Proc. R. Soc. B. 2005;272:455–460. doi: 10.1098/rspb.2004.2965. doi:10.1098/rspb.2004.2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvertown J. The ghost of competition past in the phylogeny of island endemic plants. J. Ecol. 2004;92:168–173. doi:10.1111/j.1365-2745.2004.00853.x [Google Scholar]
- Silvertown J., Francisco-Ortega J., Carine M. The monophyly of island radiations: an evaluation of niche pre-emption and some alternative explanations. J. Ecol. 2005;93:635–657. doi:10.1111/j.1365-2745.2005.01038.x [Google Scholar]
- Smith J.F. High species diversity in fleshy-fruited tropical understory plants. Am. Nat. 2001;157:646–653. doi: 10.1086/320625. doi:10.1086/320625 [DOI] [PubMed] [Google Scholar]
- Stone B.C. A review of the endemic genera of Hawaiian plants. Bot. Rev. 1967;33:216–259. doi:10.1007/BF02858638 [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 2002. PAUP*—phylogenetic analysis using parsimony (*and other methods), vers. 4, beta test version. [Google Scholar]
- Tamura K., Subramanian S., Kumar S. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol. Biol. Evol. 2004;21:36–44. doi: 10.1093/molbev/msg236. doi:10.1093/molbev/msg236 [DOI] [PubMed] [Google Scholar]
- Wagner W.L. Introduction. In: Wagner W.L., Herbst D.R., Sohmer S.H., editors. Manual of the flowering plants of Hawai `i. Bishop Museum; Honolulu, HI: 1999. pp. 1–16. [Google Scholar]
- Walker G. Geology. In: Wagner W.L., Herbst D.R., Sohmer S.H., editors. Manual of the flowering plants of Hawai `i. Bishop Museum Press; Honolulu, HI: 1990. pp. 21–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of methods, materials, and ancillary data