Abstract
Anthropogenic or natural disturbances can have a significant impact on wild animals. Therefore, understanding when, how and what type of human and natural events disturb animals is a central problem in wildlife conservation. However, it can be difficult to identify which particular environmental stressor affects an individual most. We use heart rate telemetry to quantify the energy expenditure associated with different types of human-mediated and natural disturbances in a breeding passerine, the white-eyed vireo (Vireo griseus). We fitted 0.5 g heart rate transmitters to 14 male vireos and continuously recorded heart rate and activity for two days and three nights on a military installation. We calibrated heart rate to energy expenditure for five additional males using an open-flow, push-through respirometry system showing that heart rate predicted 74 per cent of energy expenditure. We conducted standardized disturbance trials in the field to experimentally simulate a natural stressor (predator presence) and two anthropogenic stressors. Although birds initially showed behavioural and heart rate reactions to some disturbances, we could not detect an overall increase in energy expenditure during 1- or 4-hours disturbances. Similarly, overall activity rates were unaltered between control and experimental periods, and birds continued to perform parental duties despite the experimental disturbances. We suggest that vireos quickly determined that disturbances were non-threatening and thus showed no (costly) physiological response. We hypothesize that the lack of a significant response to disturbance in vireos is adaptive and may be representative of animals with fast life histories (e.g. short lifespan, high reproductive output) so as to maximize energy allocation to reproduction. Conversely, we predict that energetic cost of human-mediated disturbances will be significant in slow-living animals.
Keywords: conservation, anthropogenic disturbance, heart rate telemetry, energy expenditure, fast life histories, Vireo
1. Introduction
Anthropogenic and natural disturbances have long been thought to have deleterious impacts on wild animals and have been the focus of conservation initiatives (Steen et al. 1988; Knight & Gutzwiller 1995; Wasser et al. 1997; Creel et al. 2002; Thiel et al. 2007). Disturbances may reduce fitness because animals may use energy otherwise allotted to reproduction to cope with unpredictable disturbances (Daan et al. 1996; Fowler 1999; Weimerskirch et al. 2002). Because reproduction is considered one of the most costly activities of animals (Williams 1966; Stearns 1992), both reproduction and survival are often dependent upon the amount of energy used during the reproductive period. For example, low survival rates were correlated with the increased energy expenditure as a result of increased reproductive effort in European kestrels (Falco tinnunculus; Daan et al. 1996). Thus, selection will strongly favour cost-effective strategies that enable organisms to cope with environmental stressors while performing vital reproductive duties (Wikelski & Ricklefs 2001; Wingfield & Sapolsky 2003; Love et al. 2004; Berger et al. 2007; Romero et al. 2008). For example, Wingfield et al. (1998) proposed that birds adopt an ‘emergency’ life-history stage, and abandon reproductive activities, when faced with perturbations in their environment as a means to redirect energy towards survival.
Life-history theory predicts that the costs and benefits of responding to disturbance, and diverting energy towards survival and away from reproduction, should vary across species. Slow-living animals, i.e. those with large body size, small clutch or delayed maturity (Wikelski et al. 2003), are expected to show the strongest emergency reactions to disturbance. Under environmental challenges, such long-lived animals benefit from strategies that promote long-term survival, and thus, when faced with a trade-off, they should redirect energy allocation to activities that will enhance long-term survival over activities associated with reproduction (i.e. breeding can be postponed). On the other hand, fast-living animals, such as small songbirds—organisms with small body size, large clutch size and limited reproductive opportunities—should favour investment in current reproduction over investment in survival. Thus, short-lived organisms may appear much more tolerant towards environmental challenges, especially during breeding. Determining the energetic demands associated with unpredictable disturbances across a broad life-history spectrum can, therefore, provide a powerful conservation tool to understand long-term effects on survival and reproductive success across species. Most previous efforts have focused on slow-living species (e.g. Hunt et al. 2003; Walker et al. 2006; Wikelski & Cooke 2006); but here we studied a fast-living species to extend the scope of our understanding of anthropogenic stressors on wild animals.
Human-mediated disturbances related to military training have increasingly become a point of concern for conservation biologists. In the United States alone, a significant portion of the breeding habitat for the populations of at least 34 endangered or threatened bird species exists within military installations (e.g. Kirtland's warbler, Dendroica kirtlandii; southwestern willow flycatcher; Empidonax traillii extimus; red-cockaded woodpecker, Picoides borealis; Florida scrub jay, Aphelocoma coerulescens; golden-cheeked warbler, Dendroica chrysoparia; and black-capped vireo, Vireo atricapillus). Military training potentially presents a threat to fitness and long-term survival in birds because it is episodic, unpredictable, varying in intensity and may occur repeatedly for many days, a suite of characteristics that may cause short- and long-term (chronic) stress (Dallman & Bhatnagar 2001). Military training is also conducted at night to a greater extent than other sources of human disturbance associated with commercial or recreational activities. It is important to understand the energetic cost of such disturbances because many threatened and endangered species depend on military installations for breeding.
Until recently, measures of energy expenditure were either based on non-continuous (doubly labelled water) or non-physiological (time-activity budgets; e.g. McKinney & McWilliams 2005) estimates of energy consumption. Although comparative analyses showed that the heart rate is nearly as precise as the doubly labelled water method in estimating the energy expenditure of animals in the wild (Bevan et al. 1995; Green et al. 2001), doubly labelled water provides average daily energy expenditure only over a 24–48-hour period (Nagy 1975) and cannot give instantaneous measures of energetic demands in response to specific environmental stressors or specific activities. Alternatively, heart rate allows a continuous and instantaneous measure of the energetic demands associated with specific disturbances, and is correlated with the energy expenditure in fishes (Lucas 1994), birds (Cochran & Wikelski 2005; Cyr et al. 2008) and mammals (Boyd et al. 2006).
Measuring heart rate in response to anthropogenic disturbances may allow conservationists to directly and instantaneously quantify energy expenditure during periods of disturbance and make predictions on how best to manage such perturbation in relation to the responses elicited. In this study, we use heart rate radio telemetry to quantify the energetic cost of unpredictable anthropogenic (diurnal and nocturnal activities) and natural (predator and conspecific presence) disturbances in free-living white-eyed vireos (Vireo griseus) during the breeding season. We used the common white-eyed vireo as a surrogate for the endangered black-capped vireo (Vireo atricapilla) also breeding at our study site. Specifically, we varied experimental disturbance trials in intensity, duration and frequency because responses to stressors may vary according to the nature of the stressor (Greenberg et al. 2002). To the best of our knowledge, this is the first study to test the energetic cost of disturbance in small fast-living animals in the wild.
2. Material and methods
(a) Study system
The white-eyed vireo is a small (10 g) migratory passerine that breeds in the eastern region of North America from southern Canada to Florida. Its winter range extends from Virginia to Honduras and parts of the Caribbean (Hopp et al. 1995). Our study was conducted on Fort Hood military post near Killeen, Texas (31°7′48″ N, 97°46′49″ W), during the white-eyed vireo's breeding season from March to June 2006 and 2007. Fort Hood, an 88 000 ha installation, is one of the most intensely used ground training sites for the US military troops (Hayden et al. 2001) and therefore provides an excellent field site to test the effects of human and military activity at varying degrees of intensity. However, in order to reduce the number of stressors that we could not control, we conducted this study where military training was limited (eastern portion of the base).
(b) Heart rate transmitter placement, heart rate recording and activity monitoring
We captured 14 white-eyed vireo males 2–3 hours before roosting (approximately 17.00 hours) by luring them into mist-nets using song playbacks. Of the 14 males monitored, only two were unpaired and the remaining were nest-building (n=2), incubating (n=2) or feeding nestlings (n=1) or fledged young (n=5). We were unable to determine the breeding status for two males. Each vireo was first fitted with a unique combination of the US Fish and Wildlife Service and colour bands. We then mounted 0.5 g heart rate transmitters (Sparrow Systems, Fisher, IL, USA) on each male, following the protocol of Cochran & Wikelski (2005). The procedure lasted for an average of 15 min from the time of capture to the time of release and all males returned to territorial or nesting activities within 10–20 min after release. Heart rate transmitters emit a continuous amplitude-modulated signal by a 1800 Hz subcarrier oscillator that is frequency modulated by heart muscle potentials. The heart muscle potential was captured with two leads placed subcutaneously on the dorsum.
We obtained heart rate from 11 of the 14 transmittered vireos. However, the activity data were obtained from all males transmittered. We continuously recorded heart rate for two days and three nights (average of 60 hours) for each of the 11 male vireos using a Yagi antenna connected to an AR8000 receiver (AOR Ltd. Tokyo, Japan), PC laptop computer and/or MP3 recorder (EDIROL R09, Roland Inc.). MP3 recordings that yielded the highest quality/duration combination were obtained using a 160 kbps sampling rate and 48 kHz sampling frequency. Antennae and recording equipment were placed approximately 50 m from the edge of the vireo's territory at a location that allowed maximal tracking range. Heart rate was manually calculated using a spectrogram created in CoolEdit 2000 (sound recording analysis software; Syntrillium Software Corp., Phoenix, AZ, USA) using a fast Fourier transform. Although we recorded heart rate continuously, we sampled heart rate for a 5 s interval every 10 min during, and every 30 min outside, disturbance experiments for manual calculations.
We simultaneously recorded activity using a separate antenna connected to an automated receiver (automatic receiving unit; Sparrow Systems, Inc.). The automated receiver continuously records the signal strength of the radio transmitter in logarithmic units (i.e. dBm). Behavioural observations showed that an approximate doubling of signal strength, i.e. a change in signal strength by 4 dBm, corresponded to the activity of the individual (Crofoot et al. 2008; T. D. Lambert 2003, unpublished data).
(c) Experimental procedure for disturbance trials
For each bird transmittered, we used a repeated-measures design to compare periods of disturbance to control periods where no experimental disturbance was conducted. The 14 birds monitored were separated into three groups (table 1).
Table 1.
Standardized disturbance trial design for breeding white-eyed vireo males. (Heart rate and associated energy expenditure were continuously monitored for three nights and two days. Nights 1 (following capture and preceding day 1) and 2 (between days 1 and 2) are not shown because no disturbance trials were performed. Times are based on central daylight time zone. Sample sizes for groups 1, 2 and 3 were 4, 4 and 3, respectively, for heart rate, and 5, 5 and 4, respectively, for activity.)
| time period and disturbance type | |||
|---|---|---|---|
| groups | day 1 | day 2 | night 3 |
| 1 | no disturbance | 4 hours continuous chase 07.00–11.00 hours | no disturbance |
| 2 | 1 hour repeated human or predator 11.00–12.00 hours, 13.00–14.00 hours, and 15.00–16.00 hours | 1 hour repeated human or predator 11.00–12.00 hours, 13.00–14.00 hours, and 15.00–16.00 hours | single 1 hour at random time between 23.00 and 02.00 hours |
| 3 | no disturbance | 10 min simulated territory intrusion 07.00 hours and human 11.00–12.00 hours, 13.00–14.00 hours, and 15.00–16.00 hours | no disturbance |
To evaluate the effect of long-duration continuous human disturbance, group 1 (table 1) was subjected to a single 4-hour continuous chase (07.00–11.00 hours local time) by three different observers on the second day of the monitoring period. The first day was used as a no-disturbance control. Chases involved one observer making loud noises while following the bird on foot at close proximity (usually less than 5 m). Each observer tracked the individual bird with a handheld Yagi antenna and AR800 receiver to ensure proximity to the individual being disturbed.
To evaluate the effect of short, frequent disturbance and to compare natural (predator) versus human-mediated stressors, group 2 (table 1) was subjected to two types of 1-hour repeated disturbances from 11.00 to 12.00 hours, 13.00 to 14.00 hours and 15.00 to 16.00 hours local time, on both days of the monitoring period. The type of disturbance was randomly selected as either predator presence (screech owl Megascops asio decoy with playback in vireo territory) or human disturbance (table 1). Human disturbance included chasing the bird, passively sitting in the territory by one observer, or walking through the territory without targeting the bird by one observer, in random order. Group 2 was also subjected to 1-hour nocturnal human disturbances on the third night. The time of the 1-hour disturbance was randomly selected between 23.00 and 02.00 hours local time. The first two nights were used as experimental controls with no disturbance. Nocturnal disturbances included three observers simultaneously walking through the territory and playing loud music at 10 m from the territory.
Group 3 (table 1) was subjected to a simulated conspecific territory intrusion (10 min of white-eyed vireo playback and decoy presentation in territory) at 07.00 hours local time and three 1-hour repeated human disturbances between 11.00 and 12.00 hours, 13.00 and 14.00 hours and 15.00 and 16.00 hours local time on the second day. Similar to the disturbances for group 2, human disturbance in group 3 was randomly assigned as a chase, passive or walking, applied in random order and performed by three different observers. The first day was used as a non-disturbance control period.
(d) Behavioural observations
Behavioural observations were conducted for 4 hours for two periods: during control non-disturbance periods, at 50 m from the territory using a blind; and during disturbance trials. We specifically recorded whether males continued to perform nesting duties, such as nest-building, incubation and feeding young. All of our activities around and near the vireo territories were limited to the disturbance trials and non-disturbance behavioural observation periods.
(e) Calibrating heart rate to energy expenditure for white-eyed vireos
Heart rate measurements allowed us to indirectly estimate oxygen consumption and finally the energy expenditure, because the two variables are related to each other as derived by Fick's equation: , where is the oxygen consumption; fH is the heart rate; Vs is the cardiac stroke volume; is the oxygen content of the arterial blood; and is the oxygen content of the mixed venous blood (Fick 1870). If the oxygen pulse, Vs , remains constant, then there is a linear relationship between fH and and the former can be used to determine the latter (Green et al. 2001). However, calibration experiments to determine the exact relationship between the two variables need to be conducted for each species under study. Calibrations were performed between 9 and 11 May 2006 on five white-eyed vireos that were not included in disturbance experiments. Birds fitted with heart rate transmitters were placed in 2 l plastic metabolic chambers to simultaneously measure heart rate and in 10 s intervals. On average, birds were held in the respirometry chamber for a period of 2.3±0.6 hours (mean±s.e.). In the chamber, birds could freely move around, but when placed in a dark location, they were generally quiescent in the chambers (as determined by their low heart rate and by the lack of movement noises). Thus, we were able to obtain an estimate of over a range of heart rates. However, birds were unable to perform sustained or short bouts of true flight in the chamber as they would in the wild and therefore heart rate calibrations were not representative of such activities.
We measured oxygen consumption and carbon dioxide production in an open-flow, push-through respirometry system. External air was dried over Drierite columns and pumped through a mass flow controller (TR-FCI, Sable Systems, Nevada, USA) and a multiplexer (V2-0, Sable Systems) into the metabolic chamber and a reference chamber. Flow rate was 1000 ml min−1 and the flow controller was calibrated prior to use via a bubble meter. A previous factory calibration indicated that the flow rate errors were less than 1.2 per cent. Air leaving the chambers was dehumidified using a Peltier effect condenser (PC-1; Sable Systems, Henderson, USA), and CO2 concentration was measured from a subsample of the outlet flow (CA-1B, Sable Systems). Before (FC-1B, Sable Systems) was determined, Drierite was used to scrub potential remaining water from the air and CO2 was scrubbed from the air stream. The respirometry system was tested for leaks by pressurizing it and determining that no air was lost after 10 min of observation.
We estimated instantaneous oxygen consumption using the equation of Bartholomew et al. (1981),
where is the oxygen concentration in the excurrent air; V is the volume of the system including tubing; is the flow rate through the system; and Δt is the interval between the measurements at times t and t−1. We determined the denominator of this equation (the so-called Z-value) empirically using Datacan (Sable Systems). We then calculated the rate of oxygen consumption by eqn (4a) of Withers (1977),
where is the incurrent oxygen concentration. We also determined the respiratory quotient (the ratio of CO2 produced to O2 consumed) and used it to calculate thermal equivalents and metabolic rate (in kJ d−1, following Walsberg & Hoffman 2005). Least-squares regressions were used to determine the relationship between the heart rate and the energy expenditure (kJ d−1) for each individual.
(f) Statistical analyses
We evaluated the energetic cost in response to both human-mediated activities and natural disturbances in two ways: (i) by making repeated within-individual comparisons of the mean energy expenditure and activity between human-mediated disturbance and non-disturbance trials at the same time periods; and (ii) by testing for changes in energy expenditure before (baseline), during and after a disturbance; with the exception of long-duration disturbances because the 4 hours baseline fell within the roosting period. We used sequential Bonferroni-corrected paired sample t-tests (Holm 1979; Rice 1989) and repeated-measures analysis of variance (ANOVA) with the time of the day as a cofactor to control for circadian rhythms in activity and heart rate. Unless otherwise stated, we used one-way ANOVAs to compare the mean energy expenditure and activity between the different types of disturbance (three types of 1-hour human disturbances, 1-hour repeated versus 4-hour long duration and natural versus human). All statistical analyses were conducted using SPSS statistical software (v. 16.0; SPSS Inc., Chicago, IL, USA).
3. Results
(a) Relationship between heart rate and energy expenditure
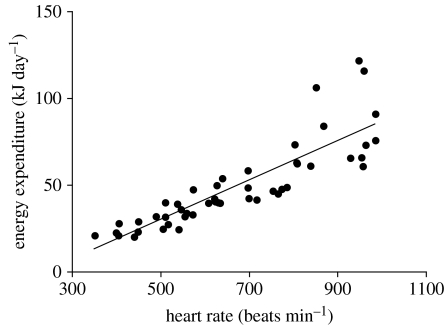
Heart rate was significantly correlated with energy expenditure in white-eyed vireos (figure 1) and the relationship across all individuals was best described by a linear fit: fH−26.2 (figure 1; mean r2=0.735). We therefore considered heart rate as a valid estimate of energy expenditure in this species and propose to use heart rate as a continuous and instantaneous estimate of energetic costs to disturbance in free-living vireo males. The empirically determined Z-value was Z=0.42±0.11.
Figure 1.
Relationship between heart rate (fH) and energy expenditure of five white-eyed vireos. The regression equation is fH−26.2, r2 =0.735. Heart rate calibrations were performed between 07.00 and 22.30 hours (local time).
(b) Response to daytime human disturbance
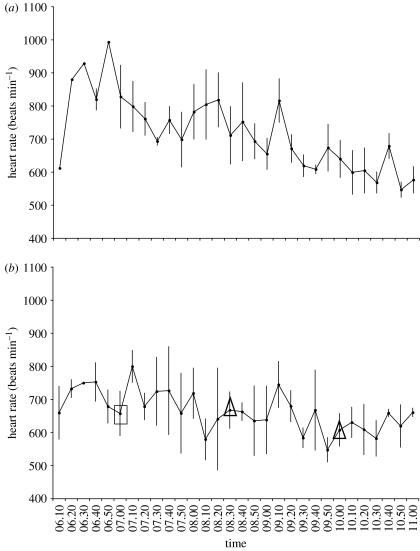
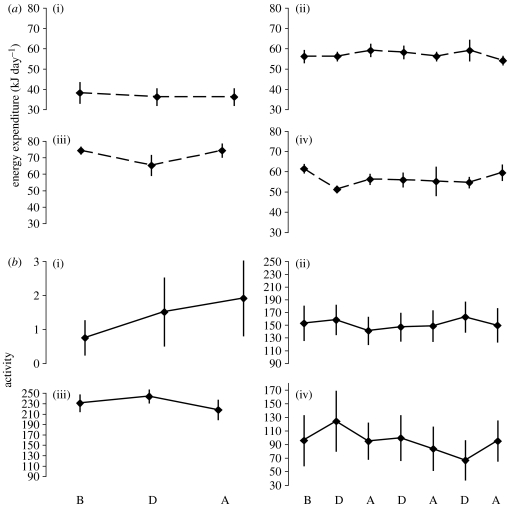
We detected a temporary (10–15 min) increase in heart rate (average 22%) shortly after the start of the long-duration (4 hour; figure 2b) human-mediated chase experiment, which was not present during non-disturbance trials on day 1 (figure 2a), indicating an initial alarm response to the chase. While heart rate decreased during the control period from 06.50 to 07.10 hours, heart rate increased within the same time frame on the day of the disturbance experiment (figure 2a,b). However, individuals did not significantly differ in activity or energy expenditure during the 4-hour chase when compared with the same time period with no disturbance (repeated-measures ANOVA, energy expenditure F2,6=1.233, p=0.356; activity F2,8=1.424, p=0.296). Similarly, repeated 1-hour disturbances (chase, passive or walking) did not elicit a significant change in energy expenditure or activity before, during and after each disturbance (repeated-measures ANOVA, p≥0.167; figure 3a,b, respectively).
Figure 2.
Mean heart rate (±s.e.) for four male white-eyed vireos (a) during the control no-disturbance trial (day 1) and (b) in response to a 4-hour chase disturbance experiment (07.00–11.00 hours local time, day 2). Rectangle indicates start of chase and triangles indicate change in observer for the chase experiment. Birds respond with increased heart rate at the start of the chase. Heart rate is shown from when birds became active (06.10 hours local time).
Figure 3.
Mean (±s.e.) (a) energy expenditure and (b) activity comparisons before (B), during (D) and after (A) experimental human and natural disturbances: (i) night disturbance, (ii) short repeated, (iii) conspecific intrusion and (iv) predator. Energy expenditure and activity did not significantly increase in response to disturbances.
We did not detect significant differences in vireo energy expenditure and activity between different types of human disturbances, i.e. whether the 1-hour disturbance was passive, walking or a chase (repeated-measures ANOVA, energy expenditure F2,12=0.197, p=0.824; activity F2,16=1.165, p=0.337). Birds that were subjected to 4-hour disturbances did not expend more energy, nor were they more active, during the disturbance than the birds that were exposed to 1-hour repeated disturbances (one-way ANOVA, energy expenditure, F1,9=3.678, p=0.087; activity F1,12=0.179, p=0.680). However, males that were subjected to 1-hour disturbances on both days and a night (group 2, table 1) were significantly less active during the day (overall mean daily activity) than males that were subjected to repeated 1-hour disturbances on day 2 only (group 3, table 1: one-way ANOVA, F1,7=14.526, p=0.007). However, we found no associated energetic costs to any such disturbances (one-way ANOVA, F1,5≤1.517, p ≥ 0.273).
(c) Response to night-time human disturbance
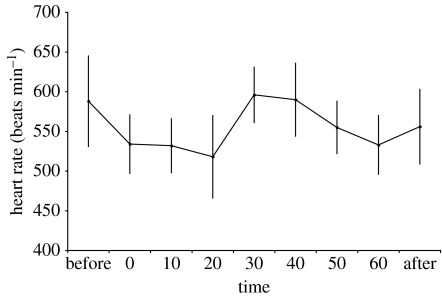
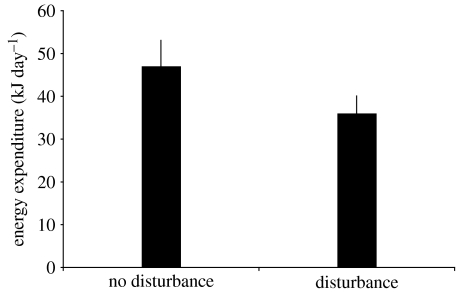
Heart rate increased towards the middle of the disturbance (figure 4), indicating an arousal in response to nocturnal disturbance that was not associated with increased activity. However, these disturbances did not cause a significant increase in energy consumption in white-eyed vireos (figure 3a; F2,6=0.632, p=0.563) when compared with energy expenditure immediately before the disturbance. Similarly, activity did not change from before to during to after the disturbance (figure 3b; repeated-measures ANOVA, F2,8=2.913, p=0.112). We also found no differences when comparing the overall mean energy expenditure during the disturbance to the control period (first night) when no disturbance was conducted (t3 =−3.539, p=0.038, not significant with a sequential Bonferroni correction requiring p≤0.007; figure 5). In fact, energy expenditure was higher on the night of no disturbance, possibly because it was the first night following transmitter placement.
Figure 4.
Mean heart rate (±s.e.) response to 1 hour of night-time human-mediated disturbance (randomly selected between 23.00 and 02.00 hours) for four male white-eyed vireos. Heart rate increase at time 30 min is not associated with increased activity (data not shown).
Figure 5.
Night-time mean energy expenditure (±s.e.) for 60 min of no-disturbance (night 1 of monitoring period) and for 60 min of experimental human-mediated disturbance (night 3 of monitoring period) for four white-eyed vireo males. Energy expenditure did not significantly differ between control and disturbance trials.
(d) Response to natural disturbances
There was no significant effect of short repeated predator calls and decoy presentations on energy expenditure or activity (repeated-measures ANOVA, energy expenditure F2,4≤9.678, p≥0.029; Bonferroni correction required p≤0.007; activity F2,8≤2.928, p>0.1; figure 4a,b) nor in relation to territorial intrusions (repeated-measures ANOVA, energy expenditure F2,4=4.177, p=0.105; activity F2,6=1.328, p=0.333; figure 3b). Birds did not expend more energy and were no more active in response to human disturbances than to natural disturbances (repeated-measures ANOVA, energy expenditure F2,2=0.133, p=0.882; activity F2,8=2.178, p=0.353).
(e) Breeding behaviour in relation to disturbances
Whereas most males continued nesting duties (e.g. incubating, feeding young) throughout human-mediated disturbance trials, two of five white-eyed vireos that were subjected to human, predator and night disturbances abandoned their territory some time after the 60-hour monitoring period. We surveyed both territories on five occasions four days after the monitoring period and were unable to locate either bird. Both males were transmittered early in the breeding season (April) and were in the early breeding stages (unpaired newly arrived male and nest-building).
4. Discussion
Birds often show negative responses to human activities, such as decreased reproductive success (Flemming et al. 1988; Piatt et al. 1990), elevated corticosterone stress responses (Wingfield et al. 1982; Nephew et al. 2003) or altered behaviour (e.g. birds flee from the disturbance; Burger 1981, Henson & Grant 1991; reviewed in Gill 2007). However, we know little about energetic costs of human disturbances in free-living birds, and what we know is restricted to large, long-lived species (Nimon et al. 1996; Ackerman et al. 2004). Our study, the first to investigate the energy expenditure in response to disturbance in a small (less than 12 g) free-living passerine, shows no evidence of elevated energetic costs to human or natural disturbances. Compared to control time periods, white-eyed vireos did not expend significantly more energy, nor were they more active, during a 4-hour continuous chase by three different observers. Even repeated 1-hour human-mediated activities, night disturbances and predator or conspecific presence did not elevate their average energy expenditure over a few hours. Whatever comparison of energy expenditure we conducted—either among baseline, during and after the stressor, or between control and experimental disturbance periods—we found no differences.
It is important to point out, however, that we did find one predicted response. Mean heart rate initially increases when birds are stressed by humans, indicating that white-eyed vireos perceive the disturbance and are mounting an ‘alarm’ response. However, this was a transitory response and the mean energy expenditure did not change (figure 3a). Similarly, we found evidence that mean heart rate increased in response to a 1-hour night disturbance (figure 4) with no change in energy expenditure (figure 3a). We cannot exclude the possibility that more frequent or longer night disturbances may have elicited significant energy consumption in white-eyed vireos. However, we consider this unlikely because daytime disturbances of varying intensity, duration and type had no impact on average energy expenditure. Similarly, we found no effect of heart rate transmitters on the activities of vireos because birds continued with breeding duties. We therefore propose that white-eyed vireos did not show measurable energetic responses to either day or night stressors.
The lack of a measurable energetic response was an unexpected result. There are several potential explanations. First, it is conceivable that vireos rapidly perceived our disturbance treatments as non-threatening. This explanation fits the parallel data indicating that similar disturbances also do not elicit hormonal responses (L. K. Butler et al., 2007, unpublished data). However, we cannot exclude that other physiological systems, such as the immune system, were affected. For example, Ots & Horak (1996) demonstrated a trade-off between health and reproductive effort. Great tits (Parus major), which are small passerines (19 g), became immunosuppressed when allocating energy towards reproduction. Such trade-offs may also occur in the face of environmental challenges, including human or natural disturbances.
A second potential explanation is that because white-eyed vireos are a fast-living species (short-lived, breed in their first year and have few breeding attempts), they quickly assess the severity of unpredictable changes in their environment and, when they perceive those changes as non-lethal, they minimize energy expenditure towards alleviating those disturbances in favour of maximizing energy available for reproduction. We propose that animals with similar fast life histories will show similar responses in energy expenditure. By contrast, when facing anthropogenic stressors, slow-living birds benefit from redirecting their physiology and behaviour towards survival. For example, slow-living species such as Humboldt penguins (Spheniscus humboldti; Ellenberg et al. 2006), wandering albatrosses (Diomedea exulans; Weimerskirch et al. 2002) and tule greater white-fronted geese (Anser albifrons elgasi; Ackerman et al. 2004) increased their heart rates and energetic costs to human-mediated stressors. All three species are long-lived (20 and 80 years in the penguin and albatross, respectively), lay small clutch sizes (one to two eggs), have large body size (more than 2 kg) or have long developmental periods, so responding to disturbances in ways that increase survival at the expense of current reproduction is consistent with our hypothesis that interspecific differences in response to unpredictable, non-life-threatening disturbances are correlated with interspecific differences in life history. Our results are also consistent with the studies that showed trade-offs in slow versus fast life histories in relation to immune defence investment (Martin et al. 2001; Tella et al. 2002). Recently, Martin et al. (2006) showed that slow-living house sparrows (Passer domesticus) from tropical populations invested more energy in costly immune activities than their temperate zone fast-living conspecifics.
Our results also hint at a difference in how anthropogenic stress is perceived during the reproductive season. Whereas two males that we disturbed early in the season abandoned their territories, the twelve males that we disturbed later in the season continued normal breeding activities. Although this observation is somewhat anecdotal, it is consistent with the interpretation that the reproductive value of an advanced brood was higher than one at the start of the breeding season. Despite the disturbance late in the breeding season, birds continued their parental investment as re-nesting opportunities would decrease as the breeding season progressed.
5. conclusion
Understanding the effect of human activity on wildlife is a major conservation concern, and military installations in particular have become areas of special conservation attention because they often provide large expanses of rare habitat for species of concern (Duncan et al. 1995; Krausman et al. 2005). Activities associated with military training are thought to act as severe disturbances for wildlife (Maier et al. 1998; Delaney et al. 1999) because of their high intensity and episodic nature. Our study was aimed at quantifying the energy expenditure of a passerine species during the breeding season in response to non-destructive and indirect human disturbance similar to military training on foot. While more chronic disturbances such as human-induced habitat changes could have effects, we failed to find evidence for an energetic cost to our study species in response to human intrusion on foot. The majority of the birds monitored continued to perform breeding duties such as incubating and feeding young. However, we do not yet know whether the lack of a response to disturbance is restricted to white-eyed vireos or whether other military activities such as live fire could have a significant impact on energy expenditure. This species is expected to be resistant to human disturbance because it can breed in urban environments (Hopp et al. 1995). It is therefore still unclear whether there is a cost of human disturbance in endangered species such as the related black-capped vireo. Nevertheless, even if there is a graded difference in disturbance sensitivity within vireos, we expect fast-living animals in general to be much less affected by human disturbances than slow-living animals. Furthermore, if military areas provide an otherwise rare optimal habitat for species, this benefit may outweigh potential disturbances associated with military training (Gill et al. 2001). We suggest that military training areas may act as preservation sites if efforts are geared towards understanding impacts on wildlife at multiple levels, as has been suggested for Sonoran pronghorn (Antilocapra americana sonoriensis; Krausman et al. 2005) and elk (Alces alces; Andersen et al. 1996).
Acknowledgments
We thank E. C. Fergus, J. Barry, J. L. Granger, S. Lovell, The Nature Conservancy (Fort Hood Chapter) and the Fort Hood Natural Resources Management Branch for their assistance in the field. We would also like to thank M. Bowlin, R. Charif and N. Hristov for their assistance with the data acquisition and processing. This project was supported by the Department of Defense, Strategic Environmental Research and Development Program, project CS-1396, through the US Army Corps of Engineers, Engineer Research and Development Center, contract no. W9132T-05-C-0023. All protocols were approved by the Institutional Animal Care and Use Committee of Princeton University.
References
- Ackerman J.T., Takekawa J.Y., Kruse K.L., Orthmeyer D.L., Yee J.L., Ely C.R., Ward D.H., Bollinger K.S., Mulcahy D.M. Using radiotelemetry to monitor cardiac response of free-living tule greater white-fronted geese (Anser albifrons elgasi) to human disturbance. Wilson Bull. 2004;116:146–151. doi:10.1676/03-110 [Google Scholar]
- Andersen R.J., Linnell D.C., Langvatn R. Short term behavioural and physiological response of moose Alces alces to military disturbance in Norway. Biol. Conserv. 1996;77:169–176. doi:10.1016/0006-3207(96)00004-3 [Google Scholar]
- Bartholomew G.A., Vleck D., Vleck C.M. Instantaneous measurements of oxygen consumption during pre-flight warm-up and post-flight cooling in sphingid and saturniid moths. J. Exp. Biol. 1981;90:17–32. [Google Scholar]
- Berger S., Wikelski M., Romero L.M., Kalko E.K., Roedl T. Behavioral and physiological adjustments to new predators in an endemic island species, the Galapagos marine iguana. Horm. Behav. 2007;52:653–663. doi: 10.1016/j.yhbeh.2007.08.004. doi:10.1016/j.yhbeh.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Bevan R.M., Speakman J.R., Butler P.J. Daily energy expenditure of tufted ducks: a comparison between indirect calorimetry, doubly labelled water and heart rate. Funct. Ecol. 1995;9:40–47. doi:10.2307/2390088 [Google Scholar]
- Boyd I.L., Bevan R.M., Woakes A.J., Butler P.J. Heart rate and behavior of fur seals: implications for measurement of field energetics. Am. J. Physiol. Heart Circ. 2006;276:844–857. doi: 10.1152/ajpheart.1999.276.3.H844. [DOI] [PubMed] [Google Scholar]
- Burger J. The effect of human activity on birds at a coastal bay. Biol. Conserv. 1981;21:231–241. doi:10.1016/0006-3207(81)90092-6 [Google Scholar]
- Cochran W.W., Wikelski M. Individual migratory tactics of new world Catharus thrushes: current knowledge and future tracking options from space. In: Greenberg R., Marra P., editors. Birds of two worlds. Johns Hopkins University Press; Washington, DC: 2005. pp. 274–289. [Google Scholar]
- Creel S., Fox J.E., Hardy A., Sands J., Garrott B., Peterson R.O. Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv. Biol. 2002;16:809–814. doi:10.1046/j.1523-1739.2002.00554.x [Google Scholar]
- Crofoot M.C., Gilby I.C., Wikelski M.C., Kays R.W. Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. Proc. Natl Acad. Sci. USA. 2008;105:577–581. doi: 10.1073/pnas.0707749105. doi:10.1073/pnas.0707749105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr N.E., Wikelski M., Romero L.M. Increased energy expenditure but decreased stress responsiveness during molt. Physiol. Biochem. Zool. 2008;81:452–462. doi: 10.1086/589547. [DOI] [PubMed] [Google Scholar]
- Daan S., Deerenberg C., Dijkstra C. Increased daily work precipitates natural death in the kestrel. J. Anim. Ecol. 1996;65:539–544. doi:10.2307/5734 [Google Scholar]
- Dallman, M. F., & Bhatnagar, S. 2001 Chronic stress and energy balance: role of the hypothalamo-pituitary-adrenal axis. In Handbook of physiology. Section 7: the endocrine system, New York, NY: Oxford University Press.
- Delaney D.K., Grubb T.G., Beier P., Pater L.L., Hildegard Reiser M. Effects of helicopter noise on Mexican spotted owls. J. Wildlife Manage. 1999;63:60–76. doi:10.2307/3802487 [Google Scholar]
- Duncan B.W., Breininger D.R., Schmalzer P.A., Larson V.L. Validating a Florida scrub jay habitat suitability model, using demography data on Kennedy Space Center. Photogram. Eng. Remote Sens. 1995;61:1361–1370. [Google Scholar]
- Ellenberg U., Mattern T., Seddon P.J., Luna Jorquera G. Physiological and reproductive consequences of human disturbance in Humboldt penguins: the need for species-specific visitor management. Biol. Conserv. 2006;133:95–106. doi:10.1016/j.biocon.2006.05.019 [Google Scholar]
- Fick A. Uber die Messung des Blutquantums in der Herzventrikeln. Sitz. Physik. Med. Ges. 1870;2:16. [Google Scholar]
- Flemming S.P., Chiasson R.D., Smith P.C., Austin-Smith P.J., Bancroft R.P. Piping plover status in Nova Scotia related to its reproductive and behavioral responses to human disturbance. J. Field Ornithol. 1988;59:321–330. [Google Scholar]
- Fowler G.S. Behavioral and hormonal responses of Magellanic penguins (Spheniscus magellanicus) to tourism and nest site visitation. Biol. Conserv. 1999;90:143–149. doi:10.1016/S0006-3207(99)00026-9 [Google Scholar]
- Gill J.A. Approaches to measuring the effects of human disturbance on birds. Ibis. 2007;149(suppl.):9–14. doi:10.1111/j.1474-919X.2007.00642.x [Google Scholar]
- Gill J.A., Norris K., William J., Sutherland W.J. Why behavioural responses may not reflect the population consequences of human disturbance. Biol. Conserv. 2001;97:265–268. doi:10.1016/S0006-3207(00)00002-1 [Google Scholar]
- Green J.A., Butler P.J., Woakes A.J., Boyd I.L., Holder R.L. Heart rate and rate of oxygen consumption of exercising Macaroni penguins. J. Exp. Biol. 2001;204:673–684. doi: 10.1242/jeb.204.4.673. [DOI] [PubMed] [Google Scholar]
- Greenberg N., Carr J.A., Summers C.H. Causes and consequences of stress. Integr. Comp. Biol. 2002;42:508–516. doi: 10.1093/icb/42.3.508. doi:10.1093/icb/42.3.508 [DOI] [PubMed] [Google Scholar]
- Hayden, T. J., Cornelius, J. D., Weinberg, H. J., Jette, L. A. & Melton, R. H. 2001 Endangered species management plan for Fort Hood, Texas; FY01-05. Technical report ERDC/CERL TR-01-26. Champaign, IL: Department of the Army, Engineer Research and Development Center, Construction Engineering Research Laboratory.
- Henson P., Grant T.A. The effects of human disturbance on trumpeter swan breeding behavior. Wildl. Soc. Bull. 1991;19:248–257. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Hopp S.L., Kirby A., Boone C.A. White-eyed vireo (Vireo griseus) In: Poole A., editor. The birds of north america online. Cornell Lab of Ornithology; Ithaca, NY: 1995. http://bna.birds.cornell.edu/bna/species/168. [Google Scholar]
- Hunt K.E., Rolland M.I., Kraus S.D., Wasser S.K. Fecal glucocorticoid analysis as a potential tool for investigating physiological stress in North Atlantic right whales (Eubalaena glacialis) Integr. Comp. Biol. 2003;43:1007. [Google Scholar]
- Knight R.L., Gutzwiller K.J. Wildlife and recreationists: coexistence through management research. Island Press; Washington, DC: 1995. pp. 1–389. [Google Scholar]
- Krausman P.R., Harris L.K., Haas S.K., Koenen K.K.G., Devers P., Bunting D., Barb M. Sonoran pronghorn habitat use on landscapes disturbed by military activities. Wildl. Soc. Bull. 2005;33:16–23. doi:10.2193/0091-7648(2005)33[16:SPHUOL]2.0.CO;2 [Google Scholar]
- Love O.P., Breuner C.W., Vezina F., Williams T.D. Mediation of a corticosterone-induced reproductive conflict. Horm. Behav. 2004;46:59–65. doi: 10.1016/j.yhbeh.2004.02.001. doi:10.1016/j.yhbeh.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Lucas M.C. Heart rate as an indicator of metabolic rate and activity in adult Atlantic salmon, Salmo salar. J. Fish Biol. 1994;44:889–903. doi:10.1111/j.1095-8649.1994.tb01262.x [Google Scholar]
- Maier J.A., Murphy S.M., White R.G., Smith M.D. Responses of caribou to overflights by low-altitude jet aircraft. J. Wildl. Manage. 1998;62:752–766. doi:10.2307/3802352 [Google Scholar]
- Martin T.E., Møller A.P., Merino S., Colbert J. Does clutch size evolve in response to parasites and immunocompetence? Proc. Natl Acad. Sci. USA. 2001;98:2071–2076. doi: 10.1073/pnas.98.4.2071. doi:10.1073/pnas.98.4.2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.B., II, Hasselquist D., Wikelski M. Investment in immune defense is linked to pace of life in house sparrows. Oecologia. 2006;147:565–575. doi: 10.1007/s00442-005-0314-y. doi:10.1007/s00442-005-0314-y [DOI] [PubMed] [Google Scholar]
- McKinney R.A., McWilliams S.R. A new model to estimate daily energy expenditure for wintering waterfowl. Wilson Bull. 2005;117:44–55. doi:10.1676/04-060 [Google Scholar]
- Nagy K.A. Water and energy budgets of free-living animals: measurement using isotopically labeled water. In: Hadley N.F., editor. Environmental physiology of desert organisms. Dowden, Hutchinson and Ross (Halsted Press); Stroudsberg, PA: 1975. pp. 227–245. [Google Scholar]
- Nephew B.C., Kahn S.A., Romero L.M. Heart rate and behavior are regulated independently of corticosterone following diverse acute stressors. Gen. Comp. Endocrinol. 2003;133:173–180. doi: 10.1016/s0016-6480(03)00165-5. doi:10.1016/S0016-6480(03)00165-5 [DOI] [PubMed] [Google Scholar]
- Nimon A.J., Schroter R.C., Oxenham R.K.C. Artificial eggs: measuring heart rate and effects of disturbance in nesting penguins. Physiol. Behav. 1996;60:1019–1022. doi: 10.1016/0031-9384(96)00079-0. [DOI] [PubMed] [Google Scholar]
- Ots I., Horak P. Great tits Parus major trade health for reproduction. Proc. R. Soc. B. 1996;263:1443–1447. doi: 10.1098/rspb.1996.0210. doi:10.1098/rspb.1996.0210 [DOI] [PubMed] [Google Scholar]
- Piatt J.F., Roberts B.D., Lidster W.W., Wells J.L., Hatch S.A. Effects of human disturbance on breeding least and crested auklets at St Lawrence Island, Alaska. Auk. 1990;107:342–350. [Google Scholar]
- Rice W. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. doi:10.2307/2409177 [DOI] [PubMed] [Google Scholar]
- Romero L.M., Meister C.J., Cyr N.E., Kenagy G.J., Wingfield J.C. Seasonal glucocorticoid responses to capture in wild free-living mammals. Am. J. Physiol. Regulatory Integrative. 2008;294:614–622. doi: 10.1152/ajpregu.00752.2007. doi:10.1152/ajpregu.00752.2007 [DOI] [PubMed] [Google Scholar]
- Stearns S. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Steen J.B., Gabrielsen G.W., Kanwisher J. Physiological aspects of freezing behavior in willow ptarmigan hens. Acta Physiol. Scand. 1988;134:299–304. doi: 10.1111/j.1748-1716.1988.tb08493.x. [DOI] [PubMed] [Google Scholar]
- Tella J.A., Scheuerlein A., Ricklefs R.E. Is cell-mediated immunity related to the evolution of life-history strategies of birds? Proc. R. Soc. B. 2002;269:1059–1066. doi: 10.1098/rspb.2001.1951. doi:10.1098/rspb.2001.1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel D., Menoni E., Brenot J.F., Jenni L. Effects of recreation and hunting on flushing of Capercaillie. J. Wildl. Manage. 2007;71:1784–1792. doi:10.2193/2006-268 [Google Scholar]
- Walker B.G., Boersma P.D., Wingfield J.C. Habituation of adult Magellanic penguins to human visitation as expressed through behavior and corticosterone secretion. Conserv. Biol. 2006;20:146–154. doi: 10.1111/j.1523-1739.2005.00271.x. doi:10.1111/j.1523-1739.2005.00271.x [DOI] [PubMed] [Google Scholar]
- Walsberg G.E., Hoffman T.C.M. Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. J. Exp. Biol. 2005;208:1035–1043. doi: 10.1242/jeb.01477. doi:10.1242/jeb.01477 [DOI] [PubMed] [Google Scholar]
- Wasser S.K., Bevis K., King G., Hanson E. Noninvasive physiological measures of disturbance in the northern spotted owl. Conserv. Biol. 1997;11:1019–1022. doi:10.1046/j.1523-1739.1997.96240.x [Google Scholar]
- Weimerskirch H., Shaffer S.A., Mabille G., Martin J., Boutard O., Rouanet J.L. Heart rate and energy expenditure of incubating wandering albatrosses: basal levels, natural variation, and the effects of human disturbance. J. Exp. Biol. 2002;205:475–483. doi: 10.1242/jeb.205.4.475. [DOI] [PubMed] [Google Scholar]
- Wikelski M., Cooke S.J. Conservation physiology. Trends Ecol. Evol. 2006;21:38–46. doi: 10.1016/j.tree.2005.10.018. doi:10.1016/j.tree.2005.10.018 [DOI] [PubMed] [Google Scholar]
- Wikelski M., Ricklefs R.E. The physiology of life histories. Trends Ecol. Evol. 2001;16:479–481. doi:10.1016/S0169-5347(01)02279-0 [Google Scholar]
- Wikelski M., Tarlow E.M., Raim A., Diehl R.H., Larkin R.P., Visser G.H. Costs of migration in free-flying songbirds. Nature. 2003;423:704. doi: 10.1038/423704a. doi:10.1038/423704a [DOI] [PubMed] [Google Scholar]
- Williams G.C. Natural selection, costs of reproduction and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]
- Wingfield J.C., Sapolsky R.M. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Wingfield J.C., Smith J.P., Farner D.S. Endocrine responses of white-crowned sparrows to environmental stress. Condor. 1982;84:399–409. doi:10.2307/1367443 [Google Scholar]
- Wingfield J.C., Maney D.L., Breuner C.W., Jacobs J.D., Lynn S., Ramenofsky M., Richardson R.D. Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Integr. Comp. Biol. 1998;38:191–206. doi:10.1093/icb/38.1.191 [Google Scholar]
- Withers P.C. Measurement of , , and evaporative water-loss with a flow-through mask. J. Appl. Physiol. 1977;42:120–123. doi: 10.1152/jappl.1977.42.1.120. [DOI] [PubMed] [Google Scholar]