Abstract
Environmental changes, such as current climate warming, can exert directional selection on reproductive phenology. In plants, evolution of earlier flowering requires that the individuals bearing genes for early flowering successfully reproduce; for non-selfing, zoophilous species, this means that early flowering individuals must be visited by pollinators. In a laboratory experiment with artificial flowers, we presented captive bumble-bees (Bombus impatiens) with flower arrays representing stages in the phenological progression of a two-species plant community: Bees that had been foraging on flowers of one colour were confronted with increasing numbers of flowers of a second colour. Early flowering individuals of the second ‘species’ were significantly under-visited, because bees avoided unfamiliar flowers, particularly when these were rare. We incorporated these aspects of bee foraging behaviour (neophobia and positive frequency dependence) in a simulation model of flowering-time evolution for a plant population experiencing selection against late flowering. Unlike simple frequency dependence, a lag in pollinator visitation prevented the plant population from responding to selection and led to declines in population size. Pollinator behaviour thus has the potential to constrain evolutionary adjustments of flowering phenology.
Keywords: climate change, colour preference, frequency dependence, hysteresis, phenology, pollination
1. Introduction
Interactions with other species can affect an organism's ecological and evolutionary responses to environmental change. Theoretical and experimental work has shown that predation and competition can influence demographic responses to changing conditions and limit a species' ability to adapt to a changing resource optimum (Ives 1995; Davis et al. 1998; Jiang & Kulczycki 2004; Johansson 2008). These results suggest that a failure to consider species interactions can lead to overly optimistic predictions about the ability of populations to cope with environmental change. However, little attention has been paid to the role of mutualists in enabling or restricting evolutionary responses to changing conditions, although it has been noted that a shortage of mutualists could act as a constraint on the range shifts expected to result from climate change (Possingham 1993). In this paper, we explore how the behaviour of a mutualist can affect a species' evolutionary response to a changing environment.
Timing of reproduction can strongly affect fitness (e.g. Réale et al. 2003; Both et al. 2006; Kudo 2006). For plants, flowering at a time when soil moisture is adequate can be critical, and, in seasonal environments, fruiting must be completed before the onset of frost or drought (reviewed by Rathcke & Lacey 1985). Changes in the abiotic environment may lead to shifts in the optimal timing of reproduction and consequent selection on flowering phenology. For example, current climate change is believed to be driving aridification in certain regions (IPCC 2007; Seager et al. 2007), a trend that may favour plants that complete reproduction earlier in the season (Stinson 2004; Franks et al. 2007). Flowering time is known to have a genetic basis in several species, and can respond to selection (Mazer & LeBuhn 1999; Geber & Griffen 2003; Franks et al. 2007). Of course, for early flowering to evolve, early flowering individuals must successfully reproduce; for outcrossing, zoophilous species, this requires that early flowering individuals receive pollinator visits. Thus, predicting a plant's evolutionary response to environmental change requires considering the dynamics of its interactions with mutualists.
Early flowering individuals within a population are both rare (relative to other plant species flowering in the community at that time) and unfamiliar to pollinators—traits that may cause these individuals to receive relatively few pollinator visits. It is already known that pollinators, similar to many consumers (Punzalan et al. 2005), tend to forage in a positively frequency-dependent fashion, at least under controlled laboratory conditions (reviewed by Smithson 2001). Bumble-bees (Bombus terrestris) faced with arrays of rewarding artificial flowers of two different colours tend to over-visit the more common type, all else being equal (Smithson & Macnair 1996, 1997). Positive frequency-dependent pollinator visitation could reduce the reproductive success of rare floral morphs of obligately animal-pollinated plants, and therefore has the potential to impose a constraint on floral evolution (Smithson 2001). In nature, rarity is likely to covary with familiarity: rare flower types may also be unfamiliar to pollinators. The avoidance of unfamiliar (often food) objects, known as neophobia, has been documented in birds (Coppinger 1970) and many other vertebrates (reviewed by Brigham & Sibly 1999), but has been largely unstudied in invertebrates. In some cases where positive frequency-dependent pollinator foraging has been observed in the field (e.g. Hersch & Roy 2007), the behaviour could result from a combination of preference for the common type (true frequency dependence) and avoidance of the unfamiliar type (neophobia).
Even if pollinators eventually learn to visit new flower types, a lag in their detection or acceptance of rare and unfamiliar flowers could penalize early flowering individuals and might impose a constraint on the evolution of early flowering. Such a lag has been inferred from observations in the field that plants often show relatively low pollinator visitation early in their flowering period, and that visitation rates can be more closely correlated with flower densities at an earlier date than with current densities (Thomson 1981, 1982). However, the existence of this type of hysteresis in plant–pollinator interactions has not been rigorously tested or quantified under controlled conditions.
Here, we test the hypotheses that (i) bees exhibit a lag in acceptance of novel flowers and (ii) such a lag could negatively affect a plant population experiencing selection for earlier flowering. To evaluate the first hypothesis, we conducted an experiment with captive bumble-bees foraging on artificial flowers of two colours, presented in a sequence that simulated the seasonal progression of flowering in a two-species plant community. We then used computer simulations to evaluate potential effects of the observed pollinator behaviour on the evolutionary trajectory of a plant population.
2. Material and methods
(a) Foraging experiments
We conducted two experiments with laboratory-reared bumble-bees (Bombus impatiens Cresson). In experiment 1, bees had prior experience with both yellow and blue artificial flowers; the results serve as a comparison with the more realistic experiment 2, in which bees were initially naive to one of the two flower colours.
(i) Study system and experimental design
We acquired bumble-bee colonies from Biobest Biological Systems (Leamington, Ontario, Canada). Colonies were connected to a screened indoor flight arena with a gated tunnel that allowed us to control entry and exit of individual bees. Worker bees were given time to learn to forage on 30 per cent (w/w) sucrose solution (henceforth ‘nectar’) provided in artificial flowers consisting of 1.5 ml Eppendorf tubes with lids removed and artificial ‘corollas’ attached to the mouth of the tube. Bees were individually marked with coloured correction fluid, and those that were consistently willing to forage were used for experiments. We used 6 bees from 2 colonies in experiment 1, and 12 bees from 3 other colonies in experiment 2. Pollen and additional nectar were provided directly to the hive as needed.
Experimental flower arrays consisted of 100 blue or yellow artificial flowers embedded in a 162×102 cm green foam-core background, positioned such that each flower was 12 cm from its six nearest neighbours. Each flower position was numbered so that an observer could record which flowers had been visited. For experimental runs, flowers were 3×3 cm squares of clear polystyrene, spray-painted either blue or yellow (see figure A1 in the electronic supplementary material), attached to Eppendorf tubes of the same colour. Each bee, over a 2-day period, sequentially encountered several arrays (see below) presenting different proportions of blue and yellow flowers; flowers were assigned to positions randomly. These different treatments are referred to as flower colour ‘frequencies’. Each bee foraged for four to seven foraging bouts per frequency (the minimum necessary to obtain 100 flower visits after the first foraging bout(s); see §2a(ii)). Flowers were washed between frequencies. At the beginning of every foraging bout, each flower contained 3 μl of nectar. Flowers were not refilled during foraging bouts, an arrangement meant to mimic dynamics in a small natural patch of flowers, in which flowers can be temporarily drained of nectar.
Experiment 1 (bees familiar with both flower colours)
Training: Bees were allowed to forage freely from a training array of two blue and two yellow flowers filled with nectar until reliable foragers were identified. Prior to an experimental run, to ensure approximately equal proficiency on both blue and yellow flowers, the selected bee was allowed to forage for one to two bouts on two yellow and two blue flowers, each containing 3 μl of nectar (refilled after being drained).
Testing: Each bee was presented with arrays of five different colour frequencies (10B (blue) : 90Y (yellow), 20B : 80Y, 50B : 50Y, 80B : 20Y and 90B : 10Y flowers). Arrays were presented in random order to avoid any lag effect that could result from bees experiencing frequencies in increasing order, as they did in experiment 2.
Experiment 2 (bees familiar with only one flower colour)
Training: The training array consisted of five flowers with white corollas. Once a reliable forager was identified and accustomed to foraging on flowers containing only 3 μl of nectar, the white training flowers were replaced by flowers of what was to be that bee's familiar colour (blue or yellow, assigned alternately). These flowers always contained only 3 μl of nectar at the base of the tube, and bees frequently had to be led into the tube by a trail of nectar drops. We allowed each bee to forage for one complete foraging bout on the coloured training flowers after its first successful (rewarded) entry.
Testing: Each bee was first presented with an array consisting of 100 flowers of the familiar colour. After four to five foraging bouts on that array, the bee encountered arrays that comprised a constantly increasing frequency of the novel colour (10, 20, 50, 80 and 90%; four consecutive bouts or above at each frequency). If bees had still not switched to the novel colour at 90 per cent, they were allowed to forage for one bout on an array of 100 per cent novel flowers.
(ii) Data collection and analysis
Because bees often encountered empty (drained) flowers and frequently rejected these flowers without fully entering them, we counted flower visits in two ways: In the first, we counted only visits in which more than half the bee's body entered the Eppendorf tube. In the second, we included all visits in which a bee landed on a flower and faced the tube entrance. In real flowers, these brief inspection visits might not transfer pollen, but they do reflect bees' decisions to investigate. We ran all analyses using both the datasets but, because the results are qualitatively identical, we present only the results based on the second (more complete) protocol.
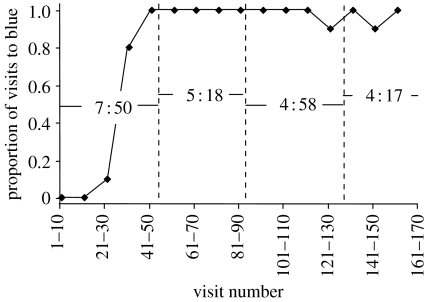
We omitted the visitation data from the first foraging bout for each bee at each frequency because we suspected that the first foraging bout would not reflect the ‘asymptotic’ behaviour pattern attained after some initial learning. Bees typically continued to do whatever they had been doing in the preceding foraging bout, and a clear switch to the new flower colour often occurred during the first bout at a given frequency (figure 1 shows a representative example). In some cases, the switch did not occur until the second or third foraging bout; in these cases, we only considered the data from bouts after the switch. This protocol gives a conservative estimate of any lag in visitation patterns. However, in four cases, bees visited a novel flower during the first foraging bout at a particular flower colour frequency and visited no others in subsequent bouts at that frequency; omitting the first bout meant ignoring that visit. We believe this is reasonable because a single visit to an outcrossing species would not result in successful pollen transfer.
Figure 1.
Data from a representative bee in experiment 2, foraging on an array of 80 blue (novel) and 20 yellow (familiar) flowers. The visit sequence is broken into 10-visit sections. Dashed lines separate the four foraging bouts, the durations of which (in minutes) are indicated in the figure.
To estimate the level of frequency dependence when bees had prior experience with both flower colours, we fitted the data from experiment 1 to a curve of the form (Smithson & Macnair 1996):
| (2.1) |
where Y is the proportion of visits to yellow flowers and A is the proportion of yellow flowers available (0.1, 0.2, 0.5, 0.8 or 0.9); the curve terminates at (0,0) and (1,1). The parameter b indicates the strength of frequency dependence, with values less than 1 indicating negative frequency dependence and values greater than 1 indicating positive frequency dependence. Values of V>1 indicate a frequency-independent preference for yellow; values less than 1 indicate blue preference. The parameters b and V were estimated using the nonlinear platform in JMP IN v. 5.1.2.
To evaluate the strength of the lag effect shown in experiment 2, we treated the visitation data as binary (i.e. each visit was to yellow or blue) and conducted a repeated-measures logistic regression on the number of visits made to flowers of a given colour, with ‘frequency’ and ‘novelty’ as continuous and categorical predictors, respectively, and individual bees as subjects. We tested visits to each colour separately (using the data from the same six bees from experiment 1 in both analyses). Because these experiments were conducted at different times, the novelty treatment is, strictly speaking, pseudo-replicated. Nevertheless, we treat individual bees as the meaningful units of replication, and argue that it is implausible that our results could be due to any conceivable confounding factors. Analyses were conducted using PROC GENMOD in SAS release v. 8.02 with a logit link function, and significance was tested using generalized estimating equations, which provide a way to deal with correlated measurements in generalized linear models (Liang & Zeger 1986). When we obtained a significant frequency×novelty interaction, we tested for differences in the proportion of visits made to novel or familiar flowers at each frequency separately, using a sign test on the number of replicates that lay above the median value for that treatment.
(b) Simulation model
Our model considers the fate of a finite, outcrossing, annual plant population, in which flowering times are genetically determined, experiencing a shift in environmental conditions that alters the ‘optimal’ flowering date. Plants flowering on dates far from the optimum are selected against. Each plant produces one flower that lasts for one time unit (‘day’) and can only be pollinated by other plants flowering in that time unit (i.e. there is strict assortative mating by flowering date). Pollinators are generalists; their population is not modelled explicitly and is assumed to be of constant size throughout each simulation run (i.e. their abundance is independent of the abundance of the focal plant). Because the trait of interest is flowering day, we are interested in the total number of plants setting seed on a given day, rather than in the specific identity of these plants; we therefore treat seed set as a cohort-level, not an individual-level, phenomenon.
For any individual plant flowering on day t, the probability of producing seed is determined by the probability that the plant receives a pollinator visit and the probability that the pollinator has visited a conspecific at some time in the past. Visitation probabilities can be modified by frequency dependence and a lag in pollinator response to floral abundance, such that visitation on day t is a sigmoid function of the focal plant's abundance at time t−m. Thus, the probability of pollen transfer for each plant on day t is
| (2.2) |
where β (‘pollinator abundance’) is a parameter that modifies the total number of pollinator visits; N is the total number of flowering plants of all species in the community on any given day; At is the abundance of the focal plant species on day t; m is the magnitude of the lag in visitation; V and b are parameters describing the strength of preference for the focal species and the strength of frequency dependence, respectively (as above); and c is a measure of pollinator constancy (table 1). Probability of pollen transfer has a maximum value of 1 (although larger values are mathematically possible for large values of β). Here, constancy is defined simply as the probability that a pollinator is carrying conspecific pollen, and reflects the tendency of pollinators to visit sequences of conspecific flowers, independent of preference or availability (Waser 1986); we treat this as a separate phenomenon from the lag effect. In the simplest scenario of a single pollinator making two flower visits within the simulated community, c determines the likelihood that this set of flower visits results in successful pollen transfer, given that the first of the two visits is to the focal species. When c=1 (perfect constancy), P(PT)t is simply the probability of receiving a single visit; when c is 0, visits are independent and P(PT)t becomes the product of the probabilities of two visits (see also Sargent & Otto 2006 for a similar treatment of constancy). We have not considered details of pollinator visit sequence, number of visits per pollinator or pollen carryover; however, varying β and c serves the same function, by controlling the total number of successful pollen transfer events at the level of the whole cohort, given the visitation probability determined by frequency dependence and the time lag.
Table 1.
Model parameters and variables.
| symbol | range of values | description |
|---|---|---|
| At | ≥0 | abundance of focal species at time t |
| b | >0 | frequency dependence exponent |
| b<1: negative frequency dependence | ||
| b>1: positive frequency dependence | ||
| β | ≥0 | pollinator abundance |
| c | 0≤c≤1 | pollinator constancy: probability that a pollinator carries conspecific pollen |
| h2 | 0≤h2≤1 | heritability of flowering time |
| K | 100 | maximum number of individuals of focal species flowering on any day (carrying capacity) |
| m | ≥0 | time lag |
| N | 120 | total number of flowering individuals of all species on any day |
| p | >0 | determines the number of seeds produced per successful pollen transfer before selection |
| s2 | >0 | variance of normal distribution defining abiotic selection |
| σ | >0 | standard deviation in offspring phenotype |
| t | 1≤t≤30 | flowering day |
| to | 15 | ‘optimal’ flowering day favoured by abiotic selection |
| V | ≥0 | pollinator preference: |
| V<1: preference for other species | ||
| V>1: preference for focal species |
For each pollen transfer event occurring on a given day, the number of surviving seeds produced by the recipient plant is described by
| (2.3) |
where the first part of the expression describes density dependence, with ep being the number of seeds produced when the number of plants flowering on day t is far from the carrying capacity K, for each day (as in the Ricker map; Mangel 2006). The use of a daily K rather than a carrying capacity for the whole population is necessary to prevent population oscillations; note that within-day density dependence tends to increase seed set for plants at the tails of the flowering distribution. This might be reasonable if, for instance, competition for the resources needed for seed maturation were most intense for plants flowering during the population peak. The second part of equation (2.3) determines the proportion of seeds surviving as a Gaussian function with mean to and variance s2, such that survival decreases as the flowering date of the parent plant becomes more distant from the optimal date to. Survival is modelled as a deterministic process, while pollen transfer is probabilistic.
The phenotype (flowering date) of the surviving offspring of all plants that flowered on day t is determined by the trait's heritability h2, which dictates mean offspring phenotype, according to (Roff 1997)
| (2.4) |
Paternal and maternal trait values are identical in this model, because of the simplifying assumption of completely assortative mating. Variation around the mean is controlled by a second parameter σ. Thus, each individual offspring produced for plants flowering on a given date is assigned a flowering date probabilistically, according to a normal distribution around the mean offspring phenotype with standard deviation σ.
For simulation runs, the plant population started with a normal distribution of flowering dates with mean=day 20, s.d.=√10 and carrying capacity=K=100 plants; N, the total number of flowering plants in the community on any day, was set at 120. We selected starting parameter values such that the population remained stable in the absence of directional selection. We then set the optimal flowering date to =day 15, and ran the algorithm for 10 generations, by which point the population's size and flowering distribution had stabilized. We systematically varied the pollinator visitation parameters β (abundance), b (frequency dependence), m (time lag), V (preference) and c (constancy), as well as the inheritance parameters h2 and σ. The response variables of interest were final population size and peak flowering date of the population. For each parameter combination, we ran the model 100 times to generate confidence intervals. We also ran simulations using different model assumptions; in particular, we replaced the Gaussian abiotic selection function by a step function to simulate truncation selection, and we modified the density-dependent function so that density dependence operated over the whole population, rather than within flowering days. Because these modifications did not alter any of our conclusions, the results we report are for the original model. The simulations were implemented in Mathematica v. 5.0.
3. Results
(a) Foraging experiments
(i) Experiment 1 (bees familiar with both flower colours)
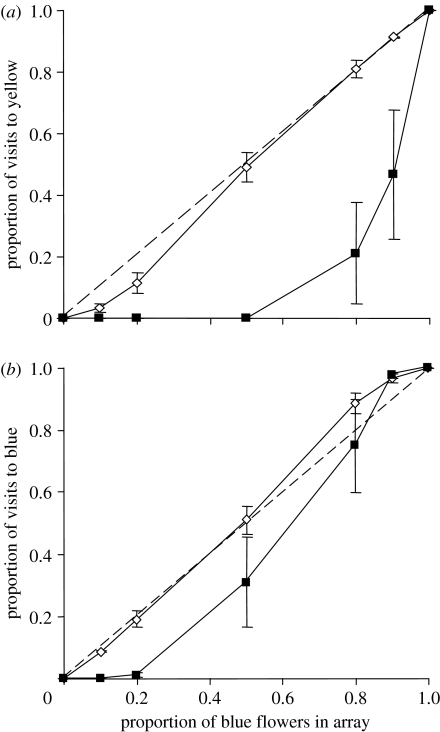
The proportion of visits to yellow flowers as a function of their relative abundance in the array is shown in figure 2a (open diamonds). The fitted value of b is 1.23±0.09 (mean±s.e.), indicating positive frequency dependence. The fitted value of V is 0.89±0.07, indicating a weak (non-significant) preference for blue.
Figure 2.
Proportion of visits received by (a) yellow and (b) blue flowers, according to the frequency with which that colour occurred within the array. Open diamonds represent means for bees with prior experience of both flower colours (familiar), and filled squares represent means for bees that were unfamiliar with that colour (n=6 bees for each treatment, but note that the ‘familiar’ data points in both plots represent the same six bees). The dashed line is the 1 : 1 line. Error bars are ±1 s.e.
(ii) Experiment 2 (bees familiar with only one flower colour)
Flowers of a given colour received a lower proportion of visits when that colour was unfamiliar compared with when bees had prior experience of both colours (table 2, figure 2). The cost of novelty was most apparent when a colour was also rare (frequency×novelty interactions, p<0.05; table 2): sign tests detected significantly lower visitation to novel than familiar yellow flowers only when these made up 50 per cent or less of the array, and to novel blue flowers when these made up 20 per cent or less of the array (sign tests, p=0.031). This difference between yellow and blue was a result of greater variation among individual yellow-trained bees when they switched to the novel colour. When bees had been trained on blue, yellow flowers received no visits until they made up 80 per cent or more of the array, whereas certain bees visited unfamiliar blue flowers when these were only 20–50 per cent of the array. Overall, bees were more reluctant to visit novel yellow than novel blue flowers: the mean frequency at which 50 per cent or above of visits were to the novel colour was 93 per cent for yellow and 72 per cent for blue (Kruskal–Wallis test, Χ12=5.77, p=0.016; figure 2).
Table 2.
Results of repeated-measures logistic regressions testing effects of flower colour frequency and novelty on the number of bee visits to each colour (frequency treated as continuous).
| explanatory variable | |Z| | d.f. | p-value |
|---|---|---|---|
| visits to yellow | |||
| frequency | 3.13 | 1 | 0.002 |
| novelty | 2.56 | 1 | 0.011 |
| novelty×frequency | 2.23 | 1 | 0.026 |
| visits to blue | |||
| frequency | 6.89 | 1 | <0.0001 |
| novelty | 2.97 | 1 | 0.003 |
| novelty×frequency | 2.38 | 1 | 0.017 |
There was striking behavioural variation among individual bees (see figure A2 in the electronic supplementary material): before switching to the unfamiliar flowers, some bees would repeatedly revisit drained flowers of their preferred colour, making as many as 38 consecutive unrewarded visits, and occasionally leaving the array to fly around the perimeter of the cage. One bee refused to visit unfamiliar yellow flowers until they made up 100 per cent of the array, and two additional bees still refused yellow flowers when given no choice (these returned to the colony and abandoned foraging). Other bees visited unfamiliar flowers without having encountered a series of drained, familiar flowers. Once bees had begun to visit the novel flower type, they quickly learned that these flowers were rewarding; some bees even developed a preference for the novel type, which was more reliably rewarding than the depleted familiar type (see figure A2 in the electronic supplementary material).
(b) Simulation model
For a population under selection for earlier flowering, increasing the frequency dependences parameter, b, slightly reduces the population response to selection and causes a decrease in population size (figure 3a). This effect is small at the level of frequency dependence observed in our experiment (b≅1.2), but is more apparent for b=1.4–1.8, values obtained by Smithson & Macnair (1996, 1997).
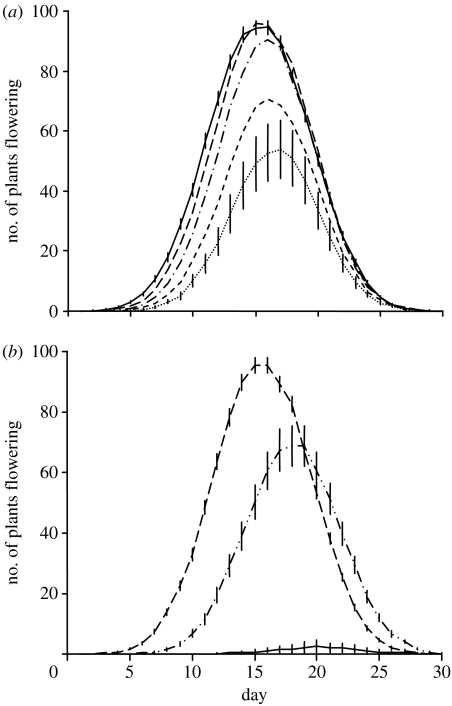
Figure 3.
Effects of varying (a) frequency dependence, b, and (b) the lag parameter, m, on the population's flowering distribution after 10 generations of selection. The starting mean of flowering day is 20; the new ‘optimal’ flowering day, to, is 15. Pollinator visitation probabilities for m=1/2 (dot-dashed curve) were obtained by averaging those for m=1 (solid curve) and m=0 (dashed curve). Parameter values are s2=30, h2=0.7, σ=3, p=5, c=0, β=1 and V=1. For (a) m=0 and (b) b=1.2. Error bars are 2 s.e., and are shown in (a) only for b=1.0 (solid curve) and b=1.8 (dotted curve) for clarity. (a) Dot-dashed curve, b=1.4; short dashed curve, b=1.6; long dashed curve, b=1.2.
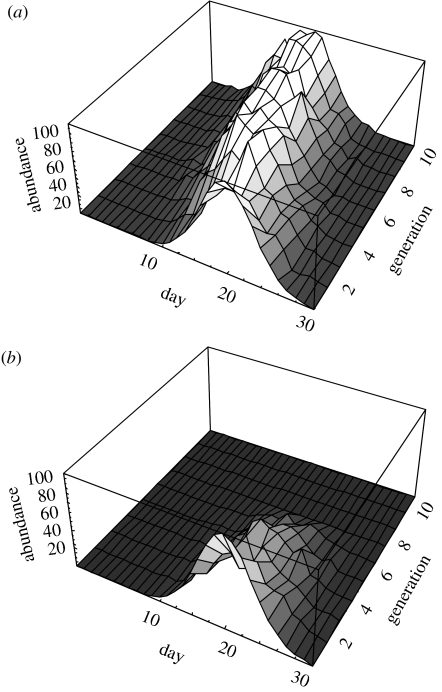
Introducing a 1-day lag in pollinator visitation causes a dramatic decline in the population response to selection and leads to reductions in population size and frequent extinction (figures 3b and 4). This is true even without frequency dependence (b=1.0), although the reduction in population size is less severe when rare plants are not under-visited. A partial lag, simulated using the mean of visitation probabilities obtained with (m=1) and without a lag (m=0), produces an intermediate reduction in population abundance (figure 3b).
Figure 4.
Examples of evolutionary trajectories for the population flowering curve over 10 generations of selection for earlier flowering at different levels of the lag parameter, m. (a) m=0 and (b) m=1. Other parameters are as in figure 3, and b=1.2.
These qualitative conclusions are unchanged by varying the inheritance parameters h2 or σ; populations are consistently significantly reduced by inclusion of a 1-day lag because of a failure to respond to selection. For almost all parameter combinations, the effect of a 1-day lag is substantially greater than the effect of a low level of frequency dependence (b=1.2), which causes no detectable decline in the response to selection. Truncation selection produces identical results. Predictably, increasing pollinator abundance (β), constancy (c) or preference for the focal species (V), or introducing autogamous seed production (seed set independent of pollinator visitation), increases seed set for all plants and consequently increases plant population size, but does not affect the population's ability to evolve earlier flowering.
4. Discussion
When our B. impatiens workers had prior experience with both flower colours, they showed positive frequency-dependent foraging behaviour, preferentially visiting the more common flower colour. These results qualitatively match those previously obtained with B. terrestris (Smithson & Macnair 1996). However, when we incorporated a more realistic sequence of foraging environments that simulated temporal change in a simple flowering plant community, we observed a marked difference in behaviour: bees were less likely to visit a flower type that they had not previously learned to associate with a reward, despite the similarity of the flower types in every respect except colour, and despite the repeated discouragement of encountering familiar flowers that had been emptied of nectar. A similar lag in the use of a food source has been observed in a laboratory study with wild-caught bumble-bees (Bombus ternarius and Bombus terricola). Bees that had been trained to recognize one colour of artificial flower as rewarding and another as unrewarding were slow to switch allegiances when the identity of the rewarding type was reversed (Heinrich et al. 1977). For an animal foraging in a changing and unpredictable environment, there should be an ‘optimal forgetting rule’ that allows it to adjust its foraging decisions on the basis of more recently acquired—but imperfect—information (Mangel 1990). The forgetting rate of our bees was apparently too slow to allow precise tracking of changes in resource availability.
It is unclear to what extent these results represent pollinator behaviour in a more natural setting. However, there is some evidence of conservatism in the foraging behaviour of wild bees: Heinrich (1976) conducted flower removal and addition experiments in the field and observed strong fidelity of worker bumble-bees to the species on which they had originally been foraging, as well as apparent avoidance of unfamiliar species. Kawaguchi et al. (2007) also found that bumble-bees (Bombus diversus) rejected flower bouquets of an unfamiliar species more often than bouquets of familiar flowers. In our experiment, when bees encountered a series of unrewarding flowers, they often left the array and flew around the perimeter of the flight cage before visiting a novel flower. This behaviour suggests that, given a larger foraging area, some bees would be more likely to search for a new patch of familiar flowers than to visit a new species when their preferred flowers become locally depleted. This suggestion is supported by Heinrich's (1979) observation that bumble-bees confined to an outdoor flight cage eventually sampled flowers of species newly introduced to the cage, but that bees released from the cage tended to maintain their original flower preferences by foraging more widely. Although our artificial flowers were undoubtedly less attractive than real ones, their morphology was simple; complex flower types with concealed nectar or pollen rewards might be more likely to experience a lag in visitation because of the time needed for bees to learn new flower-handling techniques (Laverty 1980).
Clearly, for bumble-bee colonies to survive, they must be capable of using a series of different plant species throughout a growing season. However, the colony may track changing resource availability even if behaviour of individual bees shows a lag, as long as newly emerging workers take advantage of new food sources. Our results suggest, though, that as long as a familiar species remains in bloom, new species will probably be under-visited by experienced workers. Furthermore, the available literature suggests that bumble-bees and other social bees often copy the flower choices made by other bees, particularly when faced with unfamiliar flowers (Slaa et al. 2003; Leadbeater & Chittka 2005; Worden & Papaj 2005; Kawaguchi et al. 2006, 2007).
Insects other than worker bumble-bees may be less reluctant to explore and exploit new floral resources. The foraging behaviour of non-apid pollinators is relatively unstudied, and we have little information about lags in resource use by flies or solitary bees (though see Thomson 1981). However, butterflies will preferentially visit flower species with which they have prior experience (Lewis 1986), and many solitary bee species are pollen specialists, though they may visit multiple species for nectar (Michener 2000). Flower constancy has also been noted for several taxa (Goulson 2000)—though constancy and the neophobia we have documented here are separate phenomena. Honeybees display marked flower colour constancy, and, given a choice, tend not to sample new colours of artificial flowers even when the new flowers are more rewarding (Hill et al. 1997). By comparison, bumble-bees are behaviourally flexible and intelligent foragers (Chittka et al. 2001; Gegear & Laverty 2004), making our results somewhat surprising. In fact, bumble-bees have been considered models of optimal foraging behaviour, obeying seemingly rational decision rules that maximize the rate of individual energy intake (e.g. Pyke 1978; Zimmerman 1981). Our results suggest that the behaviour of individual workers can deviate substantially—if temporarily—from ‘optimality’, and models that fail to consider lasting effects of prior experience are oversimplified. Further experiments will be necessary to document the severity and generality of pollinator neophobia. Flight cage studies in which additional floral traits (e.g. nectar volumes, odour) are manipulated would help determine factors that could increase or decrease a bee's willingness to sample new flowers. Field studies documenting visitation by wild pollinators to arrays of familiar and novel (e.g. non-native) flowers would be particularly informative.
Our simulations show that the pollinator avoidance of early flowering plants within a population can impose a significant constraint on evolution of earlier flowering phenology. In the context of the evolution of aposematism and Batesian mimicry, neophobia and learning are recognized to be important (Coppinger 1969; Servedio 2000; Puurtinen & Kaitala 2006), because predator avoidance of unfamiliar food items facilitates the spread of conspicuous warning coloration in prey populations. The possibility that an analogous phenomenon (but with opposite evolutionary consequences) might play a role in floral evolution appears not to have attracted previous theoretical or empirical investigation—perhaps in part because existing interspecific variation shows that evolutionary adjustments in flowering time have occurred in the past. Our results underscore the need to consider interactions with mutualists when attempting to predict adaptive responses to environmental change.
Our conclusion that pollinator lags in conjunction with selection against late flowering can have negative impacts on plant populations is robust to most changes in model parameters, although numerous factors (e.g. pollinator abundance and constancy) can mitigate the effect on population size. In particular, if the newly flowering species were more rewarding to pollinators than the species already in bloom, the additional pollinator visits received during the rest of its flowering season could make up for those lost by the earliest individuals. Alternatively, if the second species resembled the first, the magnitude of the lag might be lessened (cf. Gumbert 2000). Because colour generalization does not preclude constancy (Chittka & Wells 2004), the benefit of additional pollinator visits would not necessarily be negated by the cost of interspecific pollen transfer (but see Kunin 1993).
Our simulations assume a brief flowering duration, so that even a pollinator lag that is short relative to the population flowering period has dramatic consequences for the earliest individual plants. In reality, even if flower longevity is often brief (Primack 1985; Stratton 1989), lifetime reproductive opportunities of the whole plant are unlikely to be restricted to a single short-lived flower. In addition, the lag effect we have simulated is time dependent rather than experience dependent, i.e. the lag is no less severe if the plant population has a positively skewed flowering distribution. Such a distribution—or a greater investment in floral rewards early in a plant's flowering period—might tend to attract pollinators to the new flowers and reduce the delay in resource tracking (Thomson 1985; Kato & Sakai 2008). Nevertheless, our simulation results demonstrate the possibility for a severe reduction in a population's capacity for adaptation—and a consequent decline in population viability—with even a partial reduction in the probability of visitation for the earliest flowering individuals. This suggests that plants with brief flowering duration, growing in small populations, and experiencing rapid environmental change, may be susceptible to pollen limitation of the evolution of flowering time.
Acknowledgments
We thank Rob Gegear for helpful discussions and advice on experimental design. The manuscript was improved by thoughtful comments from Peter Abrams, Heather Coiner, Rob Gegear, Art Weis and our reviewers. We are also grateful to Gaurav Bhattacharya and Hsin-Yi Lin for help with laboratory work and data entry, to Mike Otterstatter for SAS code and to Biobest for bees. The Natural Sciences and Engineering Research Council of Canada and the Fonds québécois de la recherche sur la nature et les technologies provided funding.
Supplementary Material
Figure A1 shows reflectance spectra for the flowers and array background used in experiments. Figure A2 shows the proportion of vists to novel flowers as a function of frequency for individual bees in Experiments 2
References
- Both C., Bouwhuis S., Lessells C.M., Visser M.E. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441:81–83. doi: 10.1038/nature04539. doi:10.1038/nature04539 [DOI] [PubMed] [Google Scholar]
- Brigham A.J., Sibly R.M. A review of the phenomenon of neophobia. In: Cowan P.D., Feare C.J., editors. Advances in vertebrate pest management. Filander Verlag; Fürth, Germany: 1999. pp. 67–84. [Google Scholar]
- Chittka L., Wells H. Color vision in bees: mechanisms, ecology, and evolution. In: Prete F.R., editor. Complex worlds from simpler nervous systems. MIT Press; Cambridge, MA: 2004. pp. 165–191. [Google Scholar]
- Chittka L., Spaethe J., Schmidt A., Hickelsberger A. Adapatation, constraint, and chance in the evolution of flower color and pollinator color vision. In: Chittka L., Thomson J.D., editors. Cognitive ecology of pollination: animal behavior and floral evolution. Cambridge University Press; Cambridge, UK: 2001. pp. 106–126. [Google Scholar]
- Coppinger R.P. The effect of experience and novelty on avian feeding behavior with reference to the evolution of warning coloration in butterflies. Part I: Reactions of wild-caught adult blue jays to novel insects. Behaviour. 1969;35:45–60. doi:10.1163/156853970X00114 [Google Scholar]
- Coppinger R.P. The effect of experience and novelty on avian feeding behavior with reference to the evolution of warning coloration in butterflies. II. Reactions of naive birds to novel insects. Am. Nat. 1970;104:323–335. doi:10.1086/282666 [Google Scholar]
- Davis A.J., Jenkinson L.S., Lawton J.H., Shorrocks B., Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. doi:10.1038/35842 [DOI] [PubMed] [Google Scholar]
- Franks S.J., Sim S., Weis A.E. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl Acad. Sci. USA. 2007;104:1278–1282. doi: 10.1073/pnas.0608379104. doi:10.1073/pnas.0608379104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geber M.A., Griffen L.R. Inheritance and natural selection on functional traits. Int. J. Plant Sci. 2003;164:S21–S42. doi:10.1086/368233 [Google Scholar]
- Gegear R.J., Laverty T.M. Effect of a colour dimorphism on the flower constancy of honey bees and bumble bees. Can. J. Zool. 2004;82:587–593. doi:10.1139/z04-029 [Google Scholar]
- Goulson D. Are insects flower constant because they use search images to find flowers? Oikos. 2000;88:547–552. doi:10.1034/j.1600-0706.2000.880311.x [Google Scholar]
- Gumbert A. Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 2000;48:36–43. doi:10.1007/s002650000213 [Google Scholar]
- Heinrich B. The foraging specializations of individual bumblebees. Ecol. Monogr. 1976;46:105–128. doi:10.2307/1942246 [Google Scholar]
- Heinrich B. ‘Majoring’ and ‘minoring’ by foraging bumblebees, Bombus vagans: an experimental analysis. Ecology. 1979;60:245–255. doi:10.2307/1937652 [Google Scholar]
- Heinrich B., Mudge P.R., Deringis P.G. Laboratory analysis of flower constancy in foraging bumblebees: Bombus ternarius and B. terricola. Behav. Ecol. Sociobiol. 1977;2:247–265. doi:10.1007/BF00299738 [Google Scholar]
- Hersch E.I., Roy B.A. Context-dependent pollinator behavior: an explanation for patterns of hybridization among three species of Indian paintbrush. Evolution. 2007;61:111–124. doi: 10.1111/j.1558-5646.2007.00009.x. doi:10.1111/j.1558-5646.2007.00009.x [DOI] [PubMed] [Google Scholar]
- Hill P.S.M., Wells P.H., Wells H. Spontaneous flower constancy and learning in honey bees as a function of colour. Anim. Behav. 1997;54:615–627. doi: 10.1006/anbe.1996.0467. doi:10.1006/anbe.1996.0467 [DOI] [PubMed] [Google Scholar]
- IPCC. Cambridge University Press; Cambridge, UK: 2007. Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the IPCC. [Google Scholar]
- Ives A.R. Predicting the response of populations to environmental change. Ecology. 1995;76:926–941. doi:10.2307/1939357 [Google Scholar]
- Jiang L., Kulczycki A. Competition, predation and species responses to environmental change. Oikos. 2004;106:217–224. doi:10.1111/j.0030-1299.2004.13056.x [Google Scholar]
- Johansson J. Evolutionary responses to environmental changes: how does competition affect adaptation? Evolution. 2008;62:421–435. doi: 10.1111/j.1558-5646.2007.00301.x. doi:10.1111/j.1558-5646.2007.00301.x [DOI] [PubMed] [Google Scholar]
- Kato S., Sakai S. Nectar secretion strategy in three Japanese species: changes in nectar volume and sugar concentration dependent on flower age and flowering order. Botany. 2008;86:337–345. doi:10.1139/B07-131 [Google Scholar]
- Kawaguchi L.G., Ohashi K., Toquenaga Y. Do bumble bees save time when choosing novel flowers by following conspecifics? Funct. Ecol. 2006;20:239–244. doi:10.1111/j.1365-2435.2006.01086.x [Google Scholar]
- Kawaguchi L.G., Ohashi K., Toquenaga Y. Contrasting responses of bumble bees to feeding conspecifics on their familiar and unfamiliar flowers. Proc. R. Soc. B. 2007;274:2661–2667. doi: 10.1098/rspb.2007.0860. doi:10.1098/rspb.2007.0860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo G. Flowering phenologies of animal-pollinated plants: reproductive strategies and agents of selection. In: Harder L.D., Barrett S.C.H., editors. Ecology and evolution of flowers. Oxford University Press; New York, NY: 2006. pp. 139–158. [Google Scholar]
- Kunin W.E. Sex and the single mustard: population density and pollinator behavior effects on seed-set. Ecology. 1993;74:2145–2160. doi:10.2307/1940859 [Google Scholar]
- Laverty T.M. The flower-visiting behaviour of bumble bees: floral complexity and learning. Can. J. Zool. 1980;58:1324–1335. [Google Scholar]
- Leadbeater E., Chittka L. A new mode of information transfer in foraging bumblebees? Curr. Biol. 2005;15:R447–R448. doi: 10.1016/j.cub.2005.06.011. doi:10.1016/j.cub.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Lewis A.C. Memory constraints and flower choice in Pieris rapae. Science. 1986;232:863–865. doi: 10.1126/science.232.4752.863. doi:10.1126/science.232.4752.863 [DOI] [PubMed] [Google Scholar]
- Liang K.-L., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi:10.1093/biomet/73.1.13 [Google Scholar]
- Mangel M. Dynamic information in uncertain and changing worlds. J. Theor. Biol. 1990;146:317–332. doi: 10.1016/s0022-5193(05)80742-8. doi:10.1016/S0022-5193(05)80742-8 [DOI] [PubMed] [Google Scholar]
- Mangel M. Cambridge University Press; Cambridge, UK: 2006. The theoretical biologist's toolbox: quantitative methods for ecology and evolutionary biology. [Google Scholar]
- Mazer S.J., LeBuhn G. Genetic variation in life-history traits: heritability estimates within and genetic differentiation among populations. In: Vuorisalo T.O., Mutikainen P.K., editors. Life history evolution in plants. Kluwer Academic; Dordrecht, The Netherlands: 1999. pp. 85–171. [Google Scholar]
- Michener C.D. Johns Hopkins University Press; Baltimore, MD: 2000. Bees of the world. [Google Scholar]
- Possingham H.P. Impact of elevated atmospheric CO2 on biodiversity: mechanistic population-dynamic perspective. Aust. J. Bot. 1993;41:11–21. doi:10.1071/BT9930011 [Google Scholar]
- Primack R.B. Longevity of individual flowers. Annu. Rev. Ecol. Syst. 1985;16:15–37. doi:10.1146/annurev.es.16.110185.000311 [Google Scholar]
- Punzalan D., Rodd F.H., Hughes K.A. Perceptual processes and the maintenance of polymorphism through frequency-dependent predation. Evol. Ecol. 2005;19:303–320. doi:10.1007/s10682-005-2777-z [Google Scholar]
- Puurtinen M., Kaitala V. Conditions for the spread of conspicuous warning signals: a numerical model with novel insights. Evolution. 2006;60:2246–2256. doi:10.1554/06-227.1 [PubMed] [Google Scholar]
- Pyke G.H. Optimal foraging: movement patterns of bumblebees between inflorescences. Theor. Popul. Biol. 1978;13:72–98. doi: 10.1016/0040-5809(78)90036-9. doi:10.1016/0040-5809(78)90036-9 [DOI] [PubMed] [Google Scholar]
- Rathcke B., Lacey E.P. Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Syst. 1985;16:179–214. doi:10.1146/annurev.es.16.110185.001143 [Google Scholar]
- Réale D., McAdam A.G., Boutin S., Berteaux D. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. B. 2003;270:591–596. doi: 10.1098/rspb.2002.2224. doi:10.1098/rspb.2002.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff D.A.Evolutionary Quantitative Genetics1997Chapman and Hall; New York, NY, USA [Google Scholar]
- Sargent R.D., Otto S.P. The role of local species abundance in the evolution of pollinator attraction in flowering plants. American Naturalist. 2006;167:67–80. doi: 10.1086/498433. doi:10.1086/498433 [DOI] [PubMed] [Google Scholar]
- Seager R., et al. Model projections of an imminent transition to a more arid climate in southwestern North America. Science. 2007;316:1181–1184. doi: 10.1126/science.1139601. doi:10.1126/science.1139601 [DOI] [PubMed] [Google Scholar]
- Servedio M.R. The effects of predator learning, forgetting, and recognition errors on the evolution of warning coloration. Evolution. 2000;54:751–763. doi: 10.1111/j.0014-3820.2000.tb00077.x. doi:10.1554/0014-3820(2000)054[0751:TEOPLF]2.3.CO;2 [DOI] [PubMed] [Google Scholar]
- Slaa E.J., Wassenberg J., Biesmeijer J.C. The use of field-based social information in eusocial foragers: local enhancement among nestmates and heterospecifics in stingless bees. Ecol. Entomol. 2003;28:369–379. doi:10.1046/j.1365-2311.2003.00512.x [Google Scholar]
- Smithson A. Pollinator preference, frequency dependence, and floral evolution. In: Chittka L., Thomson J.D., editors. Cognitive ecology of pollination: animal behavior and floral evolution. Cambridge University Press; Cambridge, UK: 2001. pp. 237–258. [Google Scholar]
- Smithson A., Macnair M.R. Frequency-dependent selection by pollinators: mechanisms and consequences with regard to behaviour of bumblebees Bombus terrestris (L.) (Hymenoptera: Apidae) J. Evol. Biol. 1996;9:571–588. doi:10.1046/j.1420-9101.1996.9050571.x [Google Scholar]
- Smithson A., Macnair M.R. Density-dependent and frequency-dependent selection by bumblebees Bombus terrestris (L.) (Hymenoptera: Apidae) Biol. J. Linn. Soc. 1997;60:401–417. doi:10.1111/j.1095-8312.1997.tb01503.x [Google Scholar]
- Stinson K.A. Natural selection favors rapid reproductive phenology in Potentilla pulcherrima (Rosaceae) at opposite ends of a subalpine snowmelt gradient. Am. J. Bot. 2004;91:531–539. doi: 10.3732/ajb.91.4.531. doi:10.3732/ajb.91.4.531 [DOI] [PubMed] [Google Scholar]
- Stratton D.A. Longevity of individual flowers in a Costa Rican cloud forest: ecological correlates and phylogenetic constraints. Biotropica. 1989;21:308–318. doi:10.2307/2388281 [Google Scholar]
- Thomson J.D. Spatial and temporal components of resource assessment by flower-feeding insects. J. Anim. Ecol. 1981;50:49–59. doi:10.2307/4030 [Google Scholar]
- Thomson J.D. Patterns of visitation by animal pollinators. Oikos. 1982;39:241–250. doi:10.2307/3544491 [Google Scholar]
- Thomson J.D. Pollination and seed set in Diervilla lonicera (Caprifoliaceae): temporal patterns of flower and ovule deployment. Am. J. Bot. 1985;72:737–740. doi:10.2307/2443688 [Google Scholar]
- Waser N.M. Flower constancy: definition, cause, and measurement. American Naturalist. 1986;127:593–603. doi:10.1086/284507 [Google Scholar]
- Worden B.D., Papaj D.R. Flower choice copying in bumblebees. Biol. Lett. 2005;1:504–507. doi: 10.1098/rsbl.2005.0368. doi:10.1098/rsbl.2005.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M. Optimal foraging, plant density and the marginal value theorem. Oecologia. 1981;49:148–153. doi: 10.1007/BF00349181. doi:10.1007/BF00349181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A1 shows reflectance spectra for the flowers and array background used in experiments. Figure A2 shows the proportion of vists to novel flowers as a function of frequency for individual bees in Experiments 2