Abstract
Individual diversity at the major histocompatibility complex (MHC) is predicted to be optimal at intermediate rather than at maximal levels. We showed previously in sticklebacks that an intermediate MHC diversity is predominant in natural populations and provides maximal resistance in experimental multiple parasite infections in the laboratory. However, what counts ultimately is the lifetime reproductive success (LRS). Here, we measured LRS of six laboratory-bred sib-groups—to minimize the influence of non-MHC genes—three-spined sticklebacks (Gasterosteus aculeatus) during their entire breeding period, each in a seminatural enclosure in the lake of their parents, where they were exposed to the natural spectrum of parasites. We collected developing clutches at regular intervals and determined parenthood for a representative number of eggs (2279 in total) per clutch with 18 microsatellites. Both males and females with an intermediate MHC class IIB variant number had the highest LRS. The mechanistic link of MHC diversity and LRS differed between the sexes: in females, we found evidence for a trade-off between number of eggs and immunocompentence, whereas in males this correlation was concealed by different timing strategies of reproduction.
Keywords: lifetime reproductive success, major histocompatibility complex, stickleback, parasites, mating decision
1. Introduction
Genes of the major histocompatibility complex (MHC) are the most polymorphic genes and play a fundamental role in the adaptive immunity of all jawed vertebrates. They encode cell-surface proteins, which present either self-peptides or peptides derived from phagocytosed pathogens to T lymphocytes, a prerequisite for production of pathogen-specific antibodies and the development of an immunological memory (Janeway et al. 2005). The enormous allelic diversity found in natural populations is generally regarded as a consequence of parasite-mediated balancing selection (Clarke & Kirby 1966; Takahata & Nei 1990; Apanius et al. 1997; Edwards & Hedrick 1998; Jordan & Bruford 1998; Penn & Potts 1999; Penn et al. 2002; Bernatchez & Landry 2003; Mays & Hill 2004; Milinski 2006). Several reports have shown correlations between certain MHC genotypes and occurrence or severity of specific diseases and parasite infections (Briles et al. 1983; Hill et al. 1991; Godot et al. 2000; Langefors et al. 2001; Grimholt et al. 2003; Bonneaud et al. 2005, 2006; Harf & Sommer 2005). This implies that individual MHC diversity should be maximized in order to achieve resistance against as many different pathogens as possible. However, although some studies show that MHC heterozygotes are more resistant than homozygotes (Doherty & Zinkernagel 1975; McClelland et al. 2003), the overall evidence remains ambiguous especially for species harbouring recently duplicated MHC loci (Penn 2002; Penn et al. 2002; Pitcher & Neff 2006). A recent study showed that mice that were heterozygous at all MHC loci were even less resistant than mice that were homozygous at all loci when challenged with different strains of salmonella (Ilmonen et al. 2007). Therefore, it seems to be disadvantageous to have too many different MHC alleles. Indeed, each time a distinct MHC molecule is added to the individual MHC repertoire, all T-cell clones that can recognize self-peptides bound to that molecule must be removed in order to maintain self-tolerance. This process of negative T-cell selection can prevent an efficient adaptive immune response if an individual has too many different MHC alleles (Lawlor et al. 1990). Thus, theoretical models predict that maximal pathogen resistance is achieved by an intermediate, i.e. optimal, rather than a maximal number of different MHC variants (Nowak et al. 1992; De Boer & Perelson 1993; Borghans et al. 2003; Milinski 2006; Woelfing et al. 2009). The most recent mathematical model (Woelfing et al. 2009), which is based on novel findings on T-cell selection, can predict the natural range of intra-individual MHC diversity. This prediction is in line with empirical studies, showing the lowest effect of parasite infections, a fitness trait, in fish (Wegner et al. 2003a,b, 2008), and in reptiles (Madsen & Ujvari 2006) with intermediate MHC diversity. Such a beneficial amount of individual MHC diversity may not only be maintained by parasite-mediated selection, but also be amplified by MHC-based mate choice. For instance, female sticklebacks prefer males with a complementary number of MHC class II variants to their own set of variants, resulting on average in intermediate immunogenetic diversity in their offspring (Reusch et al. 2001; Aeschlimann et al. 2003; Milinski et al. 2005). Recently, Forsberg et al. (2007) found an evidence for a similar mating preference of female brown trout (Salmo trutta) for males with intermediate MHC dissimilarity. Thus, fitness should ultimately, i.e. during an individual's lifetime, be maximized when MHC allele diversity is intermediate.
In this study, we tested the effects of individual MHC class II diversity on lifetime fitness by measuring the lifetime reproductive success (LRS) of three-spined sticklebacks (Gasterosteus aculeatus) under natural conditions. Sticklebacks offer an exceptional system because, in populations of our geographical latitude, they usually have only one reproductive period in their life (Wootton 1976). Moreover, life-history traits involved in reproduction have been studied extensively, such as breeding coloration that has been shown to be a good predictor of parasitation and body condition (Milinski & Bakker 1990; Frischknecht 1993; Folstad et al. 1994; Kraak et al. 1999; Barber et al. 2000). In a field enclosure system, we analysed mating combinations and individual reproductive output of laboratory-bred sticklebacks. Within each of six enclosures, fish (eight males and eight females) were full siblings, to reduce the influence of non-MHC genes (genetic background), and fish differed almost only with respect to their MHC genotype. Experimental fish had been individually challenged twice with three common sympatric macroparasites from the lake of their parents to simulate a natural life history comparable to their free-living conspecifics. In the enclosures, we collected all developing clutches of eggs until the end of the reproductive season and allocated the most likely parents to each clutch. Therefore, using genetic methods we could estimate the LRS of each individual fish. Immunogenetic optimality should ultimately contribute to LRS. Therefore, we expect that the fish with an intermediate MHC class IIB diversity have the highest reproductive success. This would explain why individuals with an intermediate MHC diversity predominate natural populations (Reusch et al. 2001).
2. Material and methods
(a) Experimental fish
Three-spined sticklebacks caught from a natural population from the lake Großer Plöner See were used for breeding as described elsewhere (Kalbe & Kurtz 2006). Several sibships per breeding pair were raised until the age of three months when offspring from the same pair were combined and transferred to 190 l tanks in densities of 100–250 fish. Fourteen randomly chosen individuals and both parents of each sibship were analysed for their MHC class IIB genotype (see below). Six sibships with segregating numbers of MHC class IIB alleles/variants were selected, as in Wegner et al. (2003a). In such families, with a similar genetic background, MHC genotypes with different numbers of sequence variants were present: intermediate diversity (approx. six sequence variants); a low number (less than five sequence variants); and a high (more than seven sequence variants).
(b) Experimental parasite infections and time schedule
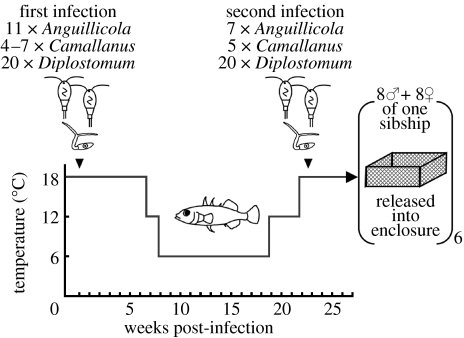
From each sibship, 30 randomly selected fish were each experimentally exposed twice to a combination of three of the most prevalent macroparasite species originating from their parents' habitat: the nematodes, Anguillicola crassus and Camallanus lacustris, as well as a digenean trematode, the eye fluke Diplostomum pseudospathaceum. All parasites originated from the Großer Plöner See or contiguous neighbouring lakes and, therefore, are regarded as sympatric to the stickleback population examined here. Infection of fish was performed as described elsewhere (Kalbe & Kurtz 2006; Krobbach et al. 2007). Fish were exposed to the combination of all three parasites in December 2004, and again in May 2005. Between treatments, to mimic natural life history including parasite exposure, sticklebacks were brought stepwise to laboratory winter conditions (6°C, 10 h light d−1) before re-experiencing summer conditions (18°C, 16 h light d−1; see figure 1 for infection dosages and schedule).
Figure 1.
Schematic of the experimental infection schedule and temperature (grey line) regime in the laboratory, prior to release of the sticklebacks into the enclosure.
(c) Enclosures
The outdoor experiment was conducted in the lake Großer Plöner See, in northern Germany (54° 9′21.16′ N, 10°25′50.14′ E) during the summer 2005. The enclosure system consisted of six stainless steel mesh cages (3 ×3 m, total height 1, 0.4–0.6 m above water surface) installed on the lake ground in a row close to the shoreline, located in the natural breeding area, i.e. where the parents of the experimental fish had been in the previous year. The 5 mm meshes allowed only small particles and most invertebrates (food items and intermediate hosts of various parasite species) to pass through. A coarse meshed net protected the fish from bird predation. Prior to release into the enclosures, we weighed (±0.1 mg), measured (±1 mm) the fish and counted the Diplostomum metacercariae in each eye lens under a dissection microscope. Finally, a 2–3 mm piece of the first dorsal spine was cut for genotyping at the end of the experiment. Each of the six enclosures was stocked with eight males and eight females, one family per cage, on 16 June 2005.
(d) Egg collection
Every week but one, because the weather did not allow it, all stickleback nests were detected in each enclosure by careful observation. All egg batches were removed and the nests were carefully replaced in their original location. Egg batches were brought to the laboratory; clutches were separated on the basis of different developmental stages. If necessary, the individual egg clutches were incubated in aerated well water (with 0.04 ppm malachite green) at 18°C until dark eye spots and the neural tube developed, to ensure a sufficient amount of DNA for further analysis (see §2f).
(e) Recapture and examination of sticklebacks
During the last egg collection on 3 August, when nests no longer contained fresh clutches, all surviving fish were caught. Immediately following capture, a picture of each male's red throat was taken within a dark box using a digital camera (Olympus E20p) with a 36 mm macro lens. For the camera parameters and the handling of fish, see Jäger et al. (2007). Intensity analysis of the red coloration was performed with IP Lab v. 3.6.2 for Mac OS v. 9.2.2 (Scanalytics, Inc.) delimitating a defined area of the red throat. Jäger et al. (2007) showed that method to be highly repeatable. Surviving fish were measured, weighed and dissected within 3 days after capture, including screening for macroparasites and weighing of organs (gonads, kidney, liver and spleen). General body condition of the fish was calculated according to Bolger & Connolly (1989).
All external and internal macroparasites and ciliates were determined to the lowest taxonomic level possible. In order to quantify the total parasite load of each fish, an individual parasite index was calculated (Kalbe et al. 2002). This allowed different combinations of rare and frequently occurring parasites to be summarized for each stickleback and their total parasite burden to be compared quantitatively.
(f) Major histocompatibility complex and microsatellite typing
DNA extractions from dorsal spines of fish before release into the enclosures and after recapturing, as well as from the developing eggs, were conducted using DNA Tissue kit (Invitek, Germany) following the manufacturer's protocol. All fish and a representative subsample of the collected fry (see below) were typed for 18 microsatellites (see the electronic supplementary material) combined in five different PCR protocols (Largiadèr et al. 1999; Peichel et al. 2001). This number of microsatellites was needed in order to guarantee a high parenthood resolution even within sibships. At the end of the experiment, survivors were retyped for identification. The MHC class IIB diversity was determined using capillary electrophoresis single-strand conformation polymorphism of the amplified exon 2 of the MHC class IIB chain as described in Binz et al. (2001) (see Reusch & Langefors 2005 for an estimation of the number loci).
(g) Parenthood analysis and heterozygosity
From each egg clutch, 16 eggs were randomly picked for parentage analysis. Paternity assignments were performed with the software PAPA for every egg (Duchesne et al. 2002). Accordingly, each egg was assigned to one of the following male categories: nest owner, sneaker and stolen. Males that fertilized the majority of the eggs in a nest were categorized as nest owners and these eggs were assumed to originate from females that have chosen this male. Eggs were assigned as being fertilized by a sneaker, if in one nest the same female had eggs fertilized by the nest owner and another male. Eggs were assigned as being stolen from other nests if the nest owner did not fertilize them and no further eggs of the same female were found in the nest. Moreover, we verified that the combination existed in other nests to exclude wrong affiliations from the software. We also calculated an heterozygosity index for each individual parent (Coulson et al. 1998).
(h) Data analysis
All statistical analyses were conducted in JMP v. 5.0.1 (SAS Institute). To start, a large multiple correlation was performed and occurring collinearities were corrected by taking the residuals of the regressions. Stepwise model selections was performed based on the AIC criterion (Sakamoto et al. 1986).
LRS was calculated for both surviving and total number of fish since some dead fish had a certain fertilization success.
(i) Lifetime reproductive success
We aimed at estimating the LRS of each fish. First, we performed an ANCOVA on the total number of eggs assigned to each parent, with enclosures, sex, parasite load, initial and final body condition (expressed as residuals of the regression with initial body condition) as the dependent variables. Second, we correlated the residuals of the previous model for LRS with MHC class IIB diversity with both linear and quadratic terms.
With our design, we had to test the correlation between MHC genotypes and LRS within sibships. We used Wegner et al.'s (2003b) method extended from Aeschlimann et al. (2003) and (Milinski 2003) to calculate the reproductive success dependent on the number of individual MHC variants using each sibship as a statistical unit. Briefly, we averaged the number of eggs within each sibship over each MHC genotype and calculated all the possible slopes between these points. For a first ‘purist’ test, we used the average number of eggs and the average slope derived as described and used this as one pair of data points from each sibship (see §3 for detailed description of analysis and the electronic supplementary material for a fictitious example explaining this method). Further tests took individual fish and thus more information into account. Because we had clear predictions with intermediate genotypes to perform better, we used directed (not one-tailed) statistical tests (Rice & Gaines 1994). This analysis was performed with all introduced fish as well as only with the survivors.
(ii) Parasite load, body condition, splenosomatic index and coloration
Surviving fish were tested for significant effects on parasite load with an ANCOVA incorporating body condition at the beginning of the experiment, body condition at the end, sex and enclosure as predictors. We also included all two-way interactions in the model. Thereafter, we correlated the residuals with MHC individual diversity.
As an estimate of the sticklebacks' immunological activation, we calculated a splenosomatic index (SSI) as follows: SSI=(spleen weight/body weight)×100. We performed an ANOVA with SSI as the dependent variable, and enclosure, sex and their interaction as predictors. We then correlated the residuals with individual MHC diversity.
For male breeding coloration, after an ANCOVA was performed to test for the effect of enclosures, body condition at the beginning and at the end of the experiment and of parasite load, we related the residuals of that model to individual MHC diversity.
3. Results
(a) Lifetime reproductive success
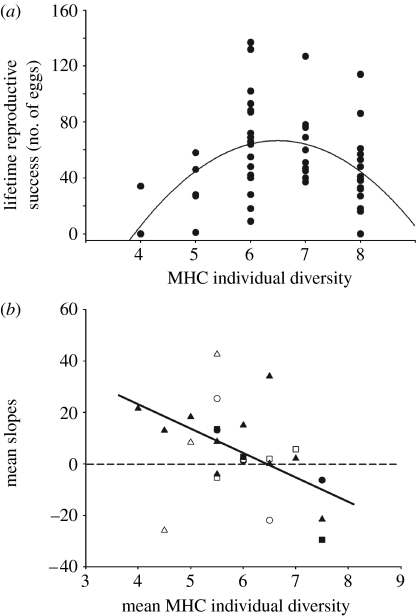
Over the entire breeding season, we collected a total of 149 egg clutches until after seven weeks the nests no longer contained fresh clutches. Using 18 microsatellite loci, 2279 out of the total of 2384 (95.6%) offspring could be unambiguously allocated to a pair of parents. The remaining offspring were removed from subsequent analyses. One per cent of the eggs were evidently stolen from other nests, whereas sneaking behaviour accounted for 12 per cent of the eggs. Hence, although female sticklebacks tend to avoid inbreeding (Frommen & Bakker 2006), but had no choice here, the sneaking rate of males within families in the enclosures was in the same range as in natural populations (Rico et al. 1992; Largiadèr et al. 2001; Blais et al. 2004). The total number of analysed offspring varied between 1 and 137 eggs per individual fish. The LRS of all fish released into the enclosures was significantly correlated only with MHC diversity: fish with an intermediate number of MHC alleles had the highest LRS (figure 2a; n=96, t=−2.40, p=0.0185; table 1, a and a1). None of our other variables could predict LRS (table 1, a). This result remained significant when restricted to surviving fish recaptured at the end of the experiment (n=53, t=−2.55, p=0.014; table 1, a2) and when recaptured males and females (from 48 of each sex originally introduced) were considered separately (female, n=28, t=−2.94, p=0.007; males, n=25, t=−2.46, p=0.022). Furthermore, LRS was not related to our individual heterozygosity index based on neutral markers (linear: F1,53=0.155, p=0.695; quadratic: F2,53=0.207, p=0.137).
Figure 2.
(a) Relationship between the LRS (shown as representative number of eggs) and the MHC class IIB diversity. Fish with an intermediate MHC II B diversity had the highest reproductive output (neggs=−0.272+0.136nMHCIIB−0.499(nMHCIIB−6.679)2, F2,52=4.29, p=0.019). For clarity, raw data of surviving fish are presented but tests were performed on residuals as stated in §2. (b) Relationship between the mean slopes of all potential pairs of surviving fish within an enclosure and their relative mean MHC diversity. All data points obtained from the same enclosure are depicted by the same symbol (filled circles, enclosure 1; open circles, enclosure 2; filled squares, enclosure 3; open squares, enclosure 4; filled triangles, enclosure 5; open triangles, enclosure 6). The equation of the linear regression is f(x)=−27.94x+178.81, directed test F1,17=33.71, p=0.0001.
Table 1.
Variables correlated with (a) LRS, (b) SSI, (c) coloration and (d) parasite load. (Models were chosen on the basis of AIC criteria. *p<0.05, **p<0.001, ***p<0.0001.)
| terms | estimates | standard error | t-ratio | prob>t | d.f. | sum of squares | F-ratio | prob>F | |
|---|---|---|---|---|---|---|---|---|---|
| (a1) LRS—polynomial regression—optimum | (a) LRS | ||||||||
| MHC class II B | 0.055 | 2.907 | 0.02 | 0.985 | enclosure | 5 | 5156.117 | 0.992 | 0.434 |
| MHC class II B (quadratic) | −4.378 | 1.826 | −2.40 | 0.019* | sex | 1 | 180.698 | 0.174 | 0.679 |
| parasite load (corrected for enclosure and sex) | 1 | 1189.776 | 1.142 | 0.291 | |||||
| (a2) LRS surviving fish—polynomial regression—optimum | initial body condition | 1 | 1175.581 | 1.129 | 0.291 | ||||
| MHC class II BMHC class II B (quadratic) | 0.136−0.499 | 0.2520.195 | 0.54−2.55 | 0.5920.014* | final body condition (corrected for parasite load and initial body condition) | 1 | 0.006 | 0.00 | 0.998 |
| (c) colouration—ANCOVA | |||||||||
| parasite load (corrected for parasite load and body condition)body condition | 11 | 207.987194.716 | 4.9974.6781 | 0.0364*0.0422* | |||||
| (b) SSI—polynomial regression—optimum | |||||||||
| MHC class II B | 0.02 | 0.0157 | 1.27 | 0.215 | (d) parasite load—ANCOVA | ||||
| MHC class II B (quadratic) | 0.0567 | 0.0169 | 3.36 | 0.0025** | enclosure | 5 | 2.212 | 4.145 | 0.0036** |
| sex | 1 | 1.401 | 13.123 | 0.0008*** | |||||
| body condition (corrected body condition and sex) | 1 | 0.086 | 0.8061 | 0.3742 | |||||
Comparing LRS among sibships: if a genotype with an optimal number of MHC alleles has the highest number of eggs, the slope between egg number of this genotype and that of a genotype having less alleles should be positive, whereas it should be negative between the optimal genotype and a genotype with more alleles. Moreover, two genotypes equally distant from the optimum should have the same egg number, and thus a slope of 0. Furthermore, the mean of the slopes should be positive when the mean of the allele numbers is lower than the optimal number and vice versa. If there is an optimal MHC allele number per individual, all single sibship data points (relationship between the mean slope and the mean MHC variant number of a sibship) should fall on one straight line with negative slope with the X-axis intercepting at the hypothesized optimum (see Wegner et al. (2003a) for a similar analysis and the electronic supplementary material). Indeed, we could detect a significant negative linear relationship between the mean slopes of LRS and the mean number of MHC class IIB sequence variants (all fish averaged per sibship, directed test F1,5=5.44, p=0.05; surviving fish, directed test F1,5=7.28, p=0.034). This analysis treated each sibship as a statistical unit. With fish averaged for each MHC individual diversity treated as statistical unit, we found similar results (all fish, directed test F1,23=4.28, p=0.031; surviving fish, directed test F1,16=19.28, p=0.0001; figure 2b). In the first case, the analysis resulted in an optimum at 6.55 MHC variants, while it resulted in an optimum of 6.40 when considering the surviving fish only. Males had a higher variance in their LRS than females (mean±s.e., males: 55.4±7.92 eggs; females: 53.11±4.57 eggs, Levene test, F1,52=5.495, p=0.023).
(b) Spleen size
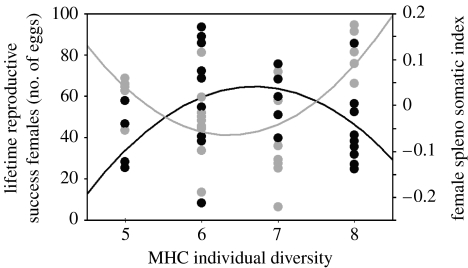
The SSI, an indicator for immune activation, was different among enclosures (F5,51=7.96, p<0.0001), and between the sexes with females showing higher values (F1,51=6.87, p=0.012). Owing to sex differences, we split the dataset and correlated SSI with an individual MHC diversity for each sex separately. Male SSI did not show a significant relationship with MHC diversity (linear, t1,25=−1.30, p=0.206; quadratic, t1,25=−1.93, p=0.083). Females with an intermediate number of MHC variants had the lowest SSI (figure 3; quadratic, t1,28=3.41, p=0.002; linear, t1,28=1.28, p=0.212; table 1, b). Moreover, SSI correlated with the total egg number per individual for females (directed test, t1,28=−1.84, p=0.049) but not for males (directed test, t1,25=0.88, p=0.242). Since spleen size is commonly used as a diagnostic tool of the immune system, this result suggests a trade-off between reproduction and immune function only in females.
Figure 3.
Graph combining the female LRS (shown as representative number of eggs) and SSI as a function of MHC II B diversity. Females with an intermediate MHC II B diversity having the highest reproductive success are shown by black circles (neggs=38.38+4.514nMHCIIB−11.98(nMHCIIB−6.481)2, F2,26=4.63, p=0.02). Females with an intermediate MHC II B diversity having the lowest SSI, representing a better immunocompetence status, are shown by grey circles (SSI=−0.193+0.02nMHCIIB+0.057(nMHCIIB−6.536)2, F2,26=6.698, p=0.004).
(c) Parasite load
We recorded 21 different parasites (for details see the electronic supplementary material) from eight taxonomic groups: Protozoa (Trichodina sp., Apiosoma sp. and Ichthyophthirius multifiliis), Monogenea (Gyrodactylus sp.), Digenea (Diplostomum sp., Apatemon cobitidis, Phyllodistomum folium, Cyathocotyle prussica, Tylodelphis clavata, Echinochasmus sp.), Cestoda (Valipora campylancristrota, Proteocephalus filicollis), Nematoda (A. crassus, C. lacustris, Contracaecum sp., Raphidascaris acus), Acanthocephala (Acanthocephalus lucii and Acanthocephalus clavula), the crustaceans, Argulus foliaceus and Ergasilus sp., and glochidia, the parasitic larval stages of freshwater mussels (Mollusca).
The total parasite load (table 1, c) varied significantly among the enclosures (F5,53=4.145, p=0.004), and females were more infected than males (F1,53=13.123, p<0.001). Infection intensities by trophically transmitted parasites (all nematodes, cestodes and acanthocephalans found in this study) were higher in females than males (t1,52=3.050, p=0.004), whereas there was no difference for directly and actively transmitted parasites (active, t1,52=1.291, p=0.203; direct, Mann–Whitney test, Z=0.632, p=0.527; see Scharsack et al. 2007).
Only for the eye fluke Diplostomum sp., but not for the two internal nematode parasites, we could determine the effect of the laboratory infection in vivo prior to release into cages. Here, parasite burden increased in the enclosure period significantly (paired t-test on log-transformed data, t1,52=15.994, p<0.001) with fish that harboured the highest number of Diplostomum sp. at the beginning also showing the highest Diplostomum sp. intensity at the end (R2=0.18, F=10.414, p=0.002).
(d) Body condition and coloration
Body condition, a function of a species-specific combination of body length and weight (Frischknecht 1993), was estimated at the beginning and at the end of the experiment for both sexes. The loss in body condition was positively correlated only with parasite burden (F1,52=9.15, p=0.004) but not with MHC individual diversity (linear, F1,52=0.001, p=0.979; quadratic, F1,52=0.532, p=0.469).
Mature male sticklebacks develop distinctive red coloration of the throat, as a secondary sexual character. The intensity of breeding coloration (table 1, d) was negatively correlated with parasite load (F1,25=4.997, p=0.0364) but increased with a higher body condition (F1,25=4.678, p=0.0422, see figures in the electronic supplementary material). We did not find any significant differences among families (F5,25=0.492, p=0.778), and the intensity of coloration of males did not predict LRS (F1,25=0.408, p=0.531). Finally, we found no significant correlation between breeding coloration and individual MHC diversity (linear, F1,25=0.416, p=0.526; quadratic, F2,25=1.00, p=0.384).
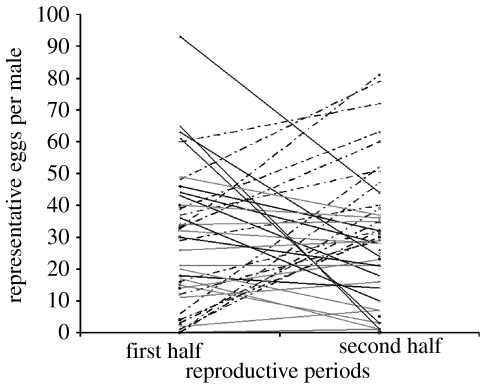
Both body condition and coloration of males were measured at the end of the experiment when all fish had ceased reproduction. The missing correlation of either measure with LRS and thus with MHC diversity might result from different males reproducing early and late in the season, as found by Bakker & Mundwiler (1994) in a field study. Also, in the present study, males differed in the time course of their reproductive effort: some reproduced predominantly early and others late (figure 4). The latter had a significantly lower body condition at the end (Welch t-test, t=−2.151, p=0.0384), which suggests that the late reproducing fish were the slightly weaker fish that could reproduce only after the stronger fish had finished, as in Bakker & Mundwiler's (1994) study. However, neither the individual MHC diversity distributions (two-sample Kolmogorov–Smirnov test, D=0.1618, p=0.9698) nor the LRS (Welch t-test, t=0.939, p=0.3589) differed significantly between the two groups.
Figure 4.
Graph showing the different male reproductive strategies during the experiment. Black dot-dashed lines represent fish that significantly increased the reproductive success in the second half, while black solid lines represent fish that decreased their activity in the second part. Grey solid lines symbolize fish for which there was no difference between the two periods.
4. Discussion
Several studies have now identified natural or sexual selection for intermediate rather than maximal MHC diversity (Reusch et al. 2001; Aeschlimann et al. 2003; Wegner et al. 2003a,b, 2008; Bonneaud et al. 2004; Milinski et al. 2005; Madsen & Ujvari 2006; Forsberg et al. 2007). Here, we show that an intermediate MHC diversity maximizes the LRS, i.e. Darwinian fitness. Our experimental design permitted the estimation of individual LRS because three-spined sticklebacks reproduce only during a single breeding season in their entire life (Wootton 1976). We found that LRS was highest in individuals with an intermediate MHC diversity, which corresponds to an immunogenetic optimum (Wegner et al. 2003a,b; Kurtz et al. 2004), even though infected by varied parasite communities in the different enclosures. While this result was significant for both males and females, the mechanistic link of MHC optimality to LRS might differ between the sexes. In our study, body condition and parasite burden are directly linked to LRS probably only in females. Overall, females harboured more parasites than males. However, this does not necessarily indicate lower immunocompetence. The disparity is only due to differential infection intensities of trophically transmitted helminths, thus most likely reflecting different feeding behaviours of females to cover their higher energy demand. The number of eggs a female stickleback can produce is dependent on its physiological capacity and nutritional status (Wootton 1977; Kraak & Bakker 1998). Hence, females need to consume more food (Wootton 1976), which also includes copepods and other potential intermediate hosts. Parasites with active transmission, such as digenean trematodes, did not differ between the sexes in infection intensity.
In males, where the variance in LRS was higher than in females, the situation is more complex, probably because different males had different time courses in their reproductive effort (figure 4), as had been shown by Bakker and Mundwiler (1994) in a field study, possibly indicating a terminal investment strategy of weaker fish. Therefore, neither breeding coloration nor body condition measured at the end of the experiment was correlated with LRS or individual MHC diversity. Because we could not take these measures repeatedly during the course of the experiment, we cannot detect any mechanistic link with individual MHC diversity. However, the reproductive success was measured continuously, allowing us to show that males with an intermediate MHC variant number achieved the highest LRS.
Among condition-dependent male traits in sticklebacks, the intensity of the red breeding coloration is one of the most conspicuous sexual traits that received a lot of attention (McLennan & McPhail 1990; Milinski & Bakker 1990, 1992; Bakker & Milinski 1991; Frischknecht 1993; Bakker & Mundwiler 1994; Candolin 1999; Kraak et al. 1999; Barber et al. 2000). Parasite infections affect breeding coloration (Milinski & Bakker 1990; Folstad et al. 1994) and body condition (Milinski & Bakker 1990; Tierney et al. 1996; Blais et al. 2004). Accordingly, as shown here, the coloration of males recaptured from the enclosures correlated positively with body condition but negatively with parasite burden measured at the end of the experiment. However, in accordance with previous studies, the quality of sexual ornaments was not greater in males with optimal MHC diversity, but rather could reveal the possession of specific MHC alleles (Buchholz et al. 2004; Jäger et al. 2007). These respective alleles probably provide resistance against the currently predominating parasite species (Jäger et al. 2007). Therefore, female mate choice is predicted to include two criteria: olfactory cues reveal a male's MHC variant diversity, whereas colour reveals the possession of currently protective alleles (Aeschlimann et al. 2003; Milinski 2006; Jäger et al. 2007). A trait correlating with individual MHC diversity in females was spleen size. Spleen size has been widely used in immunoecological studies as a measure of general activation of the immune system by multiple macroparasite infections, particularly in birds (John 1995; Møller & Erritzoe 1998; Morand & Poulin 2000; Brown & Brown 2002) and fish (Skarstein et al. 2001; Kortet et al. 2003; Lefebvre et al. 2004; Ottova et al. 2005). Females with an intermediate number of MHC class IIB variants had the lowest relative spleen size (figure 4). Spleen size negatively correlated (marginally significant) with the number of eggs assigned to the respective females. This potential link between reproduction, MHC genotype and spleen size is probably due to the activity of the immune system itself, rather than to the result of its efficiency, namely the individual parasite burden. This suggests a trade-off between an individual's LRS and the costs of immunity. Sticklebacks with a more efficient adaptive (MHC dependent) immune system can afford to invest more into their offspring, whereas fish with less optimal MHC diversity need to allocate a higher proportion of their resources for defence mechanisms to maintain their parasite load at tolerable levels. Previous studies have already shown that sticklebacks with an intermediate MHC diversity had the lowest oxidative burst activity, but were nevertheless more capable of limiting the growth of the tapeworm Schistocephalus solidus than fish with MHC variant number deviating from this optimum (Kurtz et al. 2004). Fish with an optimal MHC diversity basis seem to perform a shift from costly and self-damaging innate immune function towards a probably less costly but efficient adaptive immune strategy, and use the immunological mechanisms more concertedly and economically. Costs of immunity are predominantly regarded as metabolic constraints (Lochmiller & Deerenberg 2000). The results of the present study indicate that immunological costs might directly affect LRS, but that their impact depends to a high degree on the individual MHC genotype. Therefore, these results may further explain why sticklebacks with intermediate MHC diversity prevail in natural stickleback populations (Reusch et al. 2001; Wegner et al. 2003a).
All animal experiments described were approved by the Ministry of Nature, Environment and Country Development, Schleswig-Holstein, Germany.
Acknowledgments
We thank C. K. Krobbach for the help with and analysis of a pilot study, as well as G. Augustin, H. Deiwick, R. Derner, A. Hasselmayer, L. Janke, R. Leipnitz, D. Lemcke, S. Liedtke, H. Luttmann, I. Schultz and M. Wulf for technical assistance. Furthermore, we are indebted to H. & J. Schmidt, as well as to F. Kirfel and J. Schütt, for providing perch and eel tissue samples. We also thank C. Harrod, the editor and the referees for their helpful comments on an earlier version of the manuscript. In addition, we thank the county of Schleswig-Holstein and the city of Ploen for permitting us to run the outdoor experiments in the lake.
Supplementary Material
References
- Aeschlimann P.B., Häberli M.A., Reusch T.B.H., Boehm T., Milinski M. Female sticklebacks Gasterosteus aculeatus use self-reference to optimize MHC allele number during mate selection. Behav. Ecol. Sociobiol. 2003;54:119–126. [Google Scholar]
- Apanius V., Penn D., Slev P.R., Ruff L.R., Potts W.K. The nature of selection on the major histocompatibility complex. Crit. Rev. Immunol. 1997;17:179–224. doi: 10.1615/critrevimmunol.v17.i2.40. [DOI] [PubMed] [Google Scholar]
- Bakker T.C.M., Milinski M. Sequential female choice and the previous male effect in sticklebacks. Behav. Ecol. Sociobiol. 1991;29:205–210. doi:10.1007/BF00166402 [Google Scholar]
- Bakker T.C.M., Mundwiler B. Female mate choice and male red coloration in a natural three-spined stickleback (Gasterosteus aculeatus) population. Behav. Ecol. 1994;5:74–80. doi:10.1093/beheco/5.1.74 [Google Scholar]
- Barber I., Arnott S.A., Braithwaite V.A., Andrew J., Mullen W., Huntingford F.A. Carotenoid-based sexual coloration and body condition in nesting male sticklebacks. J. Fish Biol. 2000;57:777–790. doi:10.1111/j.1095-8649.2000.tb00274.x [Google Scholar]
- Bernatchez L., Landry C. MHC studies in nonmodel vertebrates: what have we learned about natural selection in 15 years? J. Evol. Biol. 2003;16:363–377. doi: 10.1046/j.1420-9101.2003.00531.x. doi:10.1046/j.1420-9101.2003.00531.x [DOI] [PubMed] [Google Scholar]
- Binz T., Reusch T.B.H., Wedekind C., Milinski M. SSCP analysis of MHC class II B genes in the threespine stickleback. J. Fish Biol. 2001;58:887–890. [Google Scholar]
- Blais J., Rico C., Bernatchez L. Nonlinear effects of female mate choice in wild threespine sticklebacks. Evolution. 2004;58:2498–2510. doi: 10.1111/j.0014-3820.2004.tb00879.x. doi:10.1111/j.0014-3820.2004.tb00879.x [DOI] [PubMed] [Google Scholar]
- Bolger T., Connolly P.L. The selection of suitable indexes for the measurement and analysis of fish condition. J. Fish Biol. 1989;34:171–182. doi:10.1111/j.1095-8649.1989.tb03300.x [Google Scholar]
- Bonneaud C., Mazuc J., Chastel O., Westerdahl H., Sorci G. Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution. 2004;58:2823–2830. doi: 10.1111/j.0014-3820.2004.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Bonneaud C., Richard M., Faivre B., Westerdahl H., Sorci G. An MHC class I allele associated to the expression of T-dependent immune response in the house sparrow. Immunogenetics. 2005;57:782–789. doi: 10.1007/s00251-005-0046-5. doi:10.1007/s00251-005-0046-5 [DOI] [PubMed] [Google Scholar]
- Bonneaud C., Perez-Tris J., Federici P., Chastel O., Sorci G. Major histocompatibilty alleles associated with local resistance to malaria in a passerine. Evolution. 2006;60:383–389. [PubMed] [Google Scholar]
- Borghans J.A.M., Noest A.J., De Boer R.J. Thymic selection does not limit the individual MHC diversity. Eur. J. Immunol. 2003;33:3353–3358. doi: 10.1002/eji.200324365. doi:10.1002/eji.200324365 [DOI] [PubMed] [Google Scholar]
- Briles W.E., Briles R.W., Taffs R.E., Stone H.A. Resistance to a malignant lymphoma in chickens is mapped to subregion of major histocompatibility (B) complex. Science. 1983;219:977–979. doi: 10.1126/science.6823560. doi:10.1126/science.6823560 [DOI] [PubMed] [Google Scholar]
- Brown C.R., Brown M.B. Spleen volume varies with colony size and parasite load in a colonial bird. Proc. R. Soc. B. 2002;269:1367–1373. doi: 10.1098/rspb.2002.2036. doi:10.1098/rspb.2002.2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz R., Jones Dukes M.D., Hecht S., Findley A.M. Investigating the turkey's ‘snood’ as a morphological marker of heritable disease resistance. J. Anim. Breed. Genet. 2004;121:176–185. doi:10.1111/j.1439-0388.2004.00449.x [Google Scholar]
- Candolin U. The relationship between signal quality and physical condition: is sexual signalling honest in the three-spined stickleback? Anim. Behav. 1999;58:1261. doi: 10.1006/anbe.1999.1259. doi:10.1006/anbe.1999.1259 [DOI] [PubMed] [Google Scholar]
- Clarke B., Kirby D.R.S. Maintenance of histocompatibility polymorphisms. Nature. 1966;211:999–1000. doi: 10.1038/211999a0. doi:10.1038/211999a0 [DOI] [PubMed] [Google Scholar]
- Coulson T.N., Pemberton J.M., Albon S.D., Beaumont M., Marshall T.C., Slate J., Guinness F.E., Clutton-Brock T.H. Microsatellites reveal heterosis in red deer. Proc. R. Soc. B. 1998;265:489–495. doi: 10.1098/rspb.1998.0321. doi:10.1098/rspb.1998.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer R.J., Perelson A.S. How diverse should the immune system be? Proc. R. Soc. B. 1993;252:171–175. doi: 10.1098/rspb.1993.0062. doi:10.1098/rspb.1993.0062 [DOI] [PubMed] [Google Scholar]
- Doherty P.C., Zinkernagel R.M. A biological role for the major histocompatibility antigens. Lancet. 1975;305:1406–1409. doi: 10.1016/s0140-6736(75)92610-0. doi:10.1016/S0140-6736(75)92610-0 [DOI] [PubMed] [Google Scholar]
- Duchesne P., Godbout M.H., Bernatchez L. PAPA (package for the analysis of parental allocation): a computer program for simulated and real parental allocation. Mol. Ecol. Notes. 2002;2:191–193. doi:10.1046/j.1471-8286.2002.00164.x [Google Scholar]
- Edwards S.V., Hedrick P.W. Evolution and ecology of MHC molecules: from genomics to sexual selection. Trends Ecol. Evol. 1998;13:305–311. doi: 10.1016/s0169-5347(98)01416-5. doi:10.1016/S0169-5347(98)01416-5 [DOI] [PubMed] [Google Scholar]
- Folstad I., Hope A.M., Karter A., Skorping A. Sexually selected color in male sticklebacks: a signal of both parasite exposure and parasite resistance? Oikos. 1994;69:511. doi:10.2307/3545863 [Google Scholar]
- Forsberg L.A., Dannewitz J., Petersson E., Grahn M. Influence of genetic dissimilarity in the reproductive success and mate choice of brown trout—females fishing for optimal MHC dissimilarity. J. Evol. Biol. 2007;20:1859–1869. doi: 10.1111/j.1420-9101.2007.01380.x. doi:10.1111/j.1420-9101.2007.01380.x [DOI] [PubMed] [Google Scholar]
- Frischknecht M. The breeding colouration of male three-spined sticklebacks (Gasterosteus aculeatus) as an indicator of energy investment in vigour. Evol. Ecol. 1993;7:439. doi:10.1007/BF01237640 [Google Scholar]
- Frommen J., Bakker T. Inbreeding avoidance through non-random mating in sticklebacks. Biol. Lett. 2006;2:232–235. doi: 10.1098/rsbl.2005.0432. doi:10.1098/rsbl.2005.0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godot V., Harraga S., Beurton I., Tiberghien P., Sarciron E., Gottstein B., Vuitton D.A. Resistance/susceptibility to Echinococcus multilocularis infection and cytokine profile in humans. II. Influence of the HLA B8, DR3, DQ2 haplotype. Clin. Exp. Immunol. 2000;121:491–498. doi: 10.1046/j.1365-2249.2000.01309.x. doi:10.1046/j.1365-2249.2000.01309.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimholt U., Larsen S., Nordmo R., Midtlyng P., Kjoeglum S., Storset A., Saeb S., Stet R.J.M. MHC polymorphism and disease resistance in Atlantic salmon (Salmo salar); facing pathogens with single expressed major histocompatibility class I and class II loci. Immunogenetics. 2003;55:210–219. doi: 10.1007/s00251-003-0567-8. doi:10.1007/s00251-003-0567-8 [DOI] [PubMed] [Google Scholar]
- Harf R., Sommer S. Association between MHC class II DRB alleles and parasite load in the hairy-footed gerbil, Gerbillurus paebe, in the southern Kalahari. Mol. Ecol. 2005;14:85–91. doi: 10.1111/j.1365-294X.2004.02402.x. doi:10.1111/j.1365-294X.2004.02402.x [DOI] [PubMed] [Google Scholar]
- Hill A.V.S., et al. Common West African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. doi:10.1038/352595a0 [DOI] [PubMed] [Google Scholar]
- Ilmonen P., Penn D.J., Damjanovich K., Morrison L., Ghotbi L., Potts W.K. Major histocompatibility complex heterozygosity reduces fitness in experimentally infected mice. Genetics. 2007;176:2501–2508. doi: 10.1534/genetics.107.074815. doi:10.1534/genetics.107.074815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger I., Eizaguirre C., Griffiths S.W., Kalbe M., Krobbach C.K., Reusch T.B.H., Schaschl H., Milinski M. Individual MHC class I and MHC class IIB diversities are associated with male and female reproductive traits in the three-spined stickleback. J. Evol. Biol. 2007;20:2005–2015. doi: 10.1111/j.1420-9101.2007.01366.x. doi:10.1111/j.1420-9101.2007.01366.x [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Travers P., Walport M., Sclomchik M.J. Garland Science Publishing; New York, NY: 2005. Immunobiology: the immune system in health and disease. [Google Scholar]
- John J.L. Parasites and the avian spleen—helminths. Biol. J. Linn. Soc. 1995;54:87–106. [Google Scholar]
- Jordan W.C., Bruford M.W. New perspectives on mate choice and the MHC. Heredity. 1998;81:127–133. doi: 10.1046/j.1365-2540.1998.00428.x. doi:10.1038/sj.hdy.6884281 [DOI] [PubMed] [Google Scholar]
- Kalbe M., Kurtz J. Local differences in immunocompetence reflect resistance of sticklebacks against the eye fluke Diplostomun pseudopathaceum. Parasitology. 2006;132:1–12. doi: 10.1017/S0031182005008681. doi:10.1017/S0031182006000801 [DOI] [PubMed] [Google Scholar]
- Kalbe M., Wegner K.M., Reusch T.B.H. Dispersion patterns of parasites in 0+ year three-spined sticklebacks: a cross population comparison. J. Fish Biol. 2002;60:1529–1542. doi:10.1111/j.1095-8649.2002.tb02445.x [Google Scholar]
- Kortet R., Taskinen J., Sinisalo T., Jokinen I. Breeding-related seasonal changes in immunocompetence, health state and condition of the cyprinid fish, Rutilus rutilus, L. Biol. J. Linn. Soc. 2003;78:117–127. doi:10.1046/j.1095-8312.2003.00136.x [Google Scholar]
- Kraak S.B.M., Bakker T.C.M. Mutual mate choice in sticklebacks: attractive males choose big females, which lay big eggs. Anim. Behav. 1998;56:859. doi: 10.1006/anbe.1998.0822. doi:10.1006/anbe.1998.0822 [DOI] [PubMed] [Google Scholar]
- Kraak S.B.M., Bakker T.C.M., Mundwiler B. Sexual selection in sticklebacks in the field: correlates of reproductive, mating, and paternal success. Behav. Ecol. 1999;10:696–706. doi:10.1093/beheco/10.6.696 [Google Scholar]
- Krobbach C.K., Kalbe M., Kurtz J., Scharsack J.P. Infectivity of two nematode parasites, Camallanus lacustris and Anguillicola crassus, in a paratenic host, the three-spined stickleback Gasterosteus aculeatus. Dis. Aquat. Organ. 2007;74:119–126. doi: 10.3354/dao074119. doi:10.3354/dao074119 [DOI] [PubMed] [Google Scholar]
- Kurtz J., Kalbe M., Aeschlimann P.B., Häberli M.A., Wegner K.M., Reusch T.B.H., Milinski M. Major histocompatibility complex diversity influences parasite resistance and innate immunity in sticklebacks. Proc. R. Soc. B. 2004;271:197. doi: 10.1098/rspb.2003.2567. doi:10.1098/rspb.2003.2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langefors Å., Lohm J., Grahn M., Andersen Ø., von Schantz T. Association between major histocompatibility complex class IIB alleles and resistance to Aeromonas salmonicida in Atlantic salmon. Proc. R. Soc. B. 2001;268:479–485. doi: 10.1098/rspb.2000.1378. doi:10.1098/rspb.2000.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largiadèr C.R., Fries V., Kobler B., Bakker T.C.M. Isolation and characterization of microsatellite loci from the three-spined stickleback (Gasterosteus aculeatus L.) Mol. Ecol. 1999;8:342–344. [PubMed] [Google Scholar]
- Largiadèr C.R., Fries V., Bakker T.C.M. Genetic analysis of sneaking and egg-thievery in a natural population of the three-spined stickleback (Gasterosteus aculeatus L.) Heredity. 2001;86:459–468. doi: 10.1046/j.1365-2540.2001.00850.x. doi:10.1046/j.1365-2540.2001.00850.x [DOI] [PubMed] [Google Scholar]
- Lawlor D.A., Zemmour J., Ennis P.D., Parham P. Evolution of class-I MHC genes and proteins—from natural-selection to thymic selection. Annu. Rev. Immunol. 1990;8:23–63. doi: 10.1146/annurev.iy.08.040190.000323. doi:10.1146/annurev.iy.08.040190.000323 [DOI] [PubMed] [Google Scholar]
- Lefebvre F., Mounaix B., Poizat G., Crivelli A.J. Impacts of the swimbladder nematode Anguillicola crassus on Anguilla anguilla: variations in liver and spleen masses. J. Fish Biol. 2004;64:435–447. doi:10.1111/j.0022-1112.2004.00309.x [Google Scholar]
- Lochmiller R.L., Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. doi:10.1034/j.1600-0706.2000.880110.x [Google Scholar]
- Madsen T., Ujvari B. MHC class I variation associates with parasite resistance and longevity in tropical pythons. J. Evol. Biol. 2006;19:1973–1978. doi: 10.1111/j.1420-9101.2006.01158.x. doi:10.1111/j.1420-9101.2006.01158.x [DOI] [PubMed] [Google Scholar]
- Mays H.L.J., Hill G.E. Choosing mates: good genes versus genes that are a good fit. Trends Ecol. Evol. 2004;29:564–570. doi: 10.1016/j.tree.2004.07.018. [DOI] [PubMed] [Google Scholar]
- McClelland E.E., Penn D.J., Potts W.K. Major histocompatibility complex heterozygote superiority during coinfection. Infect. Immun. 2003;71:2079–2086. doi: 10.1128/IAI.71.4.2079-2086.2003. doi:10.1128/IAI.71.4.2079-2086.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan D.A., McPhail J.D. Experimental investigations of the evolutionary significance of sexually dimorphic nuptial coloration in Gasterosteus aculeatus (L)—the relationship between male color and female behavior. Can. J. Zool. 1990;68:482–492. doi:10.1139/z90-071 [Google Scholar]
- Milinski M. The function of mate choice in sticklebacks: optimizing MHC genetics. J. Fish Biol. 2003;63:1–16. doi:10.1111/j.1095-8649.2003.00215.x [Google Scholar]
- Milinski M. The major histocompatibility complex, sexual selection, and mate choice. Annu. Rev. Ecol. Evol. Syst. 2006;37:159–186. doi:10.1146/annurev.ecolsys.37.091305.110242 [Google Scholar]
- Milinski M., Bakker T.C.M. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature. 1990;344:330–333. doi:10.1038/344330a0 [Google Scholar]
- Milinski M., Bakker T.C.M. Costs influence sequential mate choice in sticklebacks, Gasterosteus aculeatus. Proc. R. Soc. B. 1992;250:229–233. doi:10.1098/rspb.1992.0153 [Google Scholar]
- Milinski M., Griffiths S., Wegner K.M., Reusch T.B.H., Haas-Assenbaum A., Boehm T. Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc. Natl Acad. Sci. USA. 2005;102:4414–4418. doi: 10.1073/pnas.0408264102. doi:10.1073/pnas.0408264102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller A.P., Erritzoe J. Host immune defence and migration in birds. Evol. Ecol. 1998;12:945–953. doi:10.1023/A:1006516222343 [Google Scholar]
- Morand S., Poulin R. Nematode parasite species richness and the evolution of spleen size in birds. Can. J. Zool. 2000;78:1356–1360. doi:10.1139/cjz-78-8-1356 [Google Scholar]
- Nowak M.A., Tarczyhornoch K., Austyn J.M. The optimal number of major histocompatibility complex-molecules in an individual. Proc. Natl Acad. Sci. USA. 1992;89:10896–10899. doi: 10.1073/pnas.89.22.10896. doi:10.1073/pnas.89.22.10896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottova E., Simkova A., Jurajda P., Davidova M., Ondrackova M., Pecinkova M., Gelnar M. Sexual ornamentation and parasite infection in males of common bream (Abramis brama): a reflection of immunocompetence status or simple cost of reproduction? Evol. Ecol. Res. 2005;7:581–593. [Google Scholar]
- Peichel C.L., Nereng K.S., Ohgi K.A., Cole B.L.E., Colosimo P.F., Buerkle C.A., Schluter D., Kingsley D.M. The genetic architecture of divergence between threespine stickleback species. Nature. 2001;414:901–905. doi: 10.1038/414901a. doi:10.1038/414901a [DOI] [PubMed] [Google Scholar]
- Penn D.J. The scent of genetic compatibility: sexual selection and the major histocompatibility complex. Ethology. 2002;108:1–21. doi:10.1046/j.1439-0310.2002.00768.x [Google Scholar]
- Penn D.J., Potts W.K. The evolution of mating preferences and major histocompatibility complex genes. Am. Nat. 1999;153:145–164. doi: 10.1086/303166. doi:10.1086/303166 [DOI] [PubMed] [Google Scholar]
- Penn D.J., Damjanovich K., Potts W.K. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl Acad. Sci. USA. 2002;99:11260–11264. doi: 10.1073/pnas.162006499. doi:10.1073/pnas.162006499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher T.E., Neff B.D. MHC class IIB alleles contribute to both additive and nonadditive genetic effects on survival in Chinook salmon. Mol. Ecol. 2006;15:2357–2365. doi: 10.1111/j.1365-294X.2006.02942.x. doi:10.1111/j.1365-294X.2006.02942.x [DOI] [PubMed] [Google Scholar]
- Reusch T., Langefors Ã. Inter- and intralocus recombination drive MHC class IIB gene diversification in a teleost, the three-spined stickleback Gasterosteus aculeatus. J. Mol. Evol. 2005;61:531–541. doi: 10.1007/s00239-004-0340-0. doi:10.1007/s00239-004-0340-0 [DOI] [PubMed] [Google Scholar]
- Reusch T.B.H., Haberli M.A., Aeschlimann P.B., Milinski M. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature. 2001;414:300–302. doi: 10.1038/35104547. doi:10.1038/35104547 [DOI] [PubMed] [Google Scholar]
- Rice W.R., Gaines S.D. ‘Heads I win, tails you lose’: testing directional alternative hypotheses in ecological and evolutionary research. Trends Ecol. Evol. 1994;9:235. doi: 10.1016/0169-5347(94)90258-5. doi:10.1016/0169-5347(94)90258-5 [DOI] [PubMed] [Google Scholar]
- Rico C., Kuhnlein U., Fitzgerald G.J. Male reproductive tactics in the threespine stickleback—an evaluation by DNA fingerprinting. Mol. Ecol. 1992;1:79–87. doi:10.1111/j.1365-294X.1992.tb00159.x [Google Scholar]
- Sakamoto Y., Ishiguro M., Kitagawa G. D. Reidel Publing Company; Tokyo: 1986. Akaike information criterion statistics. [Google Scholar]
- Scharsack J.P., Kalbe M., Harrod C., Rauch G. Habitat-specific adaptation of immune responses of stickleback (Gasterosteus aculeatus) lake and river ecotypes. Proc. R. Soc. B. 2007;274:1523–1532. doi: 10.1098/rspb.2007.0210. doi:10.1098/rspb.2007.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstein F., Folstad I., Liljedal S. Whether to reproduce or not: immune suppression and costs of parasites during reproduction in the Arctic charr. Can. J. Zool. 2001;79:271–278. doi:10.1139/cjz-79-2-271 [Google Scholar]
- Takahata N., Nei M. Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics. 1990;124:967–978. doi: 10.1093/genetics/124.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney J.F., Huntingford F.A., Crompton D.W.T. Body condition and reproductive status in sticklebacks exposed to a single wave of Schistocephalus solidus infection. J. Fish Biol. 1996;49:483–493. [Google Scholar]
- Wegner K.M., Kalbe M., Kurtz J., Reusch T.B.H., Milinski M. Parasite selection for immunogenetic optimality. Science. 2003a;301:1343. doi: 10.1126/science.1088293. doi:10.1126/science.1088293 [DOI] [PubMed] [Google Scholar]
- Wegner K.M., Reusch T.B.H., Kalbe M. Multiple parasites are driving major histocompatibility comple polymorphism in the wild. J. Evol. Biol. 2003b;16:224–232. doi: 10.1046/j.1420-9101.2003.00519.x. doi:10.1046/j.1420-9101.2003.00519.x [DOI] [PubMed] [Google Scholar]
- Wegner K.M., Kalbe M., Milinski M., Reusch T. Mortality selection during the 2003 European heat wave in three-spined sticklebacks: effects of parasites and MHC genotype. BMC Evol. Biol. 2008;8:124. doi: 10.1186/1471-2148-8-124. doi:10.1186/1471-2148-8-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelfing B., Traulsen A., Milinski M., Boehm T. Does intra-individual MHC diversity keep a golden mean? Phil. Trans. R. Soc. B. 2009;364:117–128. doi: 10.1098/rstb.2008.0174. doi:10.1098/rstb.2008.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton R.J. Academic Press; London, UK: 1976. The biology of the sticklebacks. [Google Scholar]
- Wootton R.J. Effect of food limitation during the breeding season on the size, body components and egg production of female sticklebacks (Gasterosteus aculeatus) J. Anim. Ecol. 1977;46:823. doi:10.2307/3643 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.