Abstract
Animals generally require a dietary supply of various nutrients (vitamins, essential amino acids, etc.) because their biosynthetic capabilities are limited. The capacity of aphids to use plant phloem sap, with low essential amino acid content, has been attributed to their symbiotic bacteria, Buchnera aphidicola, which can synthesize these nutrients; but this has not been demonstrated empirically. We demonstrate here that phloem sap obtained from the severed stylets of pea aphids Acyrthosiphon pisum feeding on Vicia faba plants generally provided inadequate amounts of at least one essential amino acid to support aphid growth. Complementary analyses using aphids reared on chemically defined diets with each amino acid individually omitted revealed that the capacity of the symbiotic bacterium B. aphidicola to synthesize essential amino acids exceeded the dietary deficit of all phloem amino acids except methionine. It is proposed that this shortfall of methionine was met by aphid usage of the non-protein amino acid 5-methylmethionine in the phloem sap. This study provides the first quantitative demonstration that bacterial symbiosis can meet the nutritional demand of plant-reared aphids. It shows how symbiosis with micro-organisms has enabled this group of animals to escape from the constraint of requiring a balanced dietary supply of amino acids.
Keywords: Acyrthosiphon pisum, essential amino acids, aphid, Buchnera aphidicola, phloem sap, symbiosis
1. Introduction
The central role of nitrogen in the nutritional ecology of animals is complicated by the fact that nitrogen sources vary in their nutritional value (Karasov & Martínez del Rio 2007). In particular, animals cannot synthesize de novo the carbon skeletons of some amino acids that contribute to protein; these are called essential amino acids. As a consequence of this metabolic limitation, an adequate supply of every essential amino acid is required for animal growth, irrespective of the concentration of all other amino acids. Some animals are, however, believed to have escaped from the metabolic requirement for a balanced dietary supply of essential amino acids by forming symbioses with symbiotic micro-organisms that both synthesize and provide them with essential amino acids (Moran 2007).
Plant phloem sap is one diet with a grossly unbalanced amino acid composition (e.g. Douglas 1993; Sandström & Moran 1999; Sandström 2000). It is used through the life cycle by members of just one group of animals: insects of the order Hemiptera (Douglas 2006). The phloem sap feeding habit has evolved multiple times among the Hemiptera and all phloem feeders studied bear symbiotic micro-organisms (Buchner 1965). Research on the nutritional contribution of the symbiosis in Hemiptera has focused largely on aphids, most of which bear the γ-proteobacterium Buchnera aphidicola. There is strong dietary, metabolic and genomic evidence that these bacteria provide their insect host with essential amino acids (e.g. Douglas 1988; Febvay et al. 1999; Shigenobu et al. 2000). It is assumed widely that the bacterial-derived essential amino acids complement the supply in phloem sap (Douglas 2003; Dale & Moran 2006), but this expectation has not been tested quantitatively. One reason for this caveat to our understanding is that most research on the role of Buchnera in aphid nutrition has been conducted on aphids reared on chemically defined diets, and little experimental consideration has been given to the relevance of these studies to plant-reared aphids. Nevertheless, the phloem sap of Tithonia fruticosa has been shown to be inadequate to meet the growth requirement of the aphid Uroleucon ambrosiae for seven essential amino acids (Bernays & Klein 2002), but the contribution of the symbiosis to the nutrition of this aphid was not investigated.
The purpose of this study was to quantify the demand of plant-reared aphids for Buchnera-derived essential amino acids and the extent to which Buchnera meets this demand. The experiments were conducted on the pea aphid Acyrthosiphon pisum, which has been the focus of many nutritional studies to date and is gaining additional value as an experimental system with the availability of the complete genome sequence (http://www.hgsc.bcm.tmc.edu/projects/aphid/). The broad bean Vicia faba was selected as rearing plant because it is used by all A. pisum genotypes and the total concentration of amino acids and essential amino acids in phloem sap is similar across V. faba and various other host plants of A. pisum (Sandström & Pettersson 1994).
2. Material and methods
(a) Experimental plants and aphids
Broad bean V. faba cv. The Sutton was raised from seed at 20°C and 18 L : 6 D at irradiance 200 μmol m−2 s−1 photosynthetically active radiation (PAR). The pea aphid A. pisum clone LL01 derived originally from an alfalfa crop in France was maintained under the same conditions on three-to four-week-old pre-flowering plants and on chemically defined diets of formulation A (Prosser & Douglas 1992) containing 0.15 M amino acids and 0.5 M sucrose. The diets contained all 20 protein amino acids (the standard diet) or one essential amino acid was omitted, such that the dietary amino acid concentration was 135.7–147.1 mM. Aphids for experiments were obtained by allowing plant-reared adult apterae to larviposit on plants or diets for 24 h. These larvae were termed one day old and they were used for experiments between day two (second larval stadium) and day seven (fourth larval stadium). To obtain aphids lacking Buchnera, adults were transferred to the standard diet with 50 μgrifampicin ml−1 and larvae they deposited were maintained on this diet to day two.
(b) Performance assays
Two-day-old aphids were weighed on a Mettler MT5 microbalance, caged individually on the test diets or in mesh cages clipped to the abaxial surface of plant leaves. All surviving aphids were reweighed on day seven, and their relative growth rate (RGR) was determined as: ln(day seven weight/day two weight)/time. The protein content of the aphids was determined by the Coomassie Brilliant Blue microassay method of BioRad, following manufacturer's instructions (http://www.bio-rad.com/cmc_upload/Literature/55685/4110065A.pdf, catalogue number 500-0201), with bovine serum albumin as standard (1.25–25 μg protein ml−1). Where the experimental design precluded killing aphids, the protein contents were obtained by calculation using empirically determined values of protein per unit weight: 60±1.5, 53±1.5 and 38±1.6 μg mg−1 (mean ±s.e., n=20) for day two aphids, day seven untreated aphids and day seven antibiotic-treated aphids, respectively. The s.e. of the calculated protein content was estimated as ((s.e./mean) of weight+(s.e./mean) of protein per unit weight)×mean calculated protein content. The contribution of individual amino acids to aphid protein growth was obtained from the product of protein growth (obtained in this study; see below) and percentage contribution of each amino acid to aphid protein (Douglas et al. 2001).
(c) Phloem sap analysis
Phloem sap samples of V. faba were collected by stylectomy of A. pisum using a procedure modified from Fisher & Frame (1984) and described fully in Douglas et al. (2006). Briefly, the stylets of a feeding aphid were cut with a platinum needle using a high frequency pulse from a microcautery unit (Syntech CA-50, Hilversum, The Netherlands). Sap exuding from the stylet stump into a pool of water-saturated light white mineral oil (Sigma) was collected into a sialinized microcapillary tube backfilled with mineral oil. The collections were made at 6–10 h after the onset of the light period. Subsamples of known volume were obtained with constriction pipettes, diluted to 10 μl distilled water and stored at −20°C prior to analysis.
Phloem amino acids were quantified by reverse-phase HPLC following derivatization with o-phthaldialdehyde (Jones et al. 1981) using a Hewlett-Packard HP1100 Series autosampling CL system with C18 ZORBAX, Eclipse XDB-C8 column and fluorescence detection. The amino acids were quantified by comparison to the AA-S-18 standard amino acids (Sigma) supplemented with tryptophan, asparagine and glutamine. Data are displayed for all protein amino acids apart from cysteine and proline, which cannot be detected by this method, and histidine, which could not be separated consistently from serine. The histidine : serine in V. faba phloem sap is less than 1 : 10 (Sandström & Pettersson 1994; R. Sturmey & A. E. Douglas 2007, unpublished data) and the serine content in the few unresolved samples was, therefore, over-estimated by up to 10 per cent. The serine supply in the phloem sap was found to be greatly in excess of aphid demand (see §3, including electronic supplementary material 1), and so the error of less than 10% had no impact on the conclusions.
The volume of phloem sap ingested by two- to seven-day-old aphids reared on plants was estimated from the volume of honeydew produced. Replicate groups of five aphids were enclosed in a 0.5 cm perspex ring that was clipped to the underside of a leaf positioned above a 6 cm Petri dish containing water-saturated paraffin oil. The honeydew droplets produced by the aphids sank to the bottom of the oil. For 20 replicates in which no aphids vacated the ring, all the honeydew droplets in each dish of paraffin oil were collected into a graduated microcapillary tube (with a small volume of oil on either side of the honeydew to prevent evaporation), enabling the total honeydew volume to be determined.
3. Results
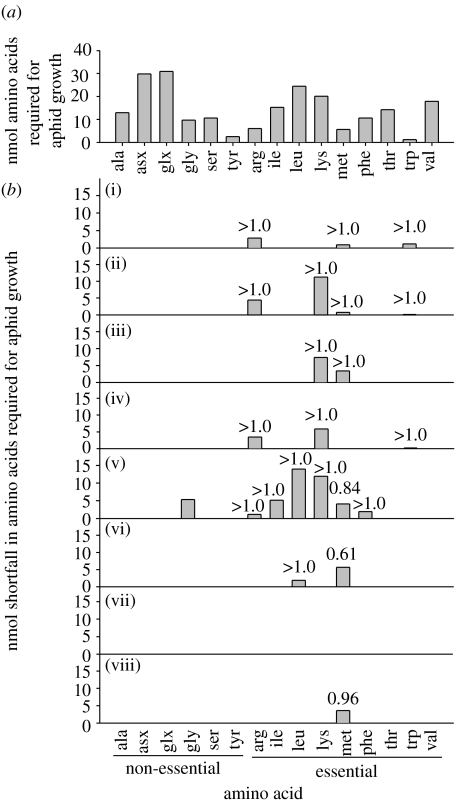
When two-day-old larvae were reared on the host plant V. faba, they increased in weight from 0.195±0.008 mg to 0.792±0.020 mg at seven days (mean ±s.e., n=15), during which period each aphid produced 5.2±0.37 μl honeydew (mean ±s.e., n=20). The mean protein contents of these aphids were 11.7±0.76 and 42.0±1.89 μg, respectively, indicating that the aphids had grown by 30.3 μg protein, equivalent to 288 nmol N. Figure 1a shows the mean contribution of each amino acid to the total aphid protein growth.
Figure 1.
Amino acid budget of pea aphids reared on V. faba. (a) Contribution of individual amino acids to protein growth of two- to seven-day-old aphids. (b) Shortfall of essential amino acids required for aphid growth in phloem sap of eight plants (i)–(viii). The total amino acid concentration of each phloem sample is: (i) 169 mM; (ii) 204 mM; (iii) 207 mM; (iv) 273 mM; (v) 280 mM; (vi) 327 mM; (vii) 460 mM; (viii) 490 mM. The proportion of the shortfall met by Buchnera-derived amino acids is shown above each bar; note that glycine is a non-essential amino acid and any shortfall can be met by aphid metabolism (ala, alanine; asx, aspartic acid and asparagine; glx, glutamic acid and glutamine; gly, glycine; ser, serine; tyr, tyrosine; arg, arginine; ile, isoleucine; leu, leucine; lys, lysine; met, methionine; phe, phenylalanine; thr, threonine; trp, tryptophan; val, valine).
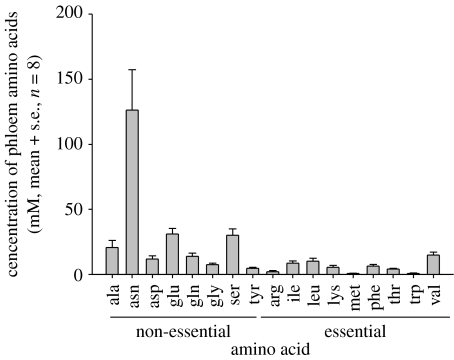
The phloem sap exuding from severed stylets of feeding aphids contained 299±41 mM amino acids (mean ±s.e., n=8). The amino acid composition is shown in figure 2. The dominant amino acid was the non-essential asparagine, at 126±31 mM, accounting for 42 per cent of the total amino acids. The essential amino acids had a total concentration of 52.3±7.26 mM (17.5% of the total). These values are comparable to published values, e.g. 46.8 mM (20.3%) of 230.5 mM total amino acids in V. faba phloem sap analysed by Sandström & Pettersson (1994).
Figure 2.
Mean amino acid composition of V. faba phloem sap (abbreviations for amino acids are provided in the legend to figure 1).
From the mean amino acid content of phloem sap and volume of aphid honeydew produced, the mean total amino acid nitrogen ingested by each two- to seven-day-old aphid was calculated as 2322 nmol. This was eight times greater than the amount required for the observed protein growth (288 nmol) of the aphids, indicating that the diet supplied sufficient nitrogen for aphid growth. However, the phloem sap ingested did not meet the aphid growth requirement for up to seven amino acids, varying among the plants tested (figure 1b; electronic supplementary material 1). Six out of the eight plants had insufficient methionine to meet the aphid requirements, and the supply of just two essential amino acids, threonine and valine, was adequate in every phloem sample. The plant supply of all non-essential amino acids was in excess of aphid demand, apart from glycine in one phloem sample.
To explore the bacterial contribution to the aphid amino acid requirements directly, the biosynthetic capacity of Buchnera was determined. This requires that the composition of the food ingested is both known precisely and could be manipulated, which is feasible for aphids reared on chemically defined diets but not on plants. Extensive research over several decades indicates that nutritional data for diet-reared aphids are broadly representative of aphids on plants (Douglas 2003). The aphids studied here could be maintained readily through one generation on the standard diet (containing all 20 protein amino acids), and the larval RGR on this diet, at 0.276±0.009 mg mg−1 d−1 (n=17), did not differ significantly from that on plants at 0.281±0.004 mg mg−1 d−1 (n=15; t22=0.05, p>0.05). These data give added confidence in the use of diet-reared aphids to quantify essential amino acid production by Buchnera.
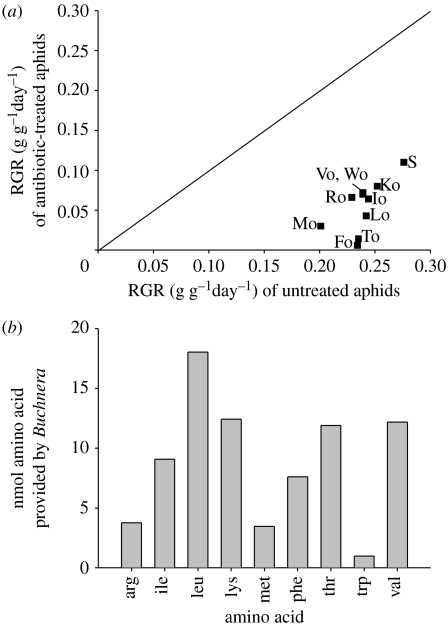
Figure 3a shows the mean RGR of 20 replicate aphids reared on diets from which each of the essential amino acids had been omitted individually. The antibiotic treatment that eliminated Buchnera significantly depressed the RGR (as demonstrated in many previous studies; see reviews of Wilkinson (1998) and Douglas (2003)), to an extent that varied with dietary amino acid composition (statistical analysis shown in legend to figure 3a).
Figure 3.
The pea aphid on chemically defined diets. (a) Mean RGR of two- to seven-day-old aphids on the standard diet containing all protein amino acids (S) and diets from which one essential amino acid was deleted (Fo, Io, etc). ANOVA: diet: F10386=5.20, 0.01>p>0.001; antibiotic treatment: F1386=1219, p<0.001; interaction: F10386=2.77, 0.01>p>0.001. The error terms are not shown for clarity and are provided in electronic supplementary material 3. (Fo, phenylalanine; Io, isoleucine; Ko, lysine; Lo, leucine; Mo, methionine; Ro, arginine; To, threonine; Vo, valine; Wo, tryptophan). (b) Contribution of Buchnera-derived amino acids to the protein growth of the aphids (abbreviations for amino acids are provided in the legend to figure 1).
The capacity of Buchnera to provide each essential amino acid to the aphids was estimated from the aphid protein growth that could be attributed to the symbiosis on the diet lacking that amino acid. This was obtained by subtracting the protein growth of antibiotic-treated aphids from that of untreated aphids (see electronic supplementary material 2). The amount varied across the amino acids, from 18 nmol for leucine to 0.98 nmol for tryptophan (figure 3b; electronic supplementary material 2). From these data, the extent to which the bacteria met aphid demand for Buchnera-derived essential amino acids on each of the eight V. faba plants was estimated. The aphids had an apparent net deficit of methionine on three plants (nos. v, vi and viii) but the remaining 18 (86%) out of 21 instances of insufficient supply of an essential amino acid in the phloem sap were met by the biosynthetic capacity of Buchnera (figure 1b).
4. Discussion
The key results from this study of the pea aphid reared on its host plant V. faba are that the supply of essential amino acids in phloem sap is generally inadequate to support the observed aphid growth and that this shortfall can be met largely by the essential amino acid biosynthetic capability of the symbiotic bacteria Buchnera (figure 1). The analysis provides an empirical demonstration of the long-held but previously untested belief that Buchnera enables aphids to subsist on a diet of plant phloem sap.
Nevertheless, the analysis is founded on certain simplifying assumptions. In one respect, it tends to underestimate the aphid demand for Buchnera amino acids. The calculations assume that phloem amino acids are assimilated across the aphid gut wall and incorporated into protein with 100 per cent efficiency. Empirical values of the amino acid assimilation efficiency of aphids are generally in excess of 80 per cent and the conversion efficiency to protein varies among amino acids, which are used to variable extents as a respiratory fuel and in other roles, e.g. neurotransmitters and pigments derived from tryptophan (Prosser et al. 1992; Febvay et al. 1999; Douglas et al. 2001; Wilkinson et al. 2001). A second simplifying assumption has the opposite effect of overestimating aphid demand. It is that aphids derive phloem essential amino acids exclusively from free amino acids. Phloem sap contains other potential sources of amino acids, including proteins, small peptides and non-protein amino acids. For most essential amino acids, the significance of these alternative sources of amino acids is probably small. The total concentration of phloem proteins in most plants is low, at 0.1–2 mg ml−1 (Douglas, 2003; Kehr 2006), which is equivalent to 0.2–5 per cent of the mean free amino acids quantified in V. faba phloem sap (figure 2). The extent to which aphids can use these nitrogen sources is uncertain. Although peptidases are expressed in aphid guts (Leckstein & Llewellyn 1974; Cristofoletti et al. 2006) and presumably released into the gut lumen, pea aphids appear to have only limited capacity to degrade ingested protein (Rahbé et al. 1995). Among non-protein amino acids, S-methylmethionine attains appreciable concentrations in the phloem sap of various plants including the lupin (Bourgis et al. 1999), a species related to V. faba used in this study; and it is a potential source of methionine for aphids. A candidate enzyme catalysing the recovery of methionine from S-methylmethionine is homocysteine S-methyltransferase (HMT). Although Buchnera probably lacks this capability (it has no homologue to the E. coli HMT, mmuM (=yagD; Thanbichler et al. 1999), a putative pea aphid HMT is represented by the EST cluster APD5842 (www.aphidests.org) with significant homology (E value 2e−50) to HMT of Drosophila melanogaster (CG10621). We hypothesize that 5-methylmethionine contributes to the methionine requirements of aphids, including, for example, the pea aphids feeding on V. faba with phloem profiles as represented by plants nos. v, vi and viii in figure 1b.
Pertinent to these considerations of the amino acid nutrition of aphids is the variability of amino acid supply in phloem sap. There is a substantial body of evidence for variation in phloem amino acid content and composition among plant species and with the developmental age of the plant, season and abiotic factors including temperature, water stress and irradiance (e.g. Sandström & Pettersson 1994; Girousse et al. 1996; Corbesier et al. 2001; Karley et al. 2002). Compounding this variation, the amino acid profile of phloem sap also varies at the fine scale of the individual sieve elements, as is highlighted by the threefold variation in total phloem amino acid content among the uniformly aged plants reared under tightly controlled conditions in this study (figure 1b), and the fourfold variation in total amino acid concentration among sieve elements from a single wheat plant studied by Gattolin et al. (2008). We can be confident that aphids use this range of sieve elements because the phloem samples are collected by stylectomy of feeding aphids (see §2), and an individual pea aphid can feed from a single sieve element of V. faba for many hours (Wilkinson & Douglas 1998). The crucial implication of the variation in phloem amino acids is that the aphid demand for Buchnera-derived essential amino acids is variable. Studies of diet-reared aphids indicate that the bacterial supply of essential amino acids varies according to that demand (Febvay et al. 1999). An important topic for future research is the mechanisms by which the profile of amino acids released from Buchnera cells is controlled and integrated into the wider regulation of the amino acid nutrition of the insect host, in the context of the variable dietary supply of these essential nutrients.
This study is also relevant to the intra- and interspecific variation in the nutritional ecology of aphids. In particular, aphid genotypes vary in their dietary requirement for essential amino acids (e.g. Srivastava et al. 1985; Wilkinson & Douglas 2003), indicative of variation in the supply of these nutrients from the symbiotic bacteria Buchnera. The methods described here to quantify the contribution of Buchnera to the nutritional demand of plant-reared aphids provide the basis to explore the impact of Buchnera variation on the plant usage traits of different aphids. This approach can also be applied more widely to investigate the nutritional contribution of symbiotic micro-organisms to a range of insect groups feeding on plant sap and other nutritionally inadequate diets.
Acknowledgments
We thank Kelly Pescod and Roger Sturmey for their assistance with the analysis of phloem amino acids and Lyn Minto for technical support. This research was conducted while AED was in receipt of a BBSRC Research Development Fellowship (grant no. BB/C520898).
Supplementary Material
Contribution of phloem amino acids to aphid requirement for growth
Contribution of Buchnera-derived amino acids to aphid requirement for growth
Relative growth rate (RGR) of untreated and antibiotic-treated aphids
References
- Bernays E.A., Klein B.A. Quantifying the symbiont contribution to essential amino acids in aphids: the importance of tryptophan for Uroleucon ambrosiae. Physiol. Entomol. 2002;27:275–284. doi:10.1046/j.1365-3032.2002.00297 [Google Scholar]
- Bourgis F., et al. S-methylmethionine plays a major role in phloem sulfur transport and is synthesised by a novel type of methyltransferase. Plant Cell. 1999;11:1485–1497. doi: 10.1105/tpc.11.8.1485. doi:10.2307/3870977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner P. John Wiley; Chichester, UK: 1965. Endosymbioses of animals with plant microorganisms. [Google Scholar]
- Corbesier L., Havelange A., Lejeune P., Bernier G., Périlleux C. N content of phloem and xylem exudates during transition to flowering in Sinapis alba and Arabidopsis thaliana. Plant Cell Environ. 2001;24:367–375. doi:10.1186/1471-2229-3-2 [Google Scholar]
- Cristofoletti P.T., de Sousa F.A.M., Rahbe Y., Terra W.R. Characterization of a membrane-bound aminopeptidase purified from Acyrthosiphon pisum midgut cells. FEBS J. 2006;273:5574–5588. doi: 10.1111/j.1742-4658.2006.05547.x. doi:10.1111/j.1742-4658.2006.05547.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale C., Moran N.A. Molecular interactions between bacterial symbionts and their hosts. Cell. 2006;126:453–465. doi: 10.1016/j.cell.2006.07.014. doi:10.1016/j.cell.2006.07.014 [DOI] [PubMed] [Google Scholar]
- Douglas A.E. Sulfate utilization in an aphid symbiosis. Insect Biochem. 1988;18:599–605. doi:10.1016/0020-1790(88)90012-1 [Google Scholar]
- Douglas A.E. The nutritional quality of phloem sap utilized by natural aphid populations. Ecol. Entomol. 1993;18:31–38. [Google Scholar]
- Douglas A.E. Nutritional physiology of aphids. Adv. Insect Physiol. 2003;31:73–140. doi:10.1016/S0065-2806(03)31002-1 [Google Scholar]
- Douglas A.E. Phloem sap feeding by animals: problems and solutions. J. Exp. Bot. 2006;57:747–754. doi: 10.1093/jxb/erj067. doi:10.1093/jxb/erj067 [DOI] [PubMed] [Google Scholar]
- Douglas A.E., Minto L.B., Wilkinson T.L. Quantifying nutrient production by the microbial symbiosis in an aphid. J. Exp. Biol. 2001;204:349–358. doi: 10.1242/jeb.204.2.349. [DOI] [PubMed] [Google Scholar]
- Douglas A.E., Price D.R.G., Minto L.B., Jones E., Pescod K.V., François C.L.M.J., Pritchard J., Boonham N. Sweet problems: insect traits defining the limits to dietary sugar utilisation by the pea aphid, Acyrthosiphon pisum. J. Exp. Biol. 2006;209:1395–1403. doi: 10.1242/jeb.02148. doi:10.1242/jeb.02148 [DOI] [PubMed] [Google Scholar]
- Febvay G., Rahbe Y., Rynkiewicz M., Guillard J., Bonnot G. Fate of dietary sucrose and neosynthesis of amino acids in the pea aphid, Acyrthosiphon pisum, reared on different diets. J. Exp. Biol. 1999;19:2639–2652. doi: 10.1242/jeb.202.19.2639. [DOI] [PubMed] [Google Scholar]
- Fisher D.B., Frame J.M. A guide to the use of the exuding stylet technique in phloem physiology. Planta. 1984;161:385–393. doi: 10.1007/BF00394567. doi:10.1007/BF00394567 [DOI] [PubMed] [Google Scholar]
- Gattolin S., Newbury H.J., Bale J.S., Tseng H.M., Barrett D.A., Pritchard J. A diurnal component to the variation in sieve tube aminoacid content in wheat (Triticum aestivum L.) Plant Physiol. 2008;147:912–921. doi: 10.1104/pp.108.116079. doi:10.1104/pp.108.116079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girousse C., Bournoville R., Bonnemain J.L. Water deficit-induced changes in concentrations of proline and some other amino acids in the phloem sap of alfalfa. Plant Physiol. 1996;111:109–113. doi: 10.1104/pp.111.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.N., Pääbo S., Stein S. Amino acid analysis and enzymatic sequence determination of peptides by an improved o-phthaldialdehyde precolumn labelling procedure. J. Liq. Chromatogr. 1981;4:565–586. doi:10.1080/01483918108059956 [Google Scholar]
- Karasov W.H., Martínez del Rio C. Princeton University Press; Princeton, NJ: 2007. Physiological ecology. [Google Scholar]
- Karley A.J., Douglas A.E., Parker W.E. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J. Exp. Biol. 2002;205:3009–3018. doi: 10.1242/jeb.205.19.3009. [DOI] [PubMed] [Google Scholar]
- Kehr J. Phloem sap proteins: their identities and potential roles in the interaction between plants and phloem-feeding insects. J. Exp. Bot. 2006;57:767–774. doi: 10.1093/jxb/erj087. doi:10.1093/jxb/erj087 [DOI] [PubMed] [Google Scholar]
- Leckstein P.M., Llewellyn M. Role of amino acids in diet intake and selection and utilization of dipeptides by Aphis fabae. J. Insect Physiol. 1974;20:877–885. doi: 10.1016/0022-1910(74)90177-2. doi:10.1016/0022-1910(74)90177-2 [DOI] [PubMed] [Google Scholar]
- Moran N.A. Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl Acad. Sci. USA. 2007;104:8627–8633. doi: 10.1073/pnas.0611659104. doi:10.1073/pnas.0611659104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser W.A., Douglas A.E. A test of the hypotheses that nitrogen is upgraded and recycled in an aphid (Acyrthosiphon pisum) symbiosis. J. Insect Physiol. 1992;38:93–99. doi:10.1016/0022-1910%2892%2990037 [Google Scholar]
- Prosser W.A., Simpson S.J., Douglas A.E. How an aphid (Acyrthosiphon pisum) symbiosis responds to variation in dietary nitrogen. J. Insect Physiol. 1992;38:301–307. doi:10.1016/0022-1910%2892%2990130-6 [Google Scholar]
- Rahbé Y., Sauvion N., Febvay G., Peumans W.J., Gatehouse A.M.R. Toxicity of lectins in symbiotic and aposymbiotic Acyrthosiphon pisum. Entomol. Exp. Appl. 1995;76:143–155. doi:10.1007/BF02383212 [Google Scholar]
- Sandström J. Nutritional quality of phloem sap in relation to host plant-alternation in the bird cherry-oat aphid. Chemoecology. 2000;10:17–24. doi:10.1007/s000490050003 [Google Scholar]
- Sandström J., Moran N.A. How nutritionally imbalanced is phloem sap for aphids? Entomol. Exp. Appl. 1999;91:203–210. doi:10.1023/A:1003605508487 [Google Scholar]
- Sandström J., Pettersson J. Amino acid composition of phloem sap and the relation to intraspecific variation in pea aphid (Acyrthosiphon pisum) performance. J. Insect Physiol. 1994;40:947–955. doi:10.1016/0022-1910(94)90133-3 [Google Scholar]
- Shigenobu S., Watanabe H., Hattori M., Sakaki Y., Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp APS. Nature. 2000;407:81–86. doi: 10.1038/35024074. doi:10.1038/35024074 [DOI] [PubMed] [Google Scholar]
- Srivastava P.N., Gao Y., Levesque J., Auclair J.L. Differences in amino acid requirements between two biotypes of the pea aphid, Acyrthosiphon pisum. Can. J. Zool. 1985;63:603–606. [Google Scholar]
- Thanbichler M., Neuhierl B., Böck A. S-methylmethionine metabolism in Escherichia coli. J. Bacteriol. 1999;181:662–665. doi: 10.1128/jb.181.2.662-665.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson T.L. The elimination of intracellular microorganisms from insects: an analysis of antibiotic-treatment in the pea aphid (Acyrthosiphon pisum) Comp. Biochem. Physiol. 1998;119:871–881. doi:10.1016/S1095-6433(98)00013-0 [Google Scholar]
- Wilkinson T.L., Douglas A.E. Plant penetration by pea aphids (Acyrthosiphon pisum) of different plant range. Entomol. Exp. Appl. 1998;87:43–50. doi:10.1023/A:1003255713767 [Google Scholar]
- Wilkinson T.L., Douglas A.E. Phloem amino acids and the host plant range of the polyphagous aphid. Aphis fabae. Ent. Exp. Appl. 2003;106:1–11. [Google Scholar]
- Wilkinson T.L., Minto L.B., Douglas A.E. Amino acids as respiratory substrates in aphids: an analysis of Aphis fabae reared on plants and diets. Physiol. Entomol. 2001;26:225–228. doi:10.1046/j.0307-6962.2001.00238 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contribution of phloem amino acids to aphid requirement for growth
Contribution of Buchnera-derived amino acids to aphid requirement for growth
Relative growth rate (RGR) of untreated and antibiotic-treated aphids