Abstract
Worldwide agriculture is one of the main drivers of biodiversity decline. Effective conservation strategies depend on the type of relationship between biodiversity and land-use intensity, but to date the shape of this relationship is unknown. We linked plant species richness with nitrogen (N) input as an indicator of land-use intensity on 130 grasslands and 141 arable fields in six European countries. Using Poisson regression, we found that plant species richness was significantly negatively related to N input on both field types after the effects of confounding environmental factors had been accounted for. Subsequent analyses showed that exponentially declining relationships provided a better fit than linear or unimodal relationships and that this was largely the result of the response of rare species (relative cover less than 1%). Our results indicate that conservation benefits are disproportionally more costly on high-intensity than on low-intensity farmland. For example, reducing N inputs from 75 to 0 and 400 to 60 kg ha−1 yr−1 resulted in about the same estimated species gain for arable plants. Conservation initiatives are most (cost-)effective if they are preferentially implemented in extensively farmed areas that still support high levels of biodiversity.
Keywords: agriculture, conservation ecology, farmland wildlife, fertilizer, plant species richness, policy implications
1. Introduction
Agricultural intensification has allowed mankind to feed the growing world population but is also one of the main drivers of worldwide biodiversity decline (e.g. Donald et al. 2001; Benton et al. 2002; Kerr & Cihlar 2004; Green et al. 2005). Extinction rates are now 100–1000 times the geological background rate and are predicted to increase 10-fold (Pimm et al. 1995). In response, nature reserves or protected areas are being created worldwide with the objective to protect species and ecosystems from harmful activities of mankind (e.g. Mittermeier et al. 2003). Furthermore, farmers are increasingly being stimulated to conserve biodiversity through the maintenance of extensive farming systems, the preservation of (semi-)natural landscape features or the extensification of intensive farming systems (Pain & Pienkowski 1997; Kleijn & Sutherland 2003; OECD 2003; American Farmland Trust 2006).
Biodiversity conservation in the wider countryside is also being advocated for pragmatic reasons. Mankind benefits from a range of services provided by nature (Daily 1997). Agriculture may benefit, for example, from services provided by wild organisms such as pollinators or natural enemies of crop pests (e.g. Rickets et al. 2004; Thies et al. 2005). Recent studies examining the provision of pollination services provided by wild bees suggest that more diverse communities of pollinators may provide more efficient pollination services and economic benefits (e.g. Kremen et al. 2002; Klein et al. 2003a,b; Albrecht et al. 2007), and that the provision of these pollination services declines with increasing land-use intensity (e.g. Kremen et al. 2002; Morandin & Winston 2006; Klein et al. 2007).
While there is ample evidence that biodiversity and related services decline with agricultural intensification, surprisingly little is known about how biodiversity declines with increasing land-use intensity (Kleijn & Sutherland 2003; Green et al. 2005; Mattison & Norris 2005). Knowing the shape of the relationship between biodiversity and land-use intensity is important for effective biodiversity conservation. If biodiversity declines linearly with land-use intensity, conservation initiatives are potentially equally effective (though not necessarily efficient) along the land-use gradient. Exponentially declining relationships would suggest that conservation efforts on farmland are more effective in extensively used farmland (Kleijn & Sutherland 2003) or even that it is more efficient to segregate conservation from farming (e.g. Green et al. 2005). Unimodal relationships, often observed in studies linking plant species richness to vegetation productivity (Grace 1999), would suggest that conservation initiatives are most effective in intermediate parts of the land-use intensity range.
Biodiversity is affected by land use not only at the small spatial scale (e.g. by the use of agrochemicals, grazing intensity, crop rotation), but also at larger spatial scales (e.g. by habitat fragmentation, hydrological changes or atmospheric deposition). Because significant differences in land-use intensity can only be found between countries or geographically distinct regions (MacDonald et al. 2000; Donald et al. 2001), they are invariably linked to differences in environmental conditions. Studies examining effects of land-use intensity in situ have to account for such differences and will be prone to high levels of environmental noise and low explanatory power of the predictor variables. Nevertheless, such studies are essential as they incorporate the entire array of changes in land use associated with agricultural intensification that would be left out with experimental approaches.
This study uses a dataset linking biodiversity with data on agricultural management on 271 agricultural fields in six European countries. Biodiversity was estimated as species richness of vascular plants and land-use intensity was characterized by nitrogen inputs. The nature of the relationship between nitrogen input and plant species richness was examined separately for grassland fields (130 fields in three countries) and arable fields (141 fields in four countries). First, we tested whether nitrogen input had significant effects on plant species richness across Europe after effects of confounding climatic, geographical and landscape factors had been accounted for. Second, we tested whether curvilinear relationships described the relationship between plant species richness and nitrogen input more adequately than linear relationships. We discuss these findings in the context of optimizing the effectiveness of efforts to conserve biodiversity and related ecosystem services on agricultural lands.
2. Material and methods
(a) Data collection
Biodiversity was estimated as plant species richness. In 2003, plant species composition was surveyed on arable fields in Germany (cereal fields), Spain (cereal fields) and the United Kingdom (mostly cereals), and on grassland fields in Hungary, Switzerland and The Netherlands. In each country, 14 fields were selected in each of three distinct areas giving a total of 252 fields. In each country, half of the fields were managed less intensively owing to the implementation of agri-environment schemes (except for Hungary; see Báldi et al. 2005 and Kleijn et al. 2006), thus increasing the range in land-use intensities sampled in each country. In 2005, an additional 31 fields were surveyed, five cereal fields in Spain, five grassland fields in the Netherlands and 21 cereal fields in Hungary. Cover of each species of vascular plant was estimated in 20 plots of 5×1 m. Ten plots, spaced 5 m apart, were located in and parallel to the field edge, ten more were located similarly in the field centre. Subsequently, relative cover per species and the total number of plant species (i.e. species richness per 100 m2) were determined for each field.
Land-use intensity is the quantity of output obtained per unit of land or time (Turner & Doolittle 1978). However, often it is used more loosely to refer to the state of agricultural industrialization and characterized by, for example, the level of mechanization or specialization or the amount of inputs used (Donald et al. 2001; Herzog et al. 2006). In this paper, land-use intensity is used in the latter, more general sense, because management (e.g. N input level, pesticide application rate, cutting frequency) is more likely to be related to biodiversity than yield per se. We used annual nitrogen input per site in kg N ha−1 yr−1 (referred to as N input) as an indicator of land-use intensity. Fertilizer use is generally correlated with yield and other variables characterizing farming (e.g. pesticide use, agricultural population density, cattle density; Donald et al. 2001) and is a key indicator used to analyse trends in farming intensity and potential impacts on the environment (European Commission 2000; Herzog et al. 2006). Nitrogen input was calculated from applications of fertilizers, as well as from inputs of atmospheric deposition (EMEP Unified model revision 1.7; EMEP 2005), as in western Europe atmospheric deposition is substantial, largely consists of ammonia (Holland et al. 2005) and agriculture produces approximately 90 per cent of the total emission of ammonia to the atmosphere (Misselbrook et al. 2000). Furthermore, N emission increases with increasing use of N in agriculture (Misselbrook et al. 2000) and most of these emissions are deposited within kilometres from the source. Atmospheric N deposition may therefore be considered as a component of land-use intensity. Data on N fertilizers applied to the study sites were obtained from farmers using questionnaires and were assumed to be typical of site management. Nitrogen input from various types of organic fertilizers was calculated using local tables of N contents.
(b) Controlling for confounding variables
Our data were from geographically distinct areas and differences in land-use intensity were correlated with differences in other factors that may affect biodiversity. To make sure that the pattern in plant species richness was due to land-use intensity, rather than some confounding factor, we included a number of biodiversity-related environmental variables (e.g. Hawkins et al. 2003; Hillebrand 2004; Herzog et al. 2005; Tscharntke et al. 2005) in our statistical models: latitude (in decimal degrees), altitude (m above sea level), precipitation (average annual rainfall, in mm, in the months April–September for the years 1961–1990; New et al. 2002), temperature (average annual temperature, in °C, for the months April–September for the years 1961–1990; New et al. 2002) and landscape diversity. Landscape diversity was calculated as the Shannon index of diversity of six habitat types (grasslands including fallows; arable fields; buildings including farmyards; forests; ditches, streams and rivers; and ‘other habitats’ consisting of a number of habitats that were typical at the national but not at the international level) located in a circular buffer of 500 m around the centre of each field. The effects of environmental variables on plant species richness will not be discussed in detail as they were solely included in the analyses to correct for possible confounding effects.
(c) Statistical analysis
Fields for which data of one or more of the explanatory variables were missing were excluded from the analyses, resulting in a total of 271 fields with complete datasets. To better understand the observed patterns in plant species richness, we also examined the response of species occupying different dominance classes: species with a relative cover of less than 1 per cent (referred to as rare species) and species with relative cover between 1 and 10 per cent (subdominants). Species with relative cover of more than 10 per cent (dominants) ranged in species richness from 0 to only 5 and were therefore not analysed. Relationships were analysed separately for arable fields (n=141) and grasslands (n=130).
We used Poisson regression to relate plant species richness to the confounding variables and to N input. Overdispersion in total, rare and subdominant species was accounted for by inflating the Poisson variance by an unknown factor and then using quasi-likelihood to estimate the parameters (McCullagh & Nelder 1989). We first tested whether all confounding variables should enter the model. To this end, all possible subsets of variables, including N input, were fitted employing a log link. This revealed that the effects of altitude, precipitation and landscape diversity were rarely significant and these variables were therefore excluded from the model. Consequently, latitude and temperature were used as correcting variables in all subsequent analyses. An alternative line of attack is to select the best model using the quasi-likelihood Akaike information criterion (QAIC; Burnham & Anderson 1998). This approach gave similar results which will not be presented here. In the second step, the relationship with N input was explored. We used Poisson regression with an identity link because we were specifically interested in the question of whether biodiversity is linearly related to land-use intensity. Poisson regression with a log link would not have allowed us to examine the fit of models with a linear relationship between species richness and N input. The linear model was compared with an exponential (brN) and a critical exponential model ((b+cN) rN, encompassing skewed unimodal relationships), all employing the identity link. In addition, a generalized additive model (GAM; Hastie & Tibshirani 1990) was used to model the relationship, now employing the conventional log link. With GAM's, the shape of the curve is dictated by the data alone. It may, therefore, serve as a check to examine whether the shape of the curve was affected by the selection of the parametric models used. The flexibility of a GAM can be expressed by its degrees of freedom, with flexible curves having more degrees of freedom. Forward significance testing at the 5 per cent level was used to examine whether adding a degree of freedom improved the fit of the GAM. All models were fitted using standard facilities in GenStat (Payne et al. 2002). The models were compared using QAIC.
4. Results
(a) Does N input significantly affect plant species richness?
Total plant species richness ranged from 19 to 94 species on grasslands and 9 to 94 species per 100 m2 on arable fields. For grassland species, none of the correcting variables contributed significantly to the fit of the model but N input was significantly negatively related to species richness (table 1). For arable species, differences in latitude and temperature explained a significant part of the variation (table 1). After effects of these variables had been accounted for, plant species richness was again highly significantly and adversely related to N input.
Table 1.
Results of Poisson regression models relating the species richness of rare, subdominant and all plant species on grasslands and arable fields to N input. (In all models, confounding effects of environmental variables were corrected for by including the variables latitude and temperature. Comparison of models with all possible subsets of variables have shown that altitude, precipitation and landscape diversity had negligible additional effects on species richness and were therefore not included. Test statistics are t values for variables and deviance ratios for models.)
| estimate | test statistic | p-values | |
|---|---|---|---|
| grasslands (n=130) | |||
| all species | |||
| latitude | −0.0216 | −1.46 | 0.148 |
| temperature | −0.0225 | −1.79 | 0.076 |
| annual nitrogen input | −0.0012 | −3.40 | <0.001 |
| model | 11.19 | <0.001 | |
| rare species | |||
| latitude | −0.0137 | −0.74 | 0.459 |
| temperature | 0.0060 | 0.38 | 0.701 |
| annual nitrogen input | −0.0015 | −3.88 | <0.001 |
| model | 11.58 | <0.001 | |
| subdominant species | |||
| latitude | −0.0425 | −2.55 | 0.012 |
| temperature | −0.1110 | −7.75 | <0.001 |
| annual nitrogen input | −0.0009 | −2.58 | 0.011 |
| model | 23.20 | <.001 | |
| arable fields (n=141) | |||
| all species | |||
| latitude | −0.1015 | −6.93 | <.001 |
| temperature | −0.1312 | −4.82 | <.001 |
| annual nitrogen input | −0.0015 | −4.77 | <.001 |
| model | 46.50 | <.001 | |
| rare species | |||
| latitude | −0.1036 | −5.45 | <.001 |
| temperature | −0.1166 | −3.29 | 0.001 |
| annual nitrogen input | −0.0017 | −4.00 | <.001 |
| model | 38.00 | <.001 | |
| subdominant species | |||
| latitude | −0.1206 | −5.69 | <.001 |
| temperature | −0.1956 | −4.96 | <.001 |
| annual nitrogen input | −0.0016 | −3.58 | <.001 |
| model | 19.42 | <.001 | |
Rare species (less than 1% cover) made up the large majority of the total number of species with on average 70 per cent (range 9–74 species per 100 m2) and 65 per cent (0–73 species) on grasslands and arable fields. On average, grassland subdominants made up 25 per cent (2–25 species) and arable subdominants 29 per cent (1–35 species) of the total number of species. The negative effects of N input on total plant species richness were mirrored in both the rare and the subdominant species (table 1). Effects of correcting variables were highly significant for rare and subdominant plants of the geographically more widely distributed arable fields and for grassland subdominants but not for rare grassland species.
(b) What function describes the species richness–N input relationship best?
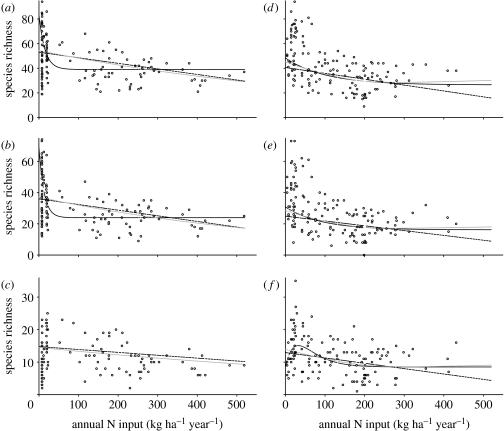
For total species richness and rare species richness of both grassland and arable plants the best-fitting parametric model used an exponential function to describe the relationship between N input and species richness (table 2, figure 1). All had a QAIC that was at least 4.2 lower than the model using a linear function. On grasslands, the most pronounced decline occurred below 30 kg N ha−1 yr−1, with hardly any decline beyond 35 kg N ha−1 yr−1. On arable fields, the decline was less rapid and was most pronounced below 100 kg N ha− 1 yr−1 with little change beyond 150 kg N ha−1 yr−1. The best-fitting parametric models for subdominant grassland and arable plants used a linear and a critical exponential function, respectively, to describe the relationship between N input and species richness (table 2).
Table 2.
The fit of the parametric models with different functions describing the relationship between plant species richness and annual N inputs and of general additive models (GAM's) with different degrees of freedom. (Additional variables included in the model to correct for confounding factors were latitude and temperature (table 1). Fit of the model is expressed as the quasi-likelihood Akaike information criterion (QAIC). p-d.f. represents significance of improved fit of the GAM by adding a degree of freedom.)
| grasslands | arable fields | |||||||
|---|---|---|---|---|---|---|---|---|
| d.f. | mean deviance | QAIC | P-d.f. | d.f. | mean deviance | QAIC | P-d.f. | |
| all species | ||||||||
| parametric model, linear | 126 | 4.89 | 136.01 | – | 137 | 3.82 | 161.43 | – |
| parametric model, exponential | 125 | 4.69 | 131.82 | – | 136 | 3.61 | 153.84 | – |
| parametric model, critical exponential | 124 | 4.71 | 133.40 | 0.512a | 135 | 3.59 | 153.97 | 0.185a |
| GAM, 1 d.f. | 126 | 4.81 | 134.00 | 0.000 | 137 | 3.65 | 154.43 | 0.000 |
| GAM, 2 d.f. | 125 | 4.76 | 133.61 | 0.123 | 136 | 3.41 | 146.00 | 0.002 |
| GAM, 3 d.f. | 124 | 4.75 | 134.56 | 0.305 | 135 | 3.36 | 145.10 | 0.089 |
| rare species | ||||||||
| parametric model, linear | 126 | 5.05 | 136.01 | – | 137 | 4.25 | 157.05 | – |
| parametric model, exponential | 125 | 4.70 | 128.10 | – | 136 | 4.10 | 152.77 | – |
| parametric model, critical exponential | 124 | 4.73 | 130.07 | 0.879a | 135 | 4.12 | 154.58 | 0.668a |
| GAM, 1 d.f. | 126 | 4.97 | 134.00 | 0.000 | 137 | 4.08 | 150.98 | 0.000 |
| GAM, 2 d.f. | 125 | 4.87 | 132.48 | 0.060 | 136 | 3.90 | 146.00 | 0.009 |
| GAM, 3 d.f. | 124 | 4.85 | 132.88 | 0.202 | 135 | 3.88 | 146.13 | 0.173 |
| subdominant species | ||||||||
| parametric model, linear | 126 | 1.64 | 135.14 | – | 137 | 2.12 | 150.95 | – |
| parametric model, exponential | 125 | 1.65 | 137.05 | – | 136 | 2.08 | 148.79 | – |
| parametric model, critical exponential | 124 | 1.66 | 138.80 | 0.621a | 135 | 1.97 | 142.59 | 0.004a |
| GAM, 1 d.f. | 126 | 1.63 | 134.00 | 0.010 | 137 | 2.10 | 149.17 | 0.000 |
| GAM, 2 d.f. | 125 | 1.64 | 135.87 | 0.724 | 136 | 2.04 | 146.00 | 0.024 |
| GAM, 3 d.f. | 124 | 1.65 | 137.58 | 0.594 | 135 | 2.02 | 145.71 | 0.130 |
Significance of the critical exponential relative to the exponential function.
Figure 1.
The relationships between plant species richness (per 100 m2) and annual nitrogen input on (a–c) grasslands and (d–f) arable fields produced by different statistical models. (a,d) all species, (b,e) rare species and (c,f) subdominant species. Black dashed lines indicate the relationships obtained with a linear function between species richness and N input; black solid curves indicate the relationships obtained with the best parametric model with a curvilinear function; and grey dashed curves indicate the relationships obtained with the best general additive model (table 2). Depicted relationships are obtained with fixed values for the environmental variables (grasslands: a site in Switzerland with latitude 47.385°, temperature 13.6°C; arable fields: a site in Germany with latitude 51.495°, temperature 13.7°C). Circles indicate the original species richness data for each site and are not corrected for confounding environmental factors.
For total and rare arable plant species richness, the best-fitting GAM's had two degrees of freedom. They had a better fit (QAIC was at least 6.7 lower) but were very similar in shape compared with the best parametric model (figure 1). For subdominant grassland species, the best GAM had one degree of freedom and was similar both in fit and shape to the best, linear, parametric model (table 2, figure 1). The QAIC of the best GAM for subdominant arable plants was 3.4 higher than that of the best parametric model, indicating a worse fit. It suggested an exponentially declining relationship rather than the critical exponential relationship that was produced by the best parametric model. The main difference was the location of the peak in species richness, but both had in common that the most pronounced decline in species richness occurred below 100 kg N ha−1 yr−1 (figure 1). The best-fitting GAM's for total and rare grassland plants had one degree of freedom and described functions that were nearly linear (figure 1). The fit of the best parametric models, using an exponential function, were better however (difference in QAIC was 2.2 and 5.9 for total and rare plant species). Furthermore, for rare grassland species the GAM with two degrees of freedom, producing an exponential relationship between species richness and N input, was marginally significant (table 2).
5. Discussion
To our knowledge, this is the first study examining the shape of the relationship between biodiversity and land-use intensity. We linked a biodiversity estimate to a management indicator on a large number of agricultural fields across Europe, thereby covering the full range from very extensive to extremely intensive land use. After differences in environmental conditions had been accounted for, we found evidence that plant species richness declined with increasing land-use intensity. Furthermore, we found that exponential functions more accurately described plant species richness than linear or unimodal functions. This implies that effects of land-use change are most pronounced in species-rich extensively managed agricultural areas while they have little effect in species-poor intensively managed areas (e.g. Kleijn & Sutherland 2003).
This study used local plant species richness as a biodiversity indicator. Beta diversity, another important biodiversity indicator, was not examined. However, in this dataset, the two measures of species richness were strongly correlated (Kleijn et al. 2006) suggesting that the result was quite robust. Plant species richness is often closely related to the diversity of other species groups (Duelli & Obrist 1998; Siemann et al. 1998, Steffan-Dewenter & Tscharntke 2001). In the fields sampled in 2003, the invertebrate species groups, bees, Orthoptera and spiders, were also surveyed (Kleijn et al. 2006). Plant species richness was positively correlated with the species richness of all three species groups in both grasslands (r>0.307, p≤0.001) and arable fields (r>0.313, p<0.001). Other species groups may, therefore, display similar relationships but further study is needed to confirm this.
In grasslands more than in arable fields, the shape of the relationship between species richness and N input seems to be determined by the upper boundary of the distribution of the data (figure 1). Species-poor grasslands occurred across the entire range of land-use intensities, and the grassland site with the lowest total species richness was in fact an unfertilized field. Restoration of plant species richness on grasslands is notoriously difficult and adverse effects of natural catastrophes or past management may have long-lasting effects (Bakker & Berendse 1999). Restoration of species-rich arable plant communities is relatively easy (Kleijn & Van der Voort 1997), and as a result these may more accurately reflect differences in land use between fields. This may explain why the results of the GAM and the parametric model differed for grassland but not for arable species. For grassland plants, the shape of the GAM more than the shape of the parametric model may have been influenced by the large number of fields with low N inputs and low species richness.
Exponentially declining functions gave the best fit for rare species in both grasslands and arable fields (figure 1). Richness of subdominant species declined linearly with increasing N input levels in grasslands and was unimodally related to it in arable fields with peak diversity at very low N input levels. This suggests that rare species are most vulnerable to increasing land-use intensity and is in line with findings of Suding et al. (2005), who observed that the least abundant species were the most likely to disappear following experimental N fertilization.
Put in a global perspective, our results may overestimate the adverse effects of land-use intensity because not many areas outside north-western Europe are farmed as intensively as some of our study areas (Giampietro et al. 1999). On the other hand, wildlife in Europe has been exposed to agriculture for a much longer period than wildlife in most areas outside Europe, and a significant proportion of the European flora and fauna is adapted to (extensive) farming practices (Poschlod & Bonn 1998). European species may, therefore, be more resilient to increases in land-use intensity than species elsewhere, and the adverse effects of land-use intensity on biodiversity may be more pronounced outside Europe.
What are the most important implications of the observed relationships between biodiversity and land-use intensity? First, it suggests that high biodiversity and the associated ecosystem services (e.g. Lyons & Schwartz 2001; Kremen et al. 2002; Albrecht et al. 2007) are largely restricted to areas where land use is very extensive. Second, it provides an explanation for the observation that species of conservation interest are concentrated in areas with low-intensity farming (e.g. Bignal & McCracken 1996), because richness of rare species declined exponentially with land-use intensity. Third, it indicates that conservation benefits are disproportionally more costly in intensively farmed than in extensively farmed areas, thus corroborating the hypothesis of Kleijn & Sutherland (2003). For example, for arable plants, reducing N inputs from 75 to 0 kg N ha−1 resulted in about the same estimated species gain as reducing N inputs from 400 to 60 kg N ha−1 yr−1 (figure 1). Of course, N input is not equivalent to land-use intensity (output per unit area or time), and change in N input is not linearly related to change in the costs of implementing changes in agricultural management. However, the financial costs of reducing farming intensity are likely to be higher in high-intensity compared with low-intensity farming systems, for example, owing to higher fixed costs associated with capital investments in the infrastructure (e.g. machinery, buildings, drainage) required for high-intensity farming. This is reflected in the costs associated with the implementation of agri-environment schemes. Agri-environmental compensations are based on cost incurred and income foregone by the farmer for participating in the agri-environmental measure. Financial compensations for farmers in intensively farmed areas tend to be much higher than those for farmers in agriculturally marginal areas (Buller et al. 2000).
The results of this study provide a conceptual framework for conservation initiatives on farmland. Initiatives are most (cost-)effective if they are preferentially implemented in low-intensity farming systems that still support high levels of biodiversity. This applies not only at the national level, but also at the international level, and highlights the importance of conservation initiatives on farmland in southern, central and eastern European countries that host some of the most species-rich farmlands but are severely threatened by abandonment or intensification (Kleijn & Báldi 2005). However, initiatives in intensively farmed areas should not be excluded if they are targeted at endangered species still inhabiting these landscapes (e.g. Verhulst et al. 2007; Wilson et al. 2007). Conservationists and policy makers should nevertheless be aware that the measures required to effectively conserve the target species in these landscapes need to more drastically reduce land-use intensity and will therefore be more costly.
Acknowledgements
We thank all land owners for giving us access to their fields. P. Duelli, F. Herzog, R. Green and J. Roelofs gave useful comments on the manuscript. Paul Goedhart's help with the statistical analyses is much appreciated. This work was funded by the EU Project QLK5-CT-2002-1495 ‘Evaluating Current European Agri-environment Schemes to Quantify and Improve Nature Conservation Efforts in Agricultural Landscapes (EASY)’.
References
- Albrecht M., Duelli P., Müller C., Kleijn D., Schmid B. Effects of a Swiss agri-environment scheme on pollinator communities and seed set of plants in nearby intensely managed farmland. J. Appl. Ecol. 2007;44:813–822. doi:10.1111/j.1365-2664.2007.01306.x [Google Scholar]
- American Farmland Trust. American Farmland Trust; Washington, DC: 2006. Agenda 2007: a new framework and direction for U.S. Farm Policy. [Google Scholar]
- Bakker J.P., Berendse F. Constraints in the restoration of ecological diversity in grassland and heathland communities. Trends Ecol. Evol. 1999;14:63–68. doi: 10.1016/s0169-5347(98)01544-4. doi:10.1016/S0169-5347(98)01544-4 [DOI] [PubMed] [Google Scholar]
- Báldi A., Batáry P., Erdős S. Effects of grazing intensity on bird assemblages and populations of Hungarian grasslands. Agricult. Ecosys. Environ. 2005;108:251–263. doi:10.1016/j.agee.2005.02.006 [Google Scholar]
- Benton T.G., Bryant D.M., Cole L., Crick H.Q.P. Linking agricultural practice to insect and bird populations: a historical study over three decades. J. Appl. Ecol. 2002;39:673–687. doi:10.1046/j.1365-2664.2002.00745.x [Google Scholar]
- Bignal E.M., McCracken D.I. Low-intensity farming systems in the conservation of the countryside. J. Appl. Ecol. 1996;33:413–424. doi:10.2307/2404973 [Google Scholar]
- Buller H., Wilson G.A., Höll A. Ashgate; Aldershot, UK: 2000. Agri-environmental policy in the European union. [Google Scholar]
- Burnham K.P., Anderson D.R. Springer; New York, NY: 1998. Model selection and inference: a practical information-theoretic approach. [Google Scholar]
- Daily G. Island Press; Washington, DC: 1997. Nature's services: societal dependence on natural ecosystems. [Google Scholar]
- Donald P.F., Green R.E., Heath M.F. Agricultural intensification and the collapse of Europe's farmland bird populations. Proc. R. Soc. B. 2001;268:25–29. doi: 10.1098/rspb.2000.1325. doi:10.1098/rspb.2000.1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duelli P., Obrist M.K. In search of the best correlates for local organismal biodiversity in cultivated areas. Biodivers. Conserv. 1998;7:297–309. doi:10.1023/A:1008873510817 [Google Scholar]
- EMEP. Transboundary acidification, eutrophication and ground level ozone in Europe in 2003. EMEP Status Report 1/05. Norwegian Meteorological Institute; Oslo, Norway: 2005. [Google Scholar]
- European Commission 2000 Indicators for the Integration of Environmental Concerns into the Common Agricultural Policy. Communication to the Council, the European Parliament, COM 2000 (0020) Final. http://eur-lex.europa.eu/LexUriServ/site/en/com/2000/com2000_0020en01.pdf Accessed 20-12-07.
- Giampietro M., Bukkens S.G.F., Pimentel D. General trends of technological changes in agriculture. Crit. Rev. Plant Sci. 1999;18:261–282. doi:10.1016/S0735-2689(99)00366-4 [Google Scholar]
- Grace J.B. The factors controlling species density in herbaceous plant communities: an assessment. Perspect. Plant Ecol. Evol. Syst. 1999;2:1–28. doi:10.1078/1433-8319-00063 [Google Scholar]
- Green R.E., Cornell S.J., Scharlemann J.P.W., Balmford A. Farming and the fate of wild nature. Science. 2005;307:550–555. doi: 10.1126/science.1106049. doi:10.1126/science.1106049 [DOI] [PubMed] [Google Scholar]
- Hastie T.J., Tibshirani R.J. Chapman and Hall; London, UK: 1990. Generalized additive models. [DOI] [PubMed] [Google Scholar]
- Hawkins B.A., et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. doi:10.1890/03-8006 [Google Scholar]
- Herzog F., et al. Assessing the intensity of temperate European agriculture at the landscape level. Eur. J. Agronomy. 2006;24:165–181. doi:10.1016/j.eja.2005.07.006 [Google Scholar]
- Herzog S.K., Kessler M., Bach K. The elevational gradient in Andean bird species richness at the local scale: a foothill peak and a high-elevation plateau. Ecography. 2005;28:209–222. doi:10.1111/j.0906-7590.2005.03935.x [Google Scholar]
- Hillebrand H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004;163:192–211. doi: 10.1086/381004. doi:10.1086/381004 [DOI] [PubMed] [Google Scholar]
- Holland E.A., Braswell B.H., Sulzman J., Lamarque J.F. Nitrogen deposition onto the United States and western Europe: synthesis of observations and models. Ecol. Appl. 2005;15:38–57. doi:10.1890/03-5162 [Google Scholar]
- Kerr J.T., Cihlar J. Patterns and causes of species endangerment in Canada. Ecol. Appl. 2004;14:743–753. doi:10.1890/02-5117 [Google Scholar]
- Kleijn D., Báldi A. Effects of set-aside land on farmland biodiversity: comments on Van Buskirk and Willi. Conserv. Biol. 2005;19:963–966. doi:10.1111/j.1523-1739.2005.00603.x [Google Scholar]
- Kleijn D., Sutherland W.J. How effective are agri-environment schemes in maintaining and conserving biodiversity? J. Appl. Ecol. 2003;40:947–969. doi:10.1111/j.1365-2664.2003.00868.x [Google Scholar]
- Kleijn D., Van der Voort L.A.C. Conservation headlands for rare arable weeds: the effects of fertilizer application and light penetration on plant growth. Biol. Conserv. 1997;81:57–67. doi:10.1016/S0006-3207(96)00153-X [Google Scholar]
- Kleijn D., et al. Mixed biodiversity benefits of agri-environment schemes in five European countries. Ecol. Lett. 2006;9:243–254. doi: 10.1111/j.1461-0248.2005.00869.x. doi:10.1111/j.1461-0248.2005.00869.x [DOI] [PubMed] [Google Scholar]
- Klein A.M., Steffan-Dewenter I., Tscharntke T. Pollination of Coffea canephora in relation to local and regional agroforestry management. J. Appl. Ecol. 2003;40:837–845. doi:10.1046/j.1365-2664.2003.00847.x [Google Scholar]
- Klein A.M., Steffan-Dewenter I., Tscharntke T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. B. 2003;270:955–961. doi: 10.1098/rspb.2002.2306. doi:10.1098/rspb.2002.2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A.M., Vaissiere B.E., Cane J.H., Steffan-Dewenter I., Cunningham S.A., Kremen C., Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. doi:10.1098/rspb.2006.3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C., Williams N.M., Thorp R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl Acad. Sci. USA. 2002;99:16 812–16 816. doi: 10.1073/pnas.262413599. doi:10.1073/pnas.262413599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K.G., Schwartz M.W. Rare species loss alters ecosystem function—invasion resistance. Ecol. Lett. 2001;4:358–365. doi:10.1046/j.1461-0248.2001.00235.x [Google Scholar]
- MacDonald D., Crabtree J.R., Wiesinger G., Dax T., Stamou N., Fleury P., Lazpita J.G., Gibon A. Agricultural abandonment in mountain areas of Europe: environmental consequences and policy response. J. Environ. Manag. 2000;59:47–69. doi:10.1006/jema.1999.0335 [Google Scholar]
- Mattison E.H.A., Norris K. Bridging the gap between agricultural policy, land-use and biodiversity. Trends Ecol. Evol. 2005;20:610–616. doi: 10.1016/j.tree.2005.08.011. doi:10.1016/j.tree.2005.08.011 [DOI] [PubMed] [Google Scholar]
- McCullagh P., Nelder J.A. 2nd edn. Chapman and Hall; London, UK: 1989. Generalized linear models. [Google Scholar]
- Misselbrook T.H., Van Der Weerden T.J., Pain B.F., Jarvis S.C., Chambers B.J., Smith K.A., Phillips V.R., Demmers T.G.M. Ammonia emission factors for UK agriculture. Atmos. Environ. 2000;34:871–880. doi:10.1016/S1352-2310(99)00350-7 [Google Scholar]
- Mittermeier R.A., Mittermeier C.G., Brooks T.M., Pilgrim J.D., Konstant W.R., da Fonseca G.A.B., Kormos C. Wilderness and biodiversity conservation. Proc. Natl Acad. Sci. USA. 2003;100:10 309–10 313. doi: 10.1073/pnas.1732458100. doi:10.1073/pnas.1732458100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandin L.A., Winston M.L. Pollinators provide economic incentive to preserve natural land in agroecosystems. Agricult. Ecosys. Environ. 2006;116:289–292. doi:10.1016/j.agee.2006.02.012 [Google Scholar]
- New M., Lister D., Hulme M., Makin I. A high-resolution data set of surface climate over global land areas. Climate Res. 2002;21:1–25. doi:10.3354/cr021001 [Google Scholar]
- OECD. Organisation for economic co-operation and development; Paris, France: 2003. Agricultural policies in OECD countries: monitoring and evaluation. [Google Scholar]
- Pain D.J., Pienkowski M.W. Academic Press; London, UK: 1997. Farming and birds in Europe: the common agricultural policy and its implications for bird conservation. [Google Scholar]
- Payne R.W., et al. 6th edn. VSN International; Oxford, UK: 2002. Genstat for windows. [Google Scholar]
- Pimm S.L., Russell G.J., Gittleman J.L., Brooks T.M. The future of biodiversity. Science. 1995;269:347–350. doi: 10.1126/science.269.5222.347. doi:10.1126/science.269.5222.347 [DOI] [PubMed] [Google Scholar]
- Poschlod P., Bonn S. Changing dispersal processes in the central European landscape since the last ice age: an explanation for the actual decrease of plant species richness in different habitats? Acta Bot. Neerl. 1998;47:27–44. [Google Scholar]
- Ricketts T.H., Daily G.C., Ehrlich P.R., Michener C.D. Economic value of tropical forest to coffee production. Proc. Natl Acad. Sci. USA. 2004;101:12 579–12 582. doi: 10.1073/pnas.0405147101. doi:10.1073/pnas.0405147101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann E., Tilman D., Haarstad J., Ritchie M. Experimental tests of the dependence of arthropod diversity on plant diversity. Am. Nat. 1998;152:738–750. doi: 10.1086/286204. doi:10.1086/286204 [DOI] [PubMed] [Google Scholar]
- Steffan-Dewenter I., Tscharntke T. Succession of bee communities on fallows. Ecography. 2001;24:83–93. doi:10.1034/j.1600-0587.2001.240110.x [Google Scholar]
- Suding K.N., Collins S.L., Gough L., Clark C., Cleland E.E., Gross K.L., Milchunas D.G., Pennings S. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl Acad. Sci. USA. 2005;102:4387–4392. doi: 10.1073/pnas.0408648102. doi:10.1073/pnas.0408648102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies C., Roschewitz I., Tscharntke T. The landscape context of cereal aphid-parasitoid interactions. Proc. R. Soc. B. 2005;272:203–210. doi: 10.1098/rspb.2004.2902. doi:10.1098/rspb.2004.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharntke T., Klein A.M., Kruess A., Steffan-Dewenter I., Thies C. Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol. Lett. 2005;8:857–874. doi:10.1111/j.1461-0248.2005.00782.x [Google Scholar]
- Turner B.L., Doolittle W.E. The concept of agricultural intensity. Prof. Geogr. 1978;30:297–301. doi:10.1111/j.0033-0124.1978.00297.x [Google Scholar]
- Verhulst J., Kleijn D., Berendse F. Direct and indirect effects of the most widely implemented Dutch agri-environment schemes on breeding waders. J. Appl. Ecol. 2007;44:70–80. doi:10.1111/j.1365-2664.2006.01238.x [Google Scholar]
- Wilson A., Vickery J., Pendlebury C. Agri-environment schemes as a tool for reversing declining populations of grassland waders: mixed benefits from environmentally sensitive areas in England. Biol. Conserv. 2007;136:128–135. doi:10.1016/j.biocon.2006.11.010 [Google Scholar]