Abstract
A major challenge in evolutionary biology is to identify the genes underlying adaptation. The oxygen-transporting haemoglobins directly link external conditions with metabolic needs and therefore represent a unique system for studying environmental effects on molecular evolution. We have discovered two haemoglobin polymorphisms in Atlantic cod populations inhabiting varying temperature and oxygen regimes in the North Atlantic. Three-dimensional modelling of the tetrameric haemoglobin structure demonstrated that the two amino acid replacements Met55β1Val and Lys62β1Ala are located at crucial positions of the α1β1 subunit interface and haem pocket, respectively. The replacements are proposed to affect the oxygen-binding properties by modifying the haemoglobin quaternary structure and electrostatic feature. Intriguingly, the same molecular mechanism for facilitating oxygen binding is found in avian species adapted to high altitudes, illustrating convergent evolution in water- and air-breathing vertebrates to reduction in environmental oxygen availability. Cod populations inhabiting the cold Arctic waters and the low-oxygen Baltic Sea seem well adapted to these conditions by possessing the high oxygen affinity Val55–Ala62 haplotype, while the temperature-insensitive Met55–Lys62 haplotype predominates in the southern populations. The distinct distributions of the functionally different haemoglobin variants indicate that the present biogeography of this ecologically and economically important species might be seriously affected by global warming.
Keywords: haemoglobin, adaptation, polymorphism, Gadus morhua, oxygen affinity, convergent evolution
1. Introduction
Elucidating the molecular mechanisms of adaptation is a central aim in evolutionary biology, yet very few studies have identified the genes underlying adaptive variation in fitness-related traits in natural populations. The haemoglobins represent a unique system for studying adaptive changes because these oxygen-carrying proteins closely connect metabolic activities with external conditions. Hence, the haemoglobins have experienced a major evolutionary pressure and acquired a number of complex features to execute their primary function under extreme variable conditions (Perutz 1983). One remarkable instance of natural selection is the mutated haemoglobin of the Andean goose (Chloephaga melanoptera) and bar-headed goose (Anser indicus) enabling these species to tolerate low oxygen pressure at high elevations in the Andean and Himalayan mountains, respectively (Hiebl et al. 1987; Liang et al. 2001). The genetic basis of high-altitude adaptation in the deer mice (Peromyscus maniculatus) has recently been elucidated by identifying several mutations in the 5′α globin gene, which showed different genotypes in the elevation zones examined (Storz et al. 2007).
Temperature change strongly affects the oxygen consumption and haemoglobin–oxygen-binding affinity of poikilothermic organisms, as well as the ambient oxygen availability. This crucial environmental factor therefore sets the limits for life by influencing the maintenance and activity of all poikilothermic animals. Accordingly, the impact of global warming on seawater temperatures has been reported to seriously affect both the distribution and production of marine species (O'Brian et al. 2000; Perry et al. 2005; Brander 2007). Thus, the northward shift of the North Sea populations of Atlantic cod was reported to be due to climate-related changes (Perry et al. 2005), which, however, seems to contrast with the lack of selective behaviour to avoid high temperatures reported in a more recent study (Neat & Righton 2007). The tolerance of marine fishes to not only high, but also low, temperatures is characterized by a discrepancy between the demand for oxygen and the capacity of oxygen supply to the tissues (Lannig et al. 2003; Sartoris et al. 2003; Pörtner & Knust 2007). This emphasizes the temperature-dependent use of functionally different haemoglobin isoforms to optimize oxygen transport. The nucleated red blood cells of fishes are generally characterized by multiple haemoglobin components, which are functionally different and appear adapted for oxygen extraction in habitats that may vary in oxygen, temperature and carbon dioxide (Pérez et al. 1995; Berenbrink et al. 2007). The heterogeneity of fish haemoglobins is further increased in Atlantic cod through the display of population-specific haemoglobin phenotypes designated HbI-1/1, HbI-2/2 and HbI-1/2 (Sick 1961).
Atlantic cod is believed to comprise several more or less reproductively isolated populations of seasonal migrants and sedentary residents (Robichaud & Rose 2004; Pampoulie et al. 2008; Wennevik et al. 2008), which seem to be differentially adapted to the varying physicochemical conditions in the Arctic and temperate regions of the North Atlantic (Nelson et al. 1996; Nissling & Westin 1997; Brix et al. 1998). The distribution of the cod haemoglobin phenotypes is extremely heterogeneous in the North Atlantic (Brix et al. 1998; Pörtner et al. 2001): the homozygote HbI-2/2 type predominates in the colder northern waters, whereas the homozygote HbI-1/1 type is prevalent at lower latitudes along the coast of Norway and in the North Sea. A similar, although less clear, cline can be seen along the North American East Coast (Sick 1965a). Accordingly, Petersen & Steffensen (2003) showed that temperature is a selective factor in the distribution of the haemoglobin phenotypes by demonstrating that HbI-2/2 cod prefer lower temperatures (8.2°C) than HbI-1/1 cod (15.4°C) under normoxic conditions. These results fit with oxygen-binding analyses demonstrating that the HbI-2/2 phenotype shows higher oxygen-binding affinity than HbI-1/1 fishes at low temperatures (less than 12°C), while the situation is reversed at higher temperatures up to 20°C (Karpov & Novikov 1980; Brix et al. 1998, 2004). The HbI-1/2 heterozygote seems to display intermediate properties of the two homozygotes.
In this study, we reveal the long-sought genetic basis of the haemoglobin polymorphism in Atlantic cod and present molecular mechanisms underlying the proposed adaptation of the cod populations to varying temperature and oxygen regimes. We first determine the relationship between the conventional haemoglobin phenotypes and sequence polymorphisms in the cod globins. Second, we infer from three-dimensional modelling of the tetrameric haemoglobin structure that the identified amino acid replacements influence the haemoglobin quaternary structure and electrostatic features, and thereby its oxygen-binding properties. Finally, we show that the allelic distribution of the haemoglobin polymorphisms in Atlantic cod populations is related to environmental temperature and oxygen saturation.
2. Material and methods
(a) Cloning of cod globin genes
Templates for polymerase chain reaction (PCR) amplification of α and β globin genes were isolated from the spleen and blood of juvenile Atlantic cod caught in the sea off Bergen and Trondheim, Norway, and in Øresund, Denmark. The tissues were stored in RNAlater (Ambion, Foster City, CA, USA) until extraction of total RNA (Trizol, Gibco BRL, Gaithersburg, MD, USA) and cDNA synthesis (Pharmacia Biotech, Piscataway, NJ, USA). Design of PCR primers was based on gadoid globin sequences available in public databases, and primer sequences are available upon request. PCR was performed under standard protocols, the amplicons were inserted in pGEM-T Easy Vector (Promega, Fitchburg, WI, USA) and globin-encoding sequences were obtained by sequencing multiple clones in both directions with BigDye v. 3.1 sequencing kit on 3730 ABI DNA analyser (Applied Biosystems, Foster City, CA, USA).
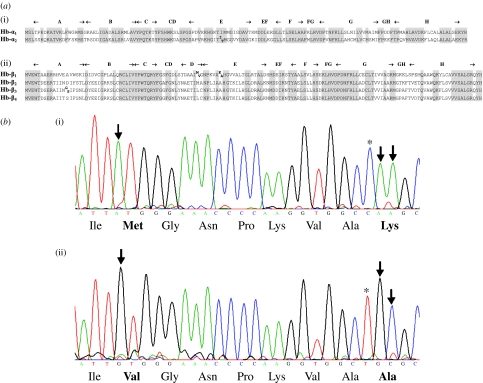
Haemoglobin genotype–phenotype relationships were determined by studying globin sequence variation in a group of 35 juvenile fish caught in the sea off Bergen, which were phenotyped by isoelectric focusing (IEF) analysis of haemolysates (Fyhn et al. 1994). The fish were genotyped using erythrocyte cDNA and genomic DNA as templates for the PCR amplification and direct sequencing (Applied Biosystems) of polymorphic sites identified in the Hb-β1, Hb-β3 and Hb-α2 genes (figure 1).
Figure 1.
Amino acid polymorphisms in globin subunits of Atlantic cod. (a) Deduced amino acid sequences of the six isolated globin genes ((i) α-globins, (ii) β-globins), including the alternative amino acids at the polymorphic sites. The sequences are available in GenBank under the accession nos FJ392681–FJ392686. The helices are conventionally designated in bold letters. Highly conserved positions are shaded. (b) Sequence chromatogram of the mutated region of Hb-β1 encoding the non-recombinant allelic globin variants (i) Met55–Lys62 and (ii) Val55–Ala62. Downward arrows, non-synonymous mutation; asterisks, synonymous mutation.
(b) Globin genotyping
A total of 363 Atlantic cod were sampled from different localities representing the stocks of northeast Arctic cod, Norwegian and Danish coastal cod, and Baltic cod. Genomic DNA was extracted from gill arches or muscle tissue, and genotyping was performed by single nucleotide polymorphism (SNP) analysis. PCR primers were designed using the software SpectroDESIGNER v. 3.0 (Sequenom) and hybridized to invariable sequences of the Hb-β1 gene. The 5′ and 3′ capture primers flanked the polymorphic positions of Met55Val and Lys62Ala, and the sequences are 5′-ACTGTTGGATGATTTCGTCCATGTGGTCCAG-3′ and 5′-ACTGTTGGATGCTACTTCGGTAGCTTTGGCG-3′, respectively. The mutation A/G in the first position of the Met (ATG) or Val (GTG) codon was determined by a downstream extension primer (5′-GCACCGACGCCGCTATT-3′), while an upstream extension primer (5′-GGCCACGACGCCGTGC-3′) recognized the mutation A/C in the second position of the Lys (AAG) or Ala (GCG) codon. No other codon combinations were found by manual inspection of multiple DNA sequences. The Sequenom MassARRAY analyser was used for allele separation, and genotypes were assigned by the MassARRAY Spectrotyper RT v. 3.4 software based on the mass peaks present. The results were manually inspected using the MassARRAY TYPER v. 3.3 software.
(c) Model building
The predicted sequences of the cod globin chains were submitted to the NCBI–Blast server and aligned with homologous sequences using ClustalW. The α- and β-globins of Antarctic rock cod Trematomus bernacchii (Protein Data Bank (PDB) code 1HBH), the Dusky notothen Trematomus newnesi (PDB code 2AA1), bluefin tuna Thunnus thynnus (PDB code 1V4W), and rainbow trout Oncorhynchus mykiss (PDB code 1OUT), shared the highest sequence identity with the cod globins and were selected as templates for homology modelling. Each chain model was built by comparative modelling using the Modeller program v. 7 (Sali & Blundell 1993) as implemented in InsightII (Accelrys Inc., San Diego, CA, USA) and using the crystallographic structures of the four fish haemoglobins. Three structural models were created for each globin chain in Atlantic cod, and the best model was selected based on the Modeller objective function (F, molecular probability density function violation) and the stereochemical criteria of Procheck (Laskowski et al. 1996). The monomeric α and β chains obtained by homology modelling were superimposed onto the crystal structure of the T. bernacchii haemoglobin to keep the same relative orientation of the four subunits. The majority of the residues of the model structures were found to occupy the most favoured regions of Ramachandran plots, and the other residues occupied the additional allowed regions. Structural manipulations and energy minimization of the four tetrameric combinations of Met55βVal and Lys62βAla were performed using the molecular modelling program InsightII (Accelrys) and the CHARMM force field (Brooks et al. 1983).
(d) GRID analysis
The hydrophobic and polar characteristics of the distal environment of the haem pocket were investigated with the GRID program. The interaction of a probe group with a protein of known structure is computed at sampled positions throughout and around the macromolecule, giving an array of energy values. GRID was used to predict the most favourable position for a water molecule near the distal His63β of the alternative pockets attributable to the Lys62βAla polymorphism. The probe used in the calculations was the water group (OH2), and the grid spacing was set to 0.25 Å. Using the flexibility option of the program, the flexible side chains of the protein can move in response to the probe and therefore mimic the adjustments that occur upon ligand binding. The results are displayed by InsightII (Accelrys) as contour maps showing regions of the β haem pocket, which favourably interact with the hydrophilic probe.
(e) Statistical analyses
Departure from Hardy–Weinberg proportions within cod samples was estimated as FIS, and population differentiation, FST, between sample pairs was evaluated using the estimator θ (Weir & Cockerham 1984). Calculations and statistical testing of FIS, FST and genotypic disequilibrium between loci were performed using Genepop on the web (Raymond & Rousset 1995).
3. Results
(a) Genotype–phenotype relationships
We searched for polymorphic genes responsible for the conventional haemoglobin phenotypes in Atlantic cod by isolating six genetically distinct globins from reverse-transcribed erythrocyte mRNA using PCR. The identified globins comprise two α chains (Hb-α1 and Hb-α2) and four β chains (Hb-β1, Hb-β2, Hb-β3 and Hb-β4), and consist of 143 and 147 amino acids, respectively (figure 1a). Amino acid polymorphisms were identified in three of these globins by studying variation in the coding sequences. The Hb-β1 gene contains three non-synonymous mutations resulting in the replacement polymorphisms of Met55Val and Lys62Ala, in which the latter codon displays two mutations (figure 1b). We found strong association between the two haplotypes Met55–Lys62 and Val55–Ala62 and the two protein alleles HbI-1 and HbI-2 by comparing the genotypes and phenotypes of 35 individuals. All the fish displayed the classic IEF patterns (Sick 1961; Fyhn et al. 1994) representing either the HbI-1/1 (n=12), HbI-1/2 (n=14) or HbI-2/2 (n=9) phenotype. Only the non-recombinant haplotypes Met55–Lys62 and Val55–Ala62 were identified in this material by direct sequencing genomic DNA and erythrocyte cDNA. Cod homozygous for the Met55–Lys62 haplotype displayed the HbI-1/1 phenotype, whereas those homozygous for the Val55–Ala62 haplotype gave the HbI-2/2 phenotype. As expected, cod harbouring both haplotypes displayed the heterozygotic HbI-1/2 phenotype. Hence, unambiguous association was found between the polymorphic Hb-β1 gene, the expressed cDNA and the haemoglobin phenotypes, in contrast to the polymorphic Hb-α2 and Hb-β3 genes (figure 1a), which showed no relationship to the phenotypes.
(b) Structure–function relationships
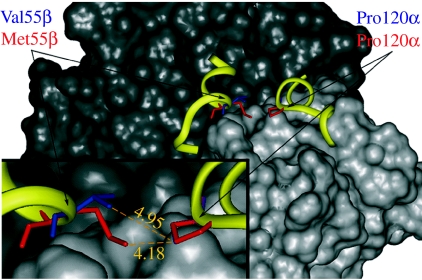
We then addressed the functional impact of the Met55βVal and Lys62βAla replacements on the oxygen-binding properties of the cod haemoglobins. As the haemoglobins in Atlantic cod comprise three different tetrameric structures designated Hb1, Hb2 and Hb3 (Verde et al. 2006), the quaternary structure of the β1-containing Hb1 tetramer α1α1β1β1 was built by three-dimensional homology modelling using the crystallographic structures of the α- and β-globins of four teleost species as templates. In the tetrameric cod haemoglobin, the position of Met55βVal is close to Pro120α at the α1β1 subunit interface (figure 2). The calculated distances of the CG atom of Pro120α to the CE atom of Met55β and to the CG2 atom of Val55β in the two model structures are 4.18 and 4.95 Å, respectively (figure 2). Hence, the replacement of Met55 with the smaller Val residue increases the distance between the α1β1 subunits that probably reduce the stability of the dimers.
Figure 2.
Met55βVal substitution in the α1β1 subunit contact of Atlantic cod haemoglobin. The three-dimensional model shows the superimposition of Met55β (red) and Val55β (blue) at the interface of the α1 (black) and β1 (grey) subunits. The D helix of β1 and the GH corner and initial H helix of α1 chain are shown in ribbon representation (yellow). The inset image depicts the distances (Å) from the CG atom of Pro120α to the CE atom of Met55β and to the CG2 atom of Val55β.
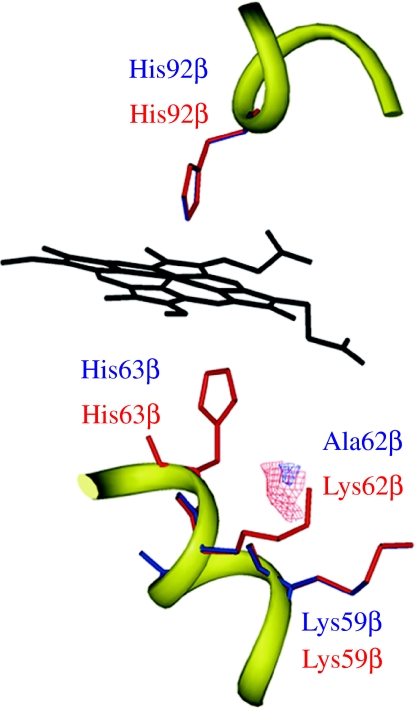
Since the distal pocket residues primarily regulate oxygen binding through electrostatic interactions (Springer et al. 1994), we evaluated the effect of the Lys62βAla polymorphism by investigating the polar characteristics of the distal environment. In the deoxygenated state, ligand access to the distal pocket is hindered by the presence of a water molecule stabilized by polar residues, whereas proton donors are required to stabilize the oxygenated state (Kachalova et al. 1999; Goldbeck et al. 2006). The relative magnitude of these two effects governs whether there is an increase or decrease in affinity (Springer et al. 1994). We estimated the binding energy of a water probe with the distal haem pocket of the Lys62β and Ala62β variants to −14.8 and −11.6 kcalmol−1, respectively, demonstrating the stronger water interaction with the polar Lys compared with the Ala residue. Differences in the water interaction patterns in the two alternative pockets are evident in the calculated GRID contour maps (figure 3). The interaction area is significantly larger in the Lys62β-containing pocket due to the presence of the relatively large and flexible polar side chain. The smaller hydrophilic contour for the Ala62β variant is due to the interaction of the water probe limited to His63β and Lys59β.
Figure 3.
Lys62βAla substitution and water interaction in the haem pocket, showing the superimposition of GRID contour maps at −11.0 kcal mol−1 for the water probe calculated for the distal haem pocket containing Lys62β (red) or Ala62β (blue). His63β, Lys59β and Lys62β were identified as the key residues contributing to the hydrophilic contour maps.
(c) Population genetic results
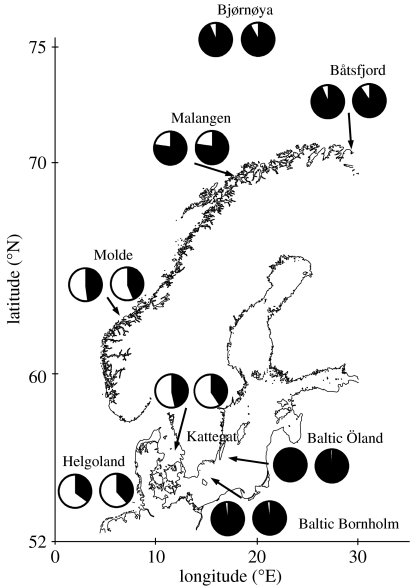
The allele frequencies of the polymorphic Hb-β1 locus showed profound differences in the cod populations examined (figure 4; table 1 in the electronic supplementary material). Both the Val55 and Ala62 alleles were completely dominant in the Baltic Sea, Barents Sea and in the northern part of the northeast Atlantic Sea. The frequencies of these alleles declined in both the north–south and east–west directions, and resulted in the predominance of the Met55 and Lys62 alleles in the southern population of the North Sea. The results are consistent with the cod population studies documenting the predominance of the protein allele HbI-2 in the waters of Greenland (99%), Iceland (98%), northern Norway (90%) and Baltic Sea (96%), decreasing to 28–36% in the warmer areas of the North Sea (Frydenberg et al. 1965; Sick 1965a,b). Allele frequencies within samples generally conformed to Hardy–Weinberg expectations, except at Malangen (table 2 in the electronic supplementary material). This sample showed a deficit of heterozygotes, possibly due to population mixing. The Val–Ala and Met–Lys haplotypes prevailed in the populations examined (table 1). Cod homozygous for the Val–Ala haplotype (corresponding to the HbI-2/2 phenotype) comprised 80–94% of the individuals in populations of the Baltic Sea and the northern waters, whereas homozygotic Met–Lys cod (HbI-1/1 phenotype) were completely lacking in these populations. Met–Lys homozygotes were mainly found in the mid- and southern populations together with the double heterozygotes. There was a significant linkage disequilibrium between the two loci (table 3 in the electronic supplementary material), but it is unclear to what extent heterozygotes are coupled (Met–Lys/Val–Ala) or repulsive (Met–Ala/Val–Lys). Intragenic recombination events have apparently occurred as indicated by the additional genotypes sparsely distributed in the populations examined (table 1). Consistently, sporadic subtypes of the cod haemoglobins have been reported along the Norwegian coast and in Danish waters, differing from the main types in IEF-banding patterns, oxygen-binding affinities and body growth rates (Fyhn et al. 1994; Brix et al. 2004; Husebø et al. 2004; Imsland et al. 2007).
Figure 4.
Relative allele frequencies of the Met55Val (left pie) and Lys62Ala (right pie) polymorphisms in the cod populations examined. The Met55 and Lys62 alleles are depicted in white, whereas the Val55 and Ala62 alleles are shown in black. Latitudes (ordinates) and longitudes (abscissas) are indicated.
Table 1.
Composite genotype frequencies (%) of the Met55βVal and Lys62βAla replacement polymorphisms in Atlantic cod populations. (Double heterozygotes represent both coupling and repulsive heterozygotes, which were not distinguished by the SNP analysis. Numbers of fish are given in parentheses. See figure 4 for collection localities of cod samples.)
| genotype | Bjørnøya (40) | Båtsfjord (41) | Malangen (40) | Molde (39) | Helgoland (36) | Kattegat (74) | Baltic B (56) | Baltic Ö (37) |
|---|---|---|---|---|---|---|---|---|
| Val–Ala/Val–Ala | 83 | 80 | 60 | 18 | 14 | 11 | 93 | 94 |
| Met–Lys/Met–Lys | 0 | 0 | 10 | 36 | 39 | 27 | 0 | 0 |
| Met–Lys/Val–Ala | 15 | 15 | 18 | 31 | 44 | 52 | 7 | 3 |
| Met–Ala/Val–Lys | ||||||||
| Val–Ala/Val–Lys | 2 | 5 | 8 | 13 | 0 | 5 | 0 | 3 |
| Met–Lys/Val–Lys | 0 | 0 | 0 | 0 | 3 | 4 | 0 | 0 |
| Val–Lys/Val–Lys | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| Met–Lys/Met–Ala | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Met–Ala/Met–Ala | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
4. Discussion
(a) Globin polymorphism and oxygen binding
This study presents strong evidence for the crucial role played by the polymorphic β1-globin in the adaptation of Atlantic cod to varying temperature and oxygen regimes. The unambiguous association between haemoglobin phenotypes and genotypes determined in both genomic DNA and expressed cDNA strongly supports Hb-β1 as the active gene responsible for the protein alleles HbI-1 and HbI-2. This is further strengthened by the different isoelectric point of the Met55–Lys62 (pI 7.68) and the Val55–Ala62 (pI 6.99) variants, which is in agreement with the HbI-1-specific cathodic band as visualized by the IEF analysis (Sick 1961; Fyhn et al. 1994). Furthermore, the Met55Val replacement explains the appearance of an HbI-1-specific cathodic fragment in the fingerprinting analysis of chymotrypsin-digested cod haemoglobin (Rattazzi & Pik 1965). This His-containing peptide matches perfectly the acidic (pI 10.81) peptide 56GNPKVAKH62 resulting from low-specific chymotrypsin cleavage at Met55, whereas treatment of the Val55 variant leaves the neutral (pI 7.55) peptide 49STDAAIVGNPKVAAH62 uncleaved. The latter peptide was apparently not recognized by Rattazzi & Pik (1965).
The Met55Val and Lys62Ala replacements occur at the key positions of the α1β1 subunit interface and haem pocket, respectively, and are therefore proposed to affect the oxygen-binding properties of cod haemoglobin. The α1β1 contact is crucial to the stability of the dimers, and any gap loosening this α1β1 interaction destabilizes the low-oxygen affinity deoxy state and facilitates transition to the high-affinity oxy state (Abbasi & Lutfullah 2002; Shikama & Matsuoka 2003). Consistently, increased oxygen affinity was induced in human haemoglobin by site-directed mutagenesis of Met55β→Ser or Pro119α→Ala (corresponding to cod Pro120α), which both excluded van der Waals contact at this subunit interface (Jessen et al. 1991). It is noteworthy that either of these crucial positions is mutated in the haemoglobin of the hypoxia-tolerant Andean goose (Leu55β→Ser) and bar-headed goose (Pro119α→Ala), when compared with the greylag goose (Anser anser) living in the plains and displaying normal oxygen affinity (Hiebl et al. 1987; Liang et al. 2001). In Atlantic cod, changing Met55 with the smaller Val residue increases the gap between the α1β1 subunits that probably destabilizes the T-structure with a resultant increase in oxygen-binding affinity, as demonstrated in the human Met55β→Ser mutant (Jessen et al. 1991). We therefore propose that, during the process of convergent evolution, the water-breathing cod and air-breathing goose have acquired the same molecular mechanism for high-efficiency oxygen binding. The exploitation of the same position for tolerating hypoxic conditions in such diverse species strongly supports the hypothesis of Perutz (1983) that adaptive changes in haemoglobins have evolved by only a few amino acid substitutions in key positions.
The second polymorphism Lys62βAla in the cod β1 globin is located close to the highly conserved His63β on the distal side of the haem. The distal His plays a key role in oxygen binding by regulating ligand access to the haem pocket and by affecting the energetics of the oxygen fixation (Olson et al. 1988; Maréchal et al. 2006). In Atlantic cod, the replacement of Ala62β with the polar Lys residue does not introduce any significant steric effects on the His63β position (figure 3), in contrast to the Ala62β→Pro substitution in the human Hb-Duarte mutant resulting in increased oxygen affinity (Ceccarelli et al. 2006). On the other hand, the mutation modifies the electrostatic feature of the haem pocket as shown by the stronger water interaction with Lys, compared with the neutral Ala, thus reducing the oxygen availability.
Binding of oxygen to haemoglobin is generally an exothermic reaction, and a decrease in temperature induces an increase in oxygen affinity (di Prisco et al. 2006). To evaluate the effect of temperature on the water interaction in the haem pocket, we considered the fact that the transfer of an amino group or an aliphatic group from a non-polar solvent to water is energetically more favourable for the amino group than the aliphatic group, with ΔG values of −132 and +8 cal mol−1 Å−2, respectively (Ooi & Oobataka 1988). Apparently, this is essentially due to the more exothermic water interaction of the former as compared with the latter group, showing a ΔH of −192 and −26 cal mol−1 Å−2, respectively (Ooi & Oobataka 1988). Consequently, at lower temperatures the oxygen path to the haem iron is restricted, being hindered by the stronger water interaction with Lys62β than with the Ala62β residue. The emerging mechanistic picture is consistent with the observation that the oxygen affinity of the HbI-1/1 (Met–Lys) form is much less temperature-sensitive than that of the HbI-2/2 (Val–Ala) form (Brix et al. 2004), due to the counterbalance between two exothermic reactions: the internal water interaction and oxygen binding to haem.
(b) Cod in the Baltic Sea
The strong shift in allele frequencies at the entrance to the Baltic coincides with the differentiation in cod at selectively neutral microsatellite DNA markers (Nielsen et al. 2003). However, the degree of differentiation found in the Hb-β1 locus between the Kattegat and Baltic cod (FST=0.480, 0.472; table 1 in the electronic supplementary material) is more than one order of magnitude higher than that shown for neutral genetic markers (FST=0.034), and is thus consistent with the divergent selection between different environments. It might be speculated that the complete dominance of cod with the Val–Ala variant in the Baltic Sea could be the result of the colonization by Barents Sea cod ca 8000–4000 yr ago, as hypothesized by Sick (1965b). This is, however, not supported by microsatellite studies demonstrating a closer relationship between the Baltic and North Sea cod than that between the Baltic cod and cod from the Barents Sea (Nielsen et al. 2001).
The brackish water of the Baltic Sea restricts cod to deeper waters with higher salinity, but this strategy is compromised by low oxygen levels near the sea bottom due to water stagnation (Tomkiewicz et al. 1998). The Baltic cod are also challenged by long periods of low temperatures during winter and spring, which might, however, be an advantage by lowering the metabolic rate and increasing the oxygen solubility. In addition to the reported local adaptation of the Baltic cod eggs and sperm to low salinity (Nissling & Westin 1997), we propose that the Baltic cod stock is adapted to environmental hypoxia by possessing the high-affinity Val–Ala (HbI-2/2) form. Owing to the temperature sensitivity of this haemoglobin variant, its oxygen affinity would further increase with decreasing temperatures because of the strong exothermic character of oxygen binding.
(c) Cod in the Arctic waters
In the cold waters of the Northern Hemisphere, fishes are well known to possess high mitochondrial densities and elevated aerobic capacities, which are crucial traits in thermal adaptation (Guderley 2004; Pörtner et al. 2006). Accordingly, permanent cold adaptation in Atlantic cod inhabiting the Barents Sea and the Norwegian coast involves elevated aerobic metabolism in white muscle compared with the North Sea cod, in line with the concept of higher maintenance costs at low temperatures (Lannig et al. 2003). Increased oxygen delivery to high mitochondria densities is also supported by the significantly higher cardiac expression of myoglobin in fish acclimatized to 4°C than 10°C, and the cold compensation was more pronounced in the northeast Arctic cod than North Sea cod (Lurman et al. 2007). HbI-2/2 cod acclimatized to 4°C showed significantly higher oxygen affinities than HbI-1/1 cod (P50 of 45.64 and 53.77 mmHg, respectively, and P80 of 130.79 and 177.28 mmHg, respectively), and larger arterial–venous differences were found in HbI-2/2 cod compared with the HbI-1/1 type when measured at 4°C (Brix et al. 2004). The importance of oxygen loading by high-affinity haemoglobins in water-breathing animals is supported by comparative studies of multiple water-breathing, bimodal and air-breathing fish species (Graham 2006). Thus, safeguarding post-branchial saturation seems to be given the first priority in a low-oxygen medium. Even though information about the ligands affecting both the arterial and venous site is limited in cod, the presented results strongly indicate that the high-affinity Val–Ala form is well adapted to the cold Arctic waters by ensuring high oxygen saturation of the arterial blood that is of importance for the high maintenance costs. The related ice cod (Arctogadus glacialis) and polar cod (Boreogadus saida) are strictly cold-water species, but the relatively stationary ice cod inhabits permanently ice-covered waters even farther north than the highly migratory polar cod. Physiological studies of a purified haemoglobin component (Hb3) revealed lower oxygen affinity, but higher Bohr effect, in the ice cod compared with both polar cod and Atlantic cod, which may be preferentially related to lifestyle (Verde et al. 2006). Intriguingly, the β1 globin of ice cod and polar cod differs by displaying Met and Val, respectively, at position 55 (similar residues Gln and Asn at position 62; SwissProt accession nos. Q1AGS3, Q1AGS7). Based on the results herein, we suggest that the low-affinity Met variant probably transport sufficient amounts of oxygen in the sluggish ice cod, whereas the polar cod is probably better fitted to higher oxygen demands by possessing the destabilizing Val residue at the α1β1 interface, similar to the Atlantic cod in these waters.
(d) Cod in the North Sea
Studying the effects of acclimation temperatures at 4 and 12°C, Brix et al. (2004) found a significant drop in oxygen affinity in HbI-2/2 cod compared with HbI-1/1 cod when acclimatized to and measured at 12°C. Cod possessing the temperature-sensitive Val–Ala form will therefore risk reduced oxygen uptake at higher temperatures. Accordingly, both oxygen-binding affinity and capacity decreased with acute temperature increases in cod from Newfoundland, which probably comprised much more than 90 per cent HbI-2/2 (Gollock et al. 2006). The large, exponential increase in blood flow measured in these Newfoundland cod differs from the hyperbolic increase in German Bight cod likely to be composed of more than 55 per cent HbI-1/1 (Lannig et al. 2004). Although different techniques may account for some of the observed differences between these studies, the unexpected resistance to warming waters specifically reported for the North Sea cod (Neat & Righton 2007) is highly suggestive of the possible great importance of the temperature insensitivity of the HbI-1/1 form. We therefore propose that cod possessing the Met–Lys form is better fitted for life in warmer waters than cod with the Val–Ala form, in agreement with the preference for higher temperatures by the HbI-1/1 cod than the HbI-2/2 fish (Petersen & Steffensen 2003). Interestingly, acclimation of heterozygotes HbI-1/2 to high temperature was shown to involve increased levels of the HbI-1 protein allele in the blood, whereas low temperature favoured the synthesis of the HbI-2 allele (Brix et al. 2004). Hence, the ability to change the ratio of the two haemoglobin forms by a hitherto unknown mechanism might be beneficial for the heterozygotic cod in these fluctuating environments.
(e) Concluding remarks
The distributions of both the exploited and unexploited North Sea fish populations have responded markedly to recent increases in sea temperatures as a consequence of global warming (O'Brian et al. 2000; Perry et al. 2005). Based on the strong relationship between the haemoglobin polymorphisms and temperature preference, we propose that the observed northward migration of Atlantic cod is due to increased temperatures exceeding that preferred by the HbI-1/1 (Met–Lys) cod in the North Sea. This implies further that a combination of increased water temperature (HELCOM 2007) and low oxygen levels would be even more unfavourable for the Baltic Sea cod, which predominantly possess the HbI-2 allele. The present frequency of the HbI-2 allele in the Baltic (approx. 97%) is, however, similar or even higher than that identified by Sick in the early 1960s (Sick 1965b), indicating either lack of environmental change or microevolutionary response during this time period.
Acknowledgments
We thank S. W. Omholt and R. Wilson for their discussions and reading of the manuscript. S. E. Fevolden is gratefully acknowledged for providing us with cod samples. This work was supported by the Norwegian Research Council and the Swedish Research Council FORMAS. The work was carried out under National Animal Board licences.
Supplementary Material
Genetic haemoglobin differentiation among pairs of samples of Atlantic cod
Deviation from Hardy–Weinberg expectations, FIS, in cod samples at the SNP-loci Met55Val and Lys62Ala
Contingency test for genotypic linkage disequilibrium
References
- Abbasi A., Lutfullah G. Molecular basis of bird respiration: primary hemoglobin structure component from tufted duck (Aythya fuligula, Anseriformes)—role of α99Arg in formation of a complex salt bridge network. Biochem. Biophys. Res. Commun. 2002;291:176–184. doi: 10.1006/bbrc.2002.6399. doi:10.1006/bbrc.2002.6399 [DOI] [PubMed] [Google Scholar]
- Berenbrink M., Koldkjær P., Klepp O., Cossins A.R. Evolution of oxygen secretion in fishes and the emergence of a complex physiological system. Science. 2007;307:1752–1757. doi: 10.1126/science.1107793. doi:10.1126/science.1107793 [DOI] [PubMed] [Google Scholar]
- Brander K.M. Global fish production and climate change. Proc. Natl Acad. Sci. USA. 2007;104:19 709–19 714. doi: 10.1073/pnas.0702059104. doi:10.1073/pnas.0702059104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix O., Forås E., Strand I. Genetic variation and functional properties of Atlantic cod hemoglobins: introducing a modified tonometric method for studying fragile hemoglobins. Comp. Biochem. Physiol. A. 1998;119:575–583. doi: 10.1016/s1095-6433(97)00469-8. doi:10.1016/S1095-6433(97)00469-8 [DOI] [PubMed] [Google Scholar]
- Brix O., Thorkildsen S., Colosimo A. Temperature acclimation modulates the oxygen binding properties of the Atlantic cod (Gadus morhua L.) genotypes-HbI*1/1, HbI*1/2, and HbI*2/2 by changing the concentrations of their major hemoglobin components (results from growth studies at different temperatures) Comp. Biochem. Physiol. A. 2004;138:241–251. doi: 10.1016/j.cbpb.2004.04.004. doi:10.1016/j.cbpb.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Brooks B.R., Bruccoleri R.E., Olafsen B.D., States D.J., Swaminathan S., Karplus M. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Comp. Chem. 1983;4:187–217. doi:10.1002/jcc.540040211 [Google Scholar]
- Ceccarelli M., Ruggerone P., Anedda R., Fais A., Era B., Sollaino M.C., Corda M., Casu M. Structure-function relationship in a variant hemoglobin: a combined computational–experimental approach. Biophys. J. 2006;91:3529–3541. doi: 10.1529/biophysj.106.083170. doi:10.1529/biophysj.106.083170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Prisco G., Condò S.G., Tamburrini M., Giardina B. Oxygen transport in extreme environments. Trends Biochem. Sci. 2006;16:471–474. doi: 10.1016/0968-0004(91)90182-u. doi:10.1016/0968-0004(91)90182-U [DOI] [PubMed] [Google Scholar]
- Frydenberg O., Møller D., Nævdal G., Sick K. Haemoglobin polymorphism in Norwegian cod populations. Hereditas. 1965;53:252–271. doi: 10.1111/j.1601-5223.1965.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Fyhn U.E.H., Brix O., Nævdal G., Johansen T. New variants of the haemoglobins of Atlantic cod: a tool for discriminating between coastal and Arctic cod populations. ICES Mar. Sci. Symp. 1994;198:666–670. [Google Scholar]
- Goldbeck R.A., Bhaskaran S., Ortega C., Mendoza J.L., Olson J.S., Soman J., Gliger D.S., Esquerra R.M. Water and ligand entry in myoglobin: assessing the speed and extent of heme pocket hydration after CO photodissociation. Proc. Natl Acad. Sci. USA. 2006;103:1254–1259. doi: 10.1073/pnas.0507840103. doi:10.1073/pnas.0507840103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollock M.J., Currie S., Petersen L.H., Gamper A.K. Cardiovascular and haematological responses of Atlantic cod (Gadus morhua) to acute temperature increase. J. Exp. Biol. 2006;209:2961–2970. doi: 10.1242/jeb.02319. doi:10.1242/jeb.02319 [DOI] [PubMed] [Google Scholar]
- Graham J.B. Aquatic and aerial respiration. In: Evans D.H., Claiborne J.B., editors. The physiology of fishes. 3rd edn. CRC Press; Boca Raton, FL: 2006. pp. 85–117. [Google Scholar]
- Guderley H. Metabolic responses to low temperature in fish muscle. Biol. Rev. 2004;79:409–427. doi: 10.1017/s1464793103006328. doi:10.1017/S1464793103006328 [DOI] [PubMed] [Google Scholar]
- HELCOM 2007 Climate change in the Baltic Sea area. Balt. Sea Environ. Proc.111, 1–49.
- Hiebl I., Braunitzer G., Schneeganss D. The primary structures of the major and minor hemoglobin-components of adult Andean goose (Chloephaga melanoptera, Anatidae): the mutation Leu–Ser in position 55 of the β-chains. Biol. Chem. Hoppe-Seyler. 1987;368:1559–1569. doi: 10.1515/bchm3.1987.368.2.1559. [DOI] [PubMed] [Google Scholar]
- Husebø Å., Imsland A.K., Nævdal G. Haemoglobin variation in cod: a description of new variants and their geographical distribution. Sarsia. 2004;90:1–11. [Google Scholar]
- Imsland A.K., Foss A., Nævdal G., Johansen T., Folkvord A., Stefansson S.O., Jonassen T.M. Variations in growth in haemoglobin genotypes of Atlantic cod. Fish. Physiol. Biochem. 2007;30:47–55. doi: 10.1016/j.cbpa.2007.03.001. doi:10.1007/s10695-004-6787-5 [DOI] [PubMed] [Google Scholar]
- Jessen T.H., Weber R.E., Fermi G., Tame J., Braunitzer G. Adaptation of bird hemoglobins to high altitudes: demonstration of molecular mechanism by protein engineering. Proc. Natl Acad. Sci. USA. 1991;88:6519–6522. doi: 10.1073/pnas.88.15.6519. doi:10.1073/pnas.88.15.6519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachalova G.S., Popov A.N., Bartunik H.D. A steric mechanism for inhibition of CO binding to heme proteins. Science. 1999;284:473–476. doi: 10.1126/science.284.5413.473. doi:10.1126/science.284.5413.473 [DOI] [PubMed] [Google Scholar]
- Karpov A.K., Novikov G.G. The hemoglobin aloforms in cod (Gadus morhua L.), their functional characteristics and distribution in the populations. J. Ichthyol. 1980;6:45–50. [Google Scholar]
- Lannig G., Eckerle L.G., Serendo I., Sartoris F.J., Fischer T., Knust R., Johansen T., Pörtner H.O. Temperature adaptation in eurythermal cod (Gadus morhua): a comparison of mitochondrial enzyme capacities in boreal and Arctic populations. Mar. Biol. 2003;142:589–599. [Google Scholar]
- Lannig G., Bock C., Sartoris F.J., Pörtner H.O. Oxygen limitation of thermal tolerance in cod, Gadus morhua L., studied by magnetic resonance imaging and on-line venous oxygen monitoring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R902–R910. doi: 10.1152/ajpregu.00700.2003. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., Rullmann J.A., MacArthur M.W., Kaptein R., Thornton J.M. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR. 1996;8:477–486. doi: 10.1007/BF00228148. doi:10.1007/BF00228148 [DOI] [PubMed] [Google Scholar]
- Liang Y., Hua Z., Liang X., Xu Q., Lu G. The crystal structure of bar-headed goose hemoglobin in deoxy form: the allosteric mechanism of a hemoglobin species with high oxygen affinity. J. Mol. Biol. 2001;313:123–137. doi: 10.1006/jmbi.2001.5028. doi:10.1006/jmbi.2001.5028 [DOI] [PubMed] [Google Scholar]
- Lurman G.J., Koschnick N., Pörtner H.O., Lucassen M. Molecular characterisation and expression of Atlantic cod (Gadus morhua) myoglobin from two populations held at two different acclimation temperatures. Comp. Biochem. Physiol. A. 2007;148:681–689. doi: 10.1016/j.cbpa.2007.08.021. doi:10.1016/j.cbpa.2007.08.021 [DOI] [PubMed] [Google Scholar]
- Maréchal J.D., Maseras F., Lledós A., Mouawad L., Perahia D. A DFT study of the relative affinity for oxygen of the α and β subunits of hemoglobin. J. Comput. Chem. 2006;27:1446–1453. doi: 10.1002/jcc.20427. doi:10.1002/jcc.20427 [DOI] [PubMed] [Google Scholar]
- Neat F., Righton D. Warm water occupancy by North Sea cod. Proc. R. Soc. B. 2007;274:789–798. doi: 10.1098/rspb.2006.0212. doi:10.1098/rspb.2006.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J.A., Tang Y., Boutilier R.G. The effects of salinity changes on the exercise performance of two Atlantic cod (Gadus morhua) populations inhabiting different environments. J. Exp. Biol. 1996;199:1295–1309. doi: 10.1242/jeb.199.6.1295. [DOI] [PubMed] [Google Scholar]
- Nielsen E.E., Hansen M.M., Schmidt C., Meldrup D., Grønkjær P. Determining the population of origin of individual cod in the Northeast Atlantic. Nature. 2001;413:272. doi: 10.1038/35095112. doi:10.1038/35095112 [DOI] [PubMed] [Google Scholar]
- Nielsen E.E., Hansen M.M., Ruzzante D.E., Meldrup D., Grønkjær P. Evidence of a hybrid-zone in Atlantic cod (Gadus morhua) in the Baltic and the Danish Belt Sea revealed by individual admixture analysis. Mol. Ecol. 2003;12:1497–1508. doi: 10.1046/j.1365-294x.2003.01819.x. doi:10.1046/j.1365-294X.2003.01819.x [DOI] [PubMed] [Google Scholar]
- Nissling A., Westin L. Salinity requirements for successful spawning of Baltic and Belt Sea cod and the potential for cod stock interactions in the Baltic Sea. Mar. Ecol. Progr. Ser. 1997;152:261–271. doi:10.3354/meps152261 [Google Scholar]
- O'Brian C.M., Fox C.J., Planque B., Casey J. Climate variability and North Sea cod. Nature. 2000;404:142. doi: 10.1038/35004654. doi:10.1038/35004654 [DOI] [PubMed] [Google Scholar]
- Olson J.S., Mathews A.J., Rohlfs R.J., Springer B.A., Egeberg K.D., Sligar S.G., Tame J., Renaud J.P., Nagai K. The role of the distal histidine in myoglobin and haemoglobin. Nature. 1988;336:265–266. doi: 10.1038/336265a0. doi:10.1038/336265a0 [DOI] [PubMed] [Google Scholar]
- Ooi T., Oobataka M. Effects of hydrated water on protein unfolding. J. Biochem. 1988;103:114–120. doi: 10.1093/oxfordjournals.jbchem.a122215. [DOI] [PubMed] [Google Scholar]
- Pampoulie C., Örn Stefansson M., Jörundsdottir T.D., Danilowicz B.S., Danielsdottir A.K. Recolonization history and large-scale dispersal in the open sea: the case study of the North Atlantic cod, Gadus morhua L. Biol. J. Linn. Soc. 2008;94:315–329. doi:10.1111/j.1095-8312.2008.00995.x [Google Scholar]
- Pérez J., Rylander K., Nirchio M. The evolution of multiple haemoglobins in fishes. Rev. Fish Biol. Fish. 1995;5:304–319. doi:10.1007/BF00043004 [Google Scholar]
- Perry A.L., Low P.J., Ellis J.R., Reynolds J.D. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. doi:10.1126/science.1111322 [DOI] [PubMed] [Google Scholar]
- Perutz M. Species adaptation in a protein molecule. Mol. Biol. Evol. 1983;1:1–28. doi: 10.1093/oxfordjournals.molbev.a040299. [DOI] [PubMed] [Google Scholar]
- Petersen M.F., Steffensen J.F. Preferred temperature of juvenile Atlantic cod Gadus morhua with different haemoglobin genotypes at normoxia and moderate hypoxia. J. Exp. Biol. 2003;206:359–364. doi: 10.1242/jeb.00111. doi:10.1242/jeb.00111 [DOI] [PubMed] [Google Scholar]
- Pörtner H.O., Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2007;315:95–97. doi: 10.1126/science.1135471. doi:10.1126/science.1135471 [DOI] [PubMed] [Google Scholar]
- Pörtner H.O., et al. Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus) Cont. Shelf Res. 2001;21:1975–1997. doi:10.1016/S0278-4343(01)00038-3 [Google Scholar]
- Pörtner H.O., Bennett A.F., Bozinovic F., Clarke A., Lardies M.A., Lucassen M., Pelster B., Schiemer F., Stillman J.H. Trade-offs in thermal adaptation: the need for a molecular to ecological integration. Physiol. Biochem. Zool. 2006;79:295–313. doi: 10.1086/499986. doi:10.1086/499986 [DOI] [PubMed] [Google Scholar]
- Rattazzi M.C., Pik C. Haemoglobin polymorphism in the cod (Gadus morhua): a single peptide difference. Nature. 1965;208:489–491. doi:10.1038/208489a0 [Google Scholar]
- Raymond M., Rousset F. Genepop on the web (v. 1.2): population genetic software for exact test and ecumenism. J. Hered. 1995;86:248–249. [Google Scholar]
- Robichaud D., Rose G.A. Migratory behaviour and range in Atlantic cod: inference from a century of tagging. Fish Fish. 2004;5:185–214. [Google Scholar]
- Sali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. doi:10.1006/jmbi.1993.1626 [DOI] [PubMed] [Google Scholar]
- Sartoris F.J., Bock C., Serendo I., Lannig G., Pörtner H.O. Temperature-dependent changes in energy metabolism, intracellular pH and blood oxygen tension in the Atlantic cod. J. Fish Biol. 2003;62:1239–1253. doi:10.1046/j.1095-8649.2003.00099.x [Google Scholar]
- Shikama K., Matsuoka A. Human haemoglobin—a new paradigm for oxygen binding involving two types of αβ contacts. Eur. J. Biochem. 2003;270:4041–4051. doi: 10.1046/j.1432-1033.2003.03791.x. doi:10.1046/j.1432-1033.2003.03791.x [DOI] [PubMed] [Google Scholar]
- Sick K. Haemoglobin polymorphism in fishes. Nature. 1961;192:894–896. doi: 10.1038/192894a0. doi:10.1038/192894a0 [DOI] [PubMed] [Google Scholar]
- Sick K. Haemoglobin polymorphism in the North Sea and North Atlantic ocean. Hereditas. 1965a;54:49–69. doi: 10.1111/j.1601-5223.1965.tb02005.x. [DOI] [PubMed] [Google Scholar]
- Sick K. Haemoglobin polymorphism of cod in the Baltic and the Danish Belt Sea. Hereditas. 1965b;54:19–48. doi: 10.1111/j.1601-5223.1965.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Springer B.A., Sligar S.G., Olson J.S., Phillips G.N. Mechanisms of ligand recognition in myoglobin. Chem. Rev. 1994;94:699–714. doi:10.1021/cr00027a007 [Google Scholar]
- Storz J.F., Sabatino S.J., Hoffman F.G., Gering E.J., Moriyama H., Ferrand N., Monteiro B., Nachman M.W. The molecular basis of high-altitude adaptation in deer mice. PLoS Genet. 2007;3:448–459. doi: 10.1371/journal.pgen.0030045. doi:10.1371/journal.pgen.0030045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkiewicz J., Lehmann K.M., St John M.A. Oceanographic influences on the distribution of Baltic cod, Gadus morhua, during spawning in the Bornholm Basin of the Baltic Sea. Fish Oceanogr. 1998;7:48–62. doi:10.1046/j.1365-2419.1998.00051.x [Google Scholar]
- Verde C., Balesrieri M., de Pascale D., Pagnozzi D., Lecointre G., di Prisco G. The oxygen transport system in three species of the boreal fish family Gadidae. J. Biol. Chem. 2006;281:22 073–22 084. doi: 10.1074/jbc.M513080200. doi:10.1074/jbc.M513080200 [DOI] [PubMed] [Google Scholar]
- Weir B.S., Cockerham C.C. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. doi:10.2307/2408641 [DOI] [PubMed] [Google Scholar]
- Wennevik V., Jørstad K.E., Dahle G., Fevolden S.-E. Mixed stock analysis and the power of different classes of molecular markers in discriminating coastal and oceanic Atlantic cod (Gadus morhua L.) on the Lofoten spawning grounds. Hydrobiologia. 2008;606:7–25. doi:10.1007/s10750-008-9349-5 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genetic haemoglobin differentiation among pairs of samples of Atlantic cod
Deviation from Hardy–Weinberg expectations, FIS, in cod samples at the SNP-loci Met55Val and Lys62Ala
Contingency test for genotypic linkage disequilibrium