SUMMARY
Background
A 29-year-old man presented to a clinic with infertility and hypogonadism in the setting of morbid obesity. On presentation, he had notable gynecomastia and a low testicular volume. The patient’s weight was 154 kg and his height was 168 cm (BMI 54.5 kg/m2). Before referral to the clinic, the patient had been treated with testosterone therapy for 4 months for hypogonadism. This treatment had caused his initially low sperm concentration to fall to undetectable levels.
Investigations
Measurement of reproductive hormone levels, pituitary MRI, and semen analysis.
Diagnosis
Infertility secondary to hypogonadotropic hypogonadism and an elevated estrogen:testosterone ratio.
Management
Treatment with an aromatase inhibitor, anastrozole, led to normalization of the patient’s testosterone, luteinizing hormone and follicle-stimulating hormone levels, suppression of serum estradiol levels, and to normalization of spermatogenesis and fertility.
Keywords: aromatase inhibitor, estradiol, male infertility, obesity-related hypogonadotropic hypogonadism, testosterone
THE CASE
A 29-year-old man presented to a clinic with a low sperm count in the setting of morbid obesity. The patient and his healthy 32-year-old wife had been unable to conceive after more than 1 year of unprotected intercourse. The patient had been seen by an outside physician and had been diagnosed as having hypogonadism with a low sperm concentration of 2 million/ml (normal concentration ≥20 million/ml). At that time he was started on testosterone therapy, testosterone enantate 200 mg intramuscularly every 2 weeks, for hypogonadism. While the patient was on testosterone therapy, a repeat sperm collection showed a complete absence of sperm (indicating a sperm concentration <2 million/ml). He then presented for a second opinion and further treatment of male infertility. At the time of evaluation at the clinic the patient had ceased testosterone therapy for 2 months.
The patient had gone through normal puberty co-incident with his peers and had never, to his knowledge, fathered a pregnancy. He had no history of testicular trauma, torsion, or infections. The patient denied alcohol, tobacco, marijuana or other illegal drug use, and was taking no prescription medications at the time of referral. He had no history of recent systemic illnesses or considerable weight change. The patient reported being overweight his entire life and denied any episodes of rapid weight gain or loss.
On physical examination, the patient had normal vital signs with a weight of 154 kg, a height of 168 cm, and a BMI of 54.5 kg/m2. Notable findings on examination were the presence of stage IV gynecomastia and morbid central obesity as well as subnormal testicular volumes of 12 cm3 on the right and 8 cm3 on the left. Testicular examination showed no varicocele or hydrocele, and the patient’s vas deferens was palpable bilaterally. He had normal male-pattern hair distribution.
Initial laboratory results (Table 1), obtained when the patient presented to the clinic, confirmed the diagnosis of hypogonadism with low total testosterone levels (5.25 nmol/l [151.2 ng/dl]; normal range 7.7–27.3 nmol/l [221.8–786.2 ng/dl]), low levels of calculated free testosterone (102.2 pmol/l [2.9 pg/ml]; normal range 105–490 pmol/l [3.0–14.1 pg/ml]), and normal levels of sex hormone-binding globulin (34.8 nmol/l; normal range 13.0–71.0 nmol/l). Luteinizing hormone (LH) levels were suppressed at <1 IU/l (normal range 1–14 IU/l) and follicle-stimulating hormone (FSH) levels were low at 2 IU/l (normal range 1–14 IU/l). Serum estradiol was within the normal range (73–275 pmol/l [20–75 pg/ml]) with a level of 172 pmol/l (47 pg/ml).
Table 1.
The case patient’s laboratory values.
| Parameters studied | Normal range | Results at initial consultation | Results after 2 months of anastrozole therapy | Results after 5 months of anastrozole therapy |
|---|---|---|---|---|
| Estradiol (pmol/l; pg/ml) | 73–275; 20–75 | 172; 47 | 81; 22 | <73; <20 |
| Follicle-stimulating hormone (IU/l) | 1–14 | 2 | 27 | 34 |
| Luteinizing hormone (IU/l) | 1–14 | <1 | 15 | 9 |
| Total testosterone (nmol/l; ng/dl) | 7.7–27.3; 221.8–786.2 | 5.3; 151.2 | 16.8; 483.8 | 14.7; 423.4 |
| Calculated free testosterone (pmol/l; pg/ml) | 105–490; 3.0–14.1 | 102; 2.9 | ND | ND |
| Prolactin (pmol; μg/l) | 0–609; 0–14 | 652; 15 | ND | ND |
| Insulin-like growth factor I (μg/l) | 117–329 | 102 | ND | ND |
| Sex hormone-binding globulin (nmol/l) | 13.0–71.0 | 34.8 | ND | ND |
| TSH (mIU/l) | 0.4–0.5 | 3.31 | ND | ND |
| Sperm concentration (spermatozoa/ml) | <20,000,000 | <1,000,000a | 15,000,000 | 21,000,000 |
Seminal fluid analysis was obtained before initial consultation. Abbreviations: ND, not determined; TSH; thyroid-stimulating hormone.
The patient’s blood counts and serum chemistry profile, including glucose levels and liver function tests, were within normal limits. In addition, an MRI of the patient’s brain was normal and, with the exception of his gonadotropin levels, his pituitary function was also normal (Table 1).
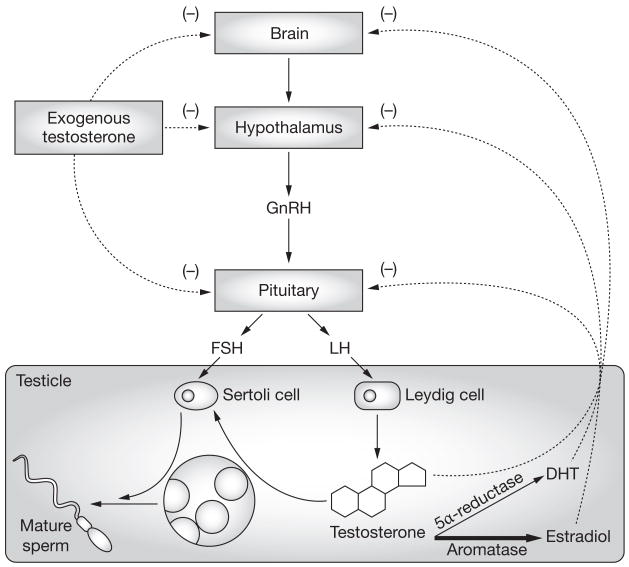
Obesity was felt to be the possible cause of secondary hypogonadism and associated infertility in the patient. He was, therefore, started on treatment with the aromatase inhibitor anastrozole. Aromatase, which converts testosterone to estradiol, is highly expressed in peripheral fat tissue1 and increased aromatase activity is thought to result in increased estradiol production, which inhibits secretion of LH and FSH from the pituitary. Reduced levels of LH and FSH lead to a reduction in testosterone synthesis and sperm production, and to infertility (Figure 1). When fertility is desired in men with hypogonadism, treatment with testosterone is not appropriate because exogenous testosterone further inhibits gonadotropin secretion and impairs spermatogenesis in men. To counteract the physiologic effects of elevated estradiol in the described patient, treatment with anastrozole was initiated.2 This treatment normalized his serum testosterone levels and spermatogenesis (Table 1), and after 6 months of therapy his wife became pregnant. At the time of writing, the couple is eagerly awaiting the birth of their first child. Now that the couple has achieved a pregnancy, the therapeutic plan is to discontinue anastrozole as there are limited long-term safety data on the use of this agent in men. The drug could be restarted if another pregnancy is desired in the future. After the cessation of anastrozole therapy a repeat semen analysis is planned in order to verify the treatment effect.
Figure 1.
The hypothalamic–pituitary–gonadal axis in men and the impact of testosterone therapy and estradiol on spermatogenesis. In obese patients, increased aromatase activity is thought to result in increased estradiol production. The increased estradiol level inhibits FSH and LH secretion from the pituitary, which results in reduced FSH and LH stimulation of the Sertoli and Leydig cells in the testes and a reduction in testosterone synthesis and sperm production. Administration of exogenous testosterone effectively raises serum testosterone levels. If fertility is desired administration of testosterone is, however, counterproductive because it provides negative feedback to the pituitary and causes inhibition of LH and FSH secretion. Decreased LH and FSH stimulation of the testes further inhibits spermatogenesis. Abbreviations: DHT, dihydrotestosterone; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
DISCUSSION OF DIAGNOSIS
There are numerous causes of male infertility, which accounts for roughly one-third of all infertility among couples (Box 1). In patients who have undergone normal puberty, the differential diagnosis of infertility is extremely broad. Some cases are thought to be due to inherited genetic defects.3 Only recently has obesity been recognized as a significant contributor to male infertility.4 Current estimates suggest that over 30% of the male population in the US are considered to be obese (defined as a BMI >30 kg/m2)—a number that continues to rise. Obesity could thus contribute substantially to the rates of hypogonadism and, potentially, to the rates of infertility.5 Several studies show a direct correlation between a rise in BMI and a decline in both free and total blood testosterone levels.1,4,6 Further studies have correlated this decline in testosterone levels with a rise in infertility rates,7 and others have found a relationship between increased BMI and decreased sperm concentrations, sperm motility, and fertility rates.8,9
Box 1 Differential diagnosis of infertility after normal puberty
Primary (testicular) hypogonadism
(characterized by high levels of FSH and LH, and low levels of testosterone)
Infectious orchitis: viral, granulomatous, bacterial
Drugs: alkylating agents, alcohol, marijuana, antiandrogens, ketoconazole, spironolactone, histamine receptor antagonists
Environmental toxins: dibromochloropropane, carbon disulfide, cadmium, lead, mercury, environmental estrogens and phytoestrogens
Radiation exposure
Hyperthermia
Systemic illness
Trauma or torsion
Klinefelter’s syndrome
Secondary hypogonadism (pituitary or hypothalamic)
(characterized by low levels of FSH, LH and testosterone)
Hemochromatosis
Pituitary and hypothalamic tumors: macroadenoma, craniopharyngioma, postsurgical resection
Infiltrative disorders: sarcoidosis, histiocytosis, tuberculosis, fungal infections
Hormonal: hyperprolactinemia, androgen excess, estrogen excess, cortisol excess
Drugs: opioids and psychotropic drugs, GnRH agonists or antagonists
Nutritional deficiency
Systemic illness
Obesity
Disorders of sperm transport
(characterized by normal levels of FSH, LH and testosterone)
Epididymal dysfunction: drugs, infection
Abnormalities of the vas deferens: vasectomy, congenital absence
Ejaculatory dysfunction: spinal cord disease, retrograde ejaculation, diabetic neuropathy, anejaculation
Idiopathic
Abbreviations: FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
In general, secondary hypogonadism is an uncommon, but usually treatable, cause of male infertility.3 In these patients, low serum testosterone levels result from loss of gonadotropin stimulation to Leydig and Sertoli cells within the testes, which leads to low testosterone production and decreased spermatogenesis, respectively (Figure 1). Aromatase is expressed in the intercellular septa of fat tissue and converts testosterone to 17β-estradiol.10
Obesity is one of the causes of secondary hypogonadism (Box 1). In obese men, increased adipose tissue results in increased aromatase activity and a relative elevation in estradiol levels, which inhibits gonadotropin secretion from the pituitary.10 Several studies have shown that men with an increased BMI have a linear rise in serum 17β-estradiol.4,10 Interestingly, estradiol levels in obese men do not frequently surpass the normal range, so some investigators have suggested that the ratio of testosterone to estradiol is more clinically useful in diagnosing estrogen excess in men than the absolute estradiol level alone.10,11 In addition, assessment of estradiol levels in men can be particularly challenging since most clinical assays are optimized to measure levels in the normal female range and are not well validated for measurement of male estradiol levels.12 Clearly, clinical symptoms such as gynecomastia as well as laboratory data should be considered in the evaluation of hypogonadotropic hypogonadism.
TREATMENT AND MANAGEMENT
Treatment of male infertility associated with hypogonadism differs considerably from the treatment of hypogonadism when fertility is not desired. If fertility is not desired, exogenous testosterone administration to hypogonadal men is the most simple and well-studied method for raising serum testosterone levels.
Testosterone therapy often improves sexual function, libido, well-being, bone density, and body composition,13 although the long-term effects of the therapy on cardiovascular health and on the risk of prostate disease have not yet been determined.14 Despite its benefits, exogenous testosterone is counterproductive if fertility is desired since it provides negative feedback to the pituitary, thereby decreasing LH and FSH stimulation to the testes and thus further inhibiting spermatogenesis (Figure 1).
Treatment of male infertility due to hypogonadotrophic hypogonadism focuses upon direct testicular stimulation to increase Leydig-cell production of testosterone, and Sertoli-cell support of spermatogenesis. Standard therapies include human chorionic gonadotropin, which acts as an LH analog within the testes, either alone or in combination with human menopausal gonadotropin or recombinant FSH. Alternatively, some men with hypothalamic hypogonadism are treated with gonadotropin-releasing hormone administered via a pump, although no data have shown that this more complex and expensive treatment improves efficacy. Although not all men with hypogonadism respond to hormonal treatment for infertility, men who previously had normal spermatogenesis are most likely to respond.14
The use of aromatase inhibitors in the treatment of male infertility is a novel approach that has not been widely used or studied. The results of an uncontrolled study of 140 heterogeneous, infertile men by Raman and Schlegel suggested a role for aromatase inhibitors in the treatment of infertility. Not all the men had infertility caused by hypogonadotrophic hypogonadism secondary to obesity, but interestingly all the men responded to treatment with aromatase inhibitors with improved sperm analyses, increased serum testosterone levels and decreased serum estradiol levels.2
Three aromatase inhibitors—anastrozole, letrozole, and testolactone—have been studied for the treatment of hyperestrogenic hypogonadotrophic hypogonadism.15 Although all three have been shown to be effective in increasing serum testosterone levels and decreasing estradiol levels, letrozole has not been evaluated in the treatment of male infertility and the appropriate dose for treatment of men with hypogonadism is less well characterized.16 Testolactone, while equally effective compared with anastrozole in improving semen parameters, is a steroidal aromatase inhibitor that requires dosing four times daily and has been shown to have other hormonal effects, including a theoretical risk of adrenal steroid inhibition.2 Anastrozole was chosen for the treatment of the case patient as it is easy to dose, has few adverse effects, and because effectiveness at improving sperm parameters has been demonstrated.
In addition to the therapeutic strategies discussed above, it is important to note that weight loss via lifestyle changes, medical therapy, or bariatric surgery should also be a primary goal of treatment for men with obesity.
CONCLUSIONS
This case illustrates the challenges in diagnosing and treating infertility in the setting of obesity. Given the unique physiologic connections between adiposity, increased conversion of testosterone to estradiol, and the effect this increase has on suppressing gonadotropin release and spermatogenesis, treatment of infertility in this population differs from that of most men with infertility. The off-label use of aromatase inhibitors to decrease peripheral conversion of testosterone to estradiol provides a unique method of manipulating the normal regulatory mechanisms of the hypothalamic–pituitary–gonadal axis to promote endogenous normalization of testosterone levels and to enhance spermatogenesis and fertility. This case suggests that aromatase inhibition could be an effective treatment for infertility in the setting of obesity-related hypogonadotropic hypogonadism. Data concerning the safety and efficacy of long-term use of aromatase inhibitors for the treatment of hypogonadism is, however, currently lacking and placebo-controlled trials are needed; therefore, even if semen parameters improve with aromatase inhibitor therapy, once fertility is achieved we recommend conventional testosterone therapy with close follow-up for long-term treatment of hypogonadism.
Acknowledgments
The authors thank WJ Bremner for helpful comments during the preparation of the manuscript. ST Page is supported, in part, by the National Institute of Aging, a Division of the NIH, by grant K23 AG027238. JK Amory is supported, in part, by the National Institute of Child Health and Human Development, a Division of the NIH, through grant K23 HD45386.
Footnotes
Competing interests
The authors declared no competing interests.
References
- 1.Hammoud AO, et al. Obesity and male reproductive potential. J Androl. 2006;27:619–626. doi: 10.2164/jandrol.106.000125. [DOI] [PubMed] [Google Scholar]
- 2.Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol. 2002;167:624–629. doi: 10.1016/S0022-5347(01)69099-2. [DOI] [PubMed] [Google Scholar]
- 3.Hull MG, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen TK, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–870. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Hedley AA, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 6.Aggerholm AS, et al. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril. 2007 doi: 10.1016/j.fertnstert. 2007.07.1292. [DOI] [PubMed] [Google Scholar]
- 7.Sallmen M, et al. Reduced fertility among overweight and obese men. Epidemiology. 2006;17:520–523. doi: 10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- 8.Hammoud AO, et al. Male obesity and alteration in sperm parameters. Fertil Steril. 2008 doi: 10.1016/j.fertnst ert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen RH, et al. Men’s body mass index and infertility. Hum Reprod. 2007;22:2488–2493. doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- 10.Fejes I, et al. Effect of body weight on testosterone/estradiol ratio in oligozoospermic patients. Arch Androl. 2006;52:97–102. doi: 10.1080/01485010500315479. [DOI] [PubMed] [Google Scholar]
- 11.Raven G, et al. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab. 2006;91:3324–3328. doi: 10.1210/jc.2006-0462. [DOI] [PubMed] [Google Scholar]
- 12.Sluss PM, et al. Mass spectrometric and physiological validation of a sensitive, automated, direct immunoassay for serum estradiol using the Architect(R) Clin Chim Acta. 2008;388:99–105. doi: 10.1016/j.cca.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Snyder PJ, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 14.Bhasin S, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 15.Zumoff B, et al. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism. 2003;52:1126–1128. doi: 10.1016/s0026-0495(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 16.de Boer H, et al. Letrozole normalizes serum testosterone in severely obese men with hypogonadotropic hypogonadism. Diabetes Obes Metab. 2005;7:211–215. doi: 10.1111/j.1463-1326.2004.00397.x. [DOI] [PubMed] [Google Scholar]