Abstract
Purpose of review
Testosterone functions as a contraceptive by suppressing the secretion of luteinizing hormone and follicle-stimulating hormone from the pituitary. Low concentrations of these hormones deprive the testes of the signals required for spermatogenesis and results in markedly decreased sperm concentrations and effective contraception in a majority of men. Male hormonal contraception is well tolerated and acceptable to most men. Unfortunately, testosterone-alone regimens fail to completely suppress spermatogenesis in all men, meaning that in some the potential for fertility remains.
Recent findings
Because of this, novel combinations of testosterone and progestins, which synergistically suppress gonadotropins, have been studied. Two recently published testosterone/progestin trials are particularly noteworthy. In the first, a long-acting injectable testosterone ester, testosterone decanoate, was combined with etonogestrel implants and resulted in 80–90% of subjects achieving a fewer than 1 million sperm per milliliter. In the second, a daily testosterone gel was combined with 3-monthly injections of depot medroxyprogesterone acetate producing similar results.
Summary
Testosterone-based hormone combinations are able to reversibly suppress human spermatogenesis; however, a uniformly effective regimen has remained elusive. Nevertheless, improvements, such as the use of injectable testosterone undecanoate, may lead to a safe, reversible and effective male contraceptive.
Keywords: etonogestrel, medroxyprogesterone acetate, progestogens, spermatogenesis, testosterone, testosterone decanoate, testosterone undecanoate
Introduction
As both of the existing methods of male contraception, condoms and vasectomy, have shortcomings, efforts are underway to develop a hormonal contraceptive for men analogous to the estrogen/progesterone pill used by women. A male hormonal contraceptive has the potential to be safe, effective and reversible. In surveys, a majority of men in most countries indicate a willingness to use such a male hormonal contraceptive [1,2], and a large majority of women in stable relationships are willing to rely on their male partner to use such a method were it available [3].
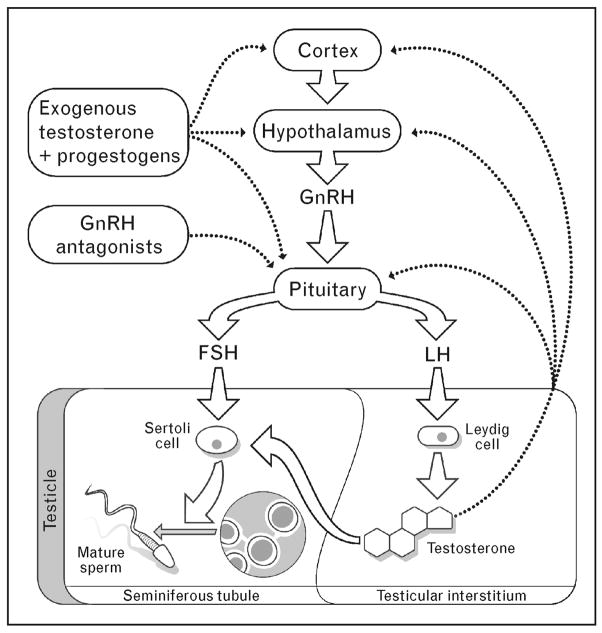
Male hormonal contraceptives all involve the administration of some form of testosterone, which functions as a contraceptive by suppressing secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary. The suppression of LH and FSH deprives the testes of the stimulatory signals required for spermatogenesis, leading to markedly decreased sperm counts and infertility in most, but not all, men (Fig. 1). Importantly, suppression of spermatogenesis by exogenous hormones appears to be uniformly reversible after a few months [4]. Unfortunately, however, the administration of testosterone alone fails to completely suppress the sperm production in a minority of men. Therefore, recent research has studied combinations of testosterone and progestins, which synergistically suppress LH and FSH, to optimize the suppression of spermatogenesis and contraceptive efficacy.
Figure 1. Spermatogenesis and male hormonal contraception.
Solid arrows, promotes spermatogenesis; dashed arrows, inhibits spermatogenesis. FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone. Negative feedback of testosterone occurs at the level of the pituitary, the hypothalamus and the cortex.
In normal men, sperm concentrations exceed 20 million sperm per milliliter of ejaculate. The absence of sperm in the ejaculate, a condition called azoospermia, makes fertilization impossible. Therefore, the universal induction of azoospermia in all men undergoing treatment is the ultimate goal of male hormonal contraceptive development. A sperm concentration below 1 million sperm per milliliter of ejaculate, or severe oligospermia, is associated with a risk of pregnancy of approximately 1% per year [5], and is considered a reasonable goal of male contraceptive development [6], as this would confer an efficacy similar to that of female hormonal contraceptives [7].
As male hormonal contraceptives inhibit sperm production, an elapse of 2–3 months until the sperm concentration in most men is suppressed is required to cause severe oligozoospermia [8], which is similar to the amount of time required before a vasectomy is fully effective. In addition, there are ethnic differences in the response to testosterone-only male hormonal contraceptive regimens. In particular, men in Asia exhibit rates of azoospermia in the 90–100% range on testosterone-alone regimens, whereas men in Europe, North America and Australia have been found to exhibit rates of azoospermia closer to 60% on very similar regimens [5,9]. Although there is no clear explanation for this ethnic difference, it is important when one compares study results between populations.
Testosterone enanthate
In the late 1980s and early 1990s, the World Health Organization (WHO) conducted two large, multicenter trials of testosterone enanthate as a male contraceptive. The first study enrolled 271 subjects who were administered 200 mg testosterone enanthate by intramuscular injection weekly for 6 months [9]. Sixty percent of the men in this study became azoospermic, and an additional 30% became severely oligospermic. The fertility of 119 of the azoospermic men was then tested in a 12-month efficacy phase. In these couples, only one pregnancy occurred despite the cessation of other forms of birth control, corresponding to a rate of 0.8 pregnancies per 100 person-years.
The second study, also sponsored by the WHO, examined the fertility of men who became either azoospermic or oligozoospermic (variably defined in this study as having less than 3–5 million sperms per milliliter of ejaculate) with injections of 200 mg of testosterone enanthate weekly [5]. A total of 399 mostly Asian men were enrolled in this study. Of these, all but eight (2%) became oligozoospermic or azoospermic. In terms of fertility, there were no pregnancies fathered by men who became azoospermic, and fertility was reduced to 8.1 pregnancies per 100 person-years in the men who suppressed their sperm concentrations to less than 3 million sperm per milliliter of ejaculate. Therefore, the overall failure rate (including the eight men who failed to suppress their fertility) was 3.4%, for an overall contraceptive efficacy of 96.6% (Table 1). All subjects had normal return of sperm production after the testosterone injections were discontinued. These two seminal studies demonstrated that testosterone is a safe, reversible and highly effective contraceptive in a majority of men. Nevertheless, they also demonstrate that pregnancy is possible even at very low sperm concentrations achieved in this study, and reinforces the desirability of azoospermia as the ultimate goal of male hormonal contraceptive development.
Table 1.
Male hormonal contraceptive efficacy trials
| Study | Number of couples | Azoospermic (%) | Oligozoospermic (%) | Failure to suppressa (%) | Pregnancies (%) | Total failuresb (%) | Overall contraceptive efficacy (95% CI) |
|---|---|---|---|---|---|---|---|
| WHO [5] | 357 | 268 (75) | 81 (23) | 8 (2.2) | 11 (3.1) | 19 (5.3) | 94.7 (92–97) |
| Gu et al. [20] | 305 | 130 (43) | 166 (54) | 9 (2.9) | 1 (0.3) | 10 (3.3) | 96.7 (95–98) |
| Turner et al. [28] | 53 | 49 (93) | 2 (3.6) | 2 (3.6) | 0 (0.0) | 2 (3.6) | 96.4 (91–100) |
| Total | 715 | 447 (62) | 249 (35) | 19 (2.6) | 12 (1.7) | 31 (4.4) | 95.7 (94–97) |
CI, confidence interval; WHO, World Health Organization.
To a sperm count less than 3–5 million depending on study.
Defined as Pregnancy and/or Failure to suppress to enrollment in the efficacy phase.
Side effects in these studies included a 10–20% decrease in serum high-density lipoprotein (HDL)-cholesterol, acne and a reversible reduction in testicular volume [10,11]. Importantly, there was no evidence that exogenous testosterone administration increased the risk of blood clots, the most serious side effect observed with female contraceptives. Indeed, in-vitro clotting studies imply that testosterone administration may exhibit an antithrombotic effect, which might have the potential to reduce the risk of heart attack and stroke [12]. Importantly, cognitive function, well-being, quality of life and sexual function on these regimens were maintained [13,14]. In terms of acceptability, the regimen was found to be better than expected by a majority of subjects [15]; however, the necessity of the weekly intramuscular injections was a deterrent, with 12% of the subjects discontinuing participation mainly because of their dislike of weekly intramuscular injections. As a result, recent research in male hormonal contraception has focused on the forms of testosterone that can be administered less frequently, such as the long-acting testosterone esters, testosterone decanoate and testosterone undecanoate, testosterone pellets, which can be implanted subdermally every 4–6 months, and the transdermal testosterone gel, which obviates the need for injections or implants entirely.
Testosterone decanoate
Testosterone decanoate can be administered by intramuscular injection every 4–6 weeks for the treatment of male hypogonadism, meaning that it can be dosed less frequently in trials of experimental male contraceptives. In one such recent trial, injections of testosterone decanoate were administered every 4–6 weeks in combination with oral etonogestrel (300 μg daily) [16]. Of 112 subjects, 111 suppressed their sperm concentrations to less than 1 million sperm per milliliter of ejaculate. Sperm suppression was more rapid in the group receiving injections every 4 weeks, and side effects were minor. These results were promising enough for the pharmaceutical company that manufactures testosterone decanoate to sponsor a follow-up study of testosterone decanoate combined with the etonogestrel implant, which they also manufacture. This trial demonstrated rates of azoospermia in the 80–90% range, with overall high acceptability and a low incidence of side effects [17]. Sadly, the company opted not to develop this promising combination further for unclear reasons.
Testosterone undecanoate
Testosterone undecanoate is another form of injectable testosterone with a half-life that is even longer than that of testosterone decanoate, allowing for injection intervals of up to 12 weeks in the treatment of hypogonadism [18,19]. For contraception, monthly administration of injections of 500 mg was studied in 308 Chinese men, 299 of whom reported suppression of sperm concentrations to below 3 million sperm per milliliter [20]. Two hundred and ninety six of these men went on to use the testosterone undecanoate injections as a sole means of contraception for 1 year. In these couples, only one pregnancy occurred. In addition, there were six individuals whose sperm concentrations rebounded to above 3 million sperm per milliliter and therefore discontinued participation, for an overall contraceptive efficacy of 97% (Table 1). More recently, an even larger study of testosterone undecanoate was conducted in China, involving 1000 couples; however, the results from this study have yet to be published.
Testosterone undecanoate has also been combined with the long-acting injectable progestin, norethisterone enanthate, which potently suppresses gonadotropins for 1–2 months [21]. In one study of testosterone undecanoate (1000 mg intramuscularly every 6 weeks) and norethisterone enanthate (200 mg every 6 weeks) 13 of 14 men achieved azoospermia after 32 weeks of treatment, with HDL suppression and mild weight gain in line with the results of prior studies of progestin/testosterone combinations [22]. More recently, this combination was tested at injection intervals of 8 and 12 weeks with 90% of subjects achieving azoospermia at the 8-week interval, but only 38% achieving azoospermia when the testosterone undecanoate was administered every 12 weeks [23]. In a follow-up to these promising trial results, the WHO is planning a large, multinational trial of this combination at 8-week intervals.
In China, testosterone undecanoate has been combined with both levonorgestrel implants [24] and injectable medroxyprogesterone acetate [25] in small-scale trials. Both of these studies demonstrated high rates of azoospermia and are attractive candidates for larger efficacy studies in Chinese men. Lastly, a follow-up trial of testosterone undecanoate combined with the etonogestrel implant involving more than 350 subjects at several European centers was recently completed; however, the final results of this study have not yet been published.
Testosterone pellets
Implantable testosterone pellets are used to treat male hypogonadism in many countries and have been combined with etonogestrel implants in two contraceptive studies examining suppression of spermatogenesis [26,27]. The overall rate of azoospermia in these studies was 85% (83/98 men). Importantly, there were no significant reductions in HDL-cholesterol with this combination, implying that the pellets/implant combination may be a safer route for the long-term administration of these hormones for contraceptive purposes.
Testosterone pellets have also been combined with injectable medroxyprogesterone acetate in a recent, notable male contraceptive study. This study combined testosterone pellets implanted subdermally every 4–6 months with medroxyprogesterone acetate administered by intramuscular injection every 3 months [28]. In this study, 53 of 55 men achieved sperm counts of less than 1 million sperm per milliliter and entered a 12-month contraceptive efficacy phase, in which no pregnancies were observed (Table 1). This important study is the first testosterone/progestin efficacy study and confirms that the high rate of pregnancy prevention observed in the original testosterone-only studies extends to androgen/progestogen combinations.
Transdermal testosterone
Testosterone patches are a successful approach to the treatment of male hypogonadism, but they do not appear to be an effective method of androgen administration for male contraception, probably because the lower peak serum levels of testosterone are unable to effectively suppress gonadotropin secretion from the pituitary. In addition, skin reactions to the patch are a problem. For example, in one study combining testosterone patches with the progestin desogestrel, 24% of participants withdrew from the study because of skin irritation caused by the patches [29].
Recently, testosterone gels have been marketed for the treatment of hypogonadism. These gels achieve higher serum testosterone concentrations and are less irritating to the skin than the patches [30]. Testosterone gel has been recently employed as the androgen in a contraceptive trial in combination with injectable medroxyprogesterone acetate with good suppression of spermatogenesis [31]. Importantly, the gel appears to be acceptable to a majority of men enrolled in the study [32], who would be interested in using this form of contraception were it available. In the future, testosterone gel, or a more concentrated testosterone cream currently in phase III clinical trials, could be combined with a progestin gel or cream as a transdermal approach to male contraception.
Why doesn’t hormonal contraception work for all men?
The major mystery in the field of male contraceptive research is why some men fail to suppress their sperm concentrations to zero despite the suppression of gonadotropins to extremely low levels. As there are few significant differences in the gonadotropin levels during treatment among men who suppress to azoospermia and those who do not, the degree of gonadotropin suppression itself is unlikely to be the answer [33–36]. Alternative explanations include the hypothesis that nonazoospermic men might have greater 5α-reductase activity in their testes [37]. Such a difference would result in higher intratesticular dihydrotestosterone (DHT) concentrations, which could then maintain spermatogenesis. In support of this theory, intratesticular DHT is relatively preserved in men on a male hormonal contraceptive regimen [38]. Nevertheless, two studies have demonstrated that the addition of the 5α-reductase inhibitor finasteride did not enhance suppression of spermatogenesis beyond that achieved by testosterone-alone or testosterone plus desogestrel regimens, as would be expected if intratesticular DHT were the cause of persistent sperm production [39,40]. Moreover, the role of residual androgens in the maintenance of spermatogenesis is unclear, since neither intratesticular testosterone nor intratesticular DHT appears to correlate with spermatogenesis after 24 weeks of treatment with testosterone gel and medroxyprogesterone acetate [41].
Genetic differences between men who suppress to azoospermia and those who do not have also been examined, but these studies have failed to provide a clear explanation for the observed differences. For example, in one study, neither the number of CAG repeats in the androgen receptor nor polymorphisms in the CYP3A4 gene appeared to influence individual responses to hormonal suppression of spermatogenesis in men [42]. On the contrary, a second study found that a CAG repeat number of greater than 22 was associated with an increased chance of azoospermia in the setting of incompletely suppressed gonadotropins [43]. These differing conclusions may be related to study design, as one study examined subjects in a single study using a testosterone-only regimen, whereas the other pooled a variety of studies using mainly combination regimens with higher overall suppression rates.
Another possible explanation for differences in the spermatogenic suppression could be the differential production of the peptide hormone insulin-like factor 3 (INSL3), which has been shown to promote germ cell survival in the setting of low gonadotropins [44]. In a recent retrospective analysis of three contraceptive trials, serum concentrations of INSL3 were shown to be higher in men with persistent spermatogenesis compared to men who completely suppressed their sperm production [45]; however, this preliminary observation requires prospective confirmation. Alternatively, INSL3 could merely be a marker of incomplete gonadotropin suppression. If INSL3 does prove to play a role in maintaining spermatogenesis in the low gonadotropin environment of a male hormonal contraceptive regimen, the addition of an INSL3 antagonist to a hormonal regimen has the potential to improve rates of sperm suppression in hormonal contraceptive trials.
Conclusion
Research has demonstrated the feasibility of the hormonal approach to male contraception. Androgen-based combinations are able to reversibly suppress human spermatogenesis without severe side effects in most men; however, a uniformly effective regimen has remained elusive. Current research is focused on both improving the method of hormone administration and finding combinations that optimize sperm suppression while minimizing side effects. Hopefully, upcoming trials of testosterone undecanoate combined with norethisterone enanthate or testosterone/progestin gel combinations will result in efficacy and acceptability that is good enough to consider for phase III testing and regulatory approval, finally bringing the promise of male hormonal contraception to fruition.
References
- 1.Martin CW, Anderson RA, Cheng L, et al. Potential impact of hormonal male contraception: cross-cultural implications for development of novel male preparations. Hum Reprod. 2000;15:637–645. doi: 10.1093/humrep/15.3.637. [DOI] [PubMed] [Google Scholar]
- 2.Heinemann K, Saad F, Wiesemes M, et al. Attitudes toward male fertility control: results of a multinational survey on four continents. Hum Reprod. 2005;20:549–556. doi: 10.1093/humrep/deh574. [DOI] [PubMed] [Google Scholar]
- 3.Glasier AF, Anakwe R, Everington D, et al. Would women trust their partners to use a male pill? Hum Reprod. 2000;15:646–649. doi: 10.1093/humrep/15.3.646. [DOI] [PubMed] [Google Scholar]
- 4.Liu PY, Swerdloff RS, Christenson PD, et al. Hormonal Male Contraception Summit Group. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006;367:1412–1420. doi: 10.1016/S0140-6736(06)68614-5. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril. 1996;65:821–829. [PubMed] [Google Scholar]
- 6.Nieschlag E. 10th Summit meeting consensus: recommendations for regulatory approval for hormonal male contraception. Contraception. 2007;75:166–167. doi: 10.1016/j.contraception.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Moreau C, Trussell J, Rodriguez G, et al. Contraceptive failure rates in France: results from a population-based survey. Hum Reprod. 2007;22:2422–2477. doi: 10.1093/humrep/dem184. [DOI] [PubMed] [Google Scholar]
- 8.Ly LP, Liu PY, Handelsman DJ. Rates of suppression and recovery of human sperm output in testosterone-based hormonal contraceptive regimens. Hum Reprod. 2005;20:1733–1740. doi: 10.1093/humrep/deh834. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet. 1990;336:995–999. [PubMed] [Google Scholar]
- 10.Bagatell CJ, Heiman JR, Matsumoto AM, et al. Metabolic and behavioral effects of high-dose, exogenous testosterone in healthy men. J Clin Endocrinol Metab. 1994;79:561–567. doi: 10.1210/jcem.79.2.8045977. [DOI] [PubMed] [Google Scholar]
- 11.Meriggiola MC, Marcovina S, Paulsen CA, Bremner WJ. Testosterone enanthate at the dose 200 mg/week decreases HDL-cholesterol levels in healthy men. Int J Androl. 1995;18:237–242. [PubMed] [Google Scholar]
- 12.Zitzmann M, Junker R, Kamischke A, Nieschlag E. Contraceptive steroids influence the hemostatic activation state in healthy men. J Androl. 2002;23:503–511. [PubMed] [Google Scholar]
- 13.Cherrier MM, Anawalt BD, Herbst KL, et al. Cognitive effects of short-term manipulation of serum sex steroids in healthy young men. J Clin Endocrinol Metab. 2002;87:3090–3096. doi: 10.1210/jcem.87.7.8570. [DOI] [PubMed] [Google Scholar]
- 14.Sjogren B, Gottlieb C. Testosterone for male contraception during one year: attitudes, well being and quality of sex life. Contraception. 2001;64:59–65. doi: 10.1016/s0010-7824(01)00223-2. [DOI] [PubMed] [Google Scholar]
- 15.Ringheim K. Evidence for the acceptability of an injectable hormonal method for men. Fam Plan Perspect. 1995;27:123–128. [PubMed] [Google Scholar]
- 16.Hay CJ, Brady DM, Zitzmann M, et al. A multicenter phase IIb study of a novel combination of intramuscular androgen (testosterone decanoate) and oral progestogen (etonogestrel) for male hormonal contraception. J Clin Endocrinol Metab. 2005;90:2042–2049. doi: 10.1210/jc.2004-0895. [DOI] [PubMed] [Google Scholar]
- 17.Brady DM, Amory JK, Perheentupa A, et al. A multicentre study investigating subcutaneous etonogestrel implants with injectable testosterone decanoate as a potential long-acting male contraceptive. Hum Reprod. 2006;21:285–294. doi: 10.1093/humrep/dei300. [DOI] [PubMed] [Google Scholar]
- 18.Zhang GY, Gu YQ, Wang XH, et al. A pharmacokinetic study of injectable testosterone undecanoate in hypogonadal men. J Androl. 1998;19:761–768. [PubMed] [Google Scholar]
- 19.Behre AM, Abshagen K, Oettel M, et al. Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies. Eur J Endrocrinol. 1999;140:414–419. doi: 10.1530/eje.0.1400414. [DOI] [PubMed] [Google Scholar]
- 20.Gu Y-Q, Wang X-H, Xu D, et al. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab. 2003;88:562–568. doi: 10.1210/jc.2002-020447. [DOI] [PubMed] [Google Scholar]
- 21.Kamischke A, Diebacker J, Nieschlag E. Potential of norethisterone enanthate for male contraception: pharmacokinetics and suppression of pituitary and gonadal function. Clin Endocrinol (Oxford) 2000;53:351–358. doi: 10.1046/j.1365-2265.2000.01097.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamischke A, Venherm S, Ploger D, et al. Intramuscular testosterone undecanoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab. 2001;86:303–309. doi: 10.1210/jcem.86.1.7057. [DOI] [PubMed] [Google Scholar]
- 23.Meriggiola MC, Constantino A, Saad F, et al. Norethisterone enanthate plus testosterone undecanoate for male contraception: effects of various injection intervals of spermatogenesis, reproductive hormones, testis and prostate. J Clin Endocrinol Metab. 2005;90:2005–2014. doi: 10.1210/jc.2004-1852. [DOI] [PubMed] [Google Scholar]
- 24.Gui YL, He CH, Amory JK, et al. Male hormonal contraception: suppression of spermatogenesis by injectable testosterone undecanoate alone or with levonorgestrel implants in Chinese men. J Androl. 2004;25:720–727. doi: 10.1002/j.1939-4640.2004.tb02846.x. [DOI] [PubMed] [Google Scholar]
- 25.Gu YQ, Tong JS, Ma DZ, et al. Male hormonal contraception: effects of injections of testosterone undecanoate and depot medroxyprogesterone acetate at eight-week intervals in Chinese men. J Clin Endocrinol Metab. 2004;89:2254–2262. doi: 10.1210/jc.2003-031307. [DOI] [PubMed] [Google Scholar]
- 26.Kinniburgh D, Zhu H, Cheng L, et al. Oral desogestrel with testosterone pellets induces consistent suppression of spermatogenesis to azoospermia in both Caucasian and Chinese men. Hum Reprod. 2002;17:1490–1501. doi: 10.1093/humrep/17.6.1490. [DOI] [PubMed] [Google Scholar]
- 27.Anderson RA, Van Der Spuy ZM, Dada OA, et al. Investigation of hormonal male contraception in African men: suppression of spermatogenesis by oral desogestrel with depot testosterone. Hum Reprod. 2002;17:2869–2877. doi: 10.1093/humrep/17.11.2869. [DOI] [PubMed] [Google Scholar]
- 28.Turner L, Conway AJ, Jimenez M, et al. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab. 2003;88:4659–4667. doi: 10.1210/jc.2003-030107. [DOI] [PubMed] [Google Scholar]
- 29.Hair WM, Kitteridge K, O’Conner DB, Wu FC. A novel male contraceptive pill–patch combination: oral desogestrel and transdermal testosterone in the suppression of spermatogenesis in normal men. J Clin Endocrinol Metab. 2001;86:5201–5209. doi: 10.1210/jcem.86.11.8028. [DOI] [PubMed] [Google Scholar]
- 30.Steidle C, Schwartz S, Jacoby K, et al. North American AA2500 T Gel Study Group. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 31.Page ST, Amory JK, Anawalt BD, et al. Testosterone gel combined with depomedroxyprogesterone acetate (DMPA) is an effective male hormonal contraceptive regimen but is not enhanced by the addition of the GnRH antagonist acyline. J Clin Endocrinol Metab. 2006;91:4374–4380. doi: 10.1210/jc.2006-1411. [DOI] [PubMed] [Google Scholar]
- 32.Amory JK, Page ST, Anawalt BD, et al. Acceptability of a combination testosterone gel and depomedroxyprogesterone acetate male contraceptive regimen. Contraception. 2007;75:218–223. doi: 10.1016/j.contraception.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Wallace EM, Gow SM, Wu FC. Comparison between testosterone enanthate-induced azoospermia and oligoazoospermia in a male contraceptive study I: plasma luteinizing hormone, follicle stimulating hormone, testosterone, estradiol and inhibin concentrations. J Clin Endocrinol Metab. 1993;77:290–293. doi: 10.1210/jcem.77.1.8325955. [DOI] [PubMed] [Google Scholar]
- 34.Handelsman DJ, Farley TM, Peregoudov A, Waites GM. Factors in nonuniform induction of azoospermia by testosterone enanthate in normal men. Fertil Steril. 1995;63:125–133. [PubMed] [Google Scholar]
- 35.Amory JK, Anawalt BD, Bremner WJ, Matsumoto AM. Daily testosterone and gonadotropin levels are similar in azoospermic and nonazoospermic normal men administered weekly testosterone: implications for male contraceptive development. J Androl. 2001;22:1053–1060. doi: 10.1002/j.1939-4640.2001.tb03445.x. [DOI] [PubMed] [Google Scholar]
- 36.McLachlan RI, Robertson DM, Pruysers E. Relationship between serum gonadotropins and spermatogenic suppression in men undergoing steroidal contraceptive treatment. J Clin Endocrinol Metab. 2004;89:142–149. doi: 10.1210/jc.2003-030616. [DOI] [PubMed] [Google Scholar]
- 37.Anderson RA, Wallace AM, Wu FC. Comparison between testosterone enanthate-induced azoospermia and oligozoospermia in a male contraceptive study. III. Higher 5α-reductase activity in oligozoospermic men administered supraphysiological doses of testosterone. J Clin Endocrinol Metab. 1996;81:902–908. doi: 10.1210/jcem.81.3.8772548. [DOI] [PubMed] [Google Scholar]
- 38.McLachlan RI, O’Donnell L, Stanton PG, et al. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002;87:546–556. doi: 10.1210/jcem.87.2.8231. [DOI] [PubMed] [Google Scholar]
- 39.McLachlan RI, McDonald J, Rushford D, et al. Efficacy and acceptability of testosterone implants, alone or in combination with a 5α-reductase inhibitor, for male hormonal contraception. Contraception. 2000;62:73–78. doi: 10.1016/s0010-7824(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 40.Kinniburgh D, Zhu H, Cheng L, et al. Suppression of spermatogenesis with desogestrel and testosterone pellets is not enhanced by addition of finasteride. J Androl. 2001;22:88–95. [PubMed] [Google Scholar]
- 41.Page ST, Kalhorn TF, Bremner WJ, et al. Intratesticular androgens and spermatogenesis during severe gonadotropin suppression induced by male hormonal contraceptive treatment. J Androl. 2007;28:734–741. doi: 10.2164/jandrol.107.002790. [DOI] [PubMed] [Google Scholar]
- 42.Yu B, Handelsman DJ. Pharmacogenetic polymorphisms of the AR and metabolism and susceptibility to hormone-induced azoospermia. J Clin Endocrinol Metab. 2001;86:4406–4411. doi: 10.1210/jcem.86.9.7793. [DOI] [PubMed] [Google Scholar]
- 43.Eckardstein SV, Schmidt A, Kamischke A, et al. CAG repeat length in the androgen receptor gene and gonadotropin suppression influence the effectiveness of hormonal male contraception. Clin Endocrinol (Oxford) 2002;57:647–655. doi: 10.1046/j.1365-2265.2002.01652.x. [DOI] [PubMed] [Google Scholar]
- 44.Kawamura K, Kumagi J, Sudo S, et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci U S A. 2004;101:7323–7328. doi: 10.1073/pnas.0307061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amory JK, Page ST, Anawalt BD, et al. Elevated end-of-treatment serum INSL3 is associated with failure to completely suppress spermatogenesis in men receiving male hormonal contraception. J Anrol. 2007;28:548–554. doi: 10.2164/jandrol.106.002345. [DOI] [PubMed] [Google Scholar]