Abstract
Many essential organelles and endosymbionts exhibit a strict matrilineal pattern of inheritance. The absence of paternal transmission of such extra-nuclear components is thought to preclude a response to selection on their effects on male viability and fertility. We overturn this dogma by showing two mechanisms, inbreeding and kin selection, which allow mitochondria to respond to selection on both male viability and fertility fitness. Even modest levels of inbreeding allow such a response to selection when there are direct fitness effects of mitochondria on male fertility, because inbreeding associates male fertility traits with mitochondrial matrilines. Male viability effects of mitochondria are also selectable whenever there are indirect fitness effects of males on the fitness of their sisters. When either of these effects is sufficiently strong, we show that there are conditions that allow the spread of mitochondria with direct effects harmful to females, contrary to standard expectation. We discuss the implications of our findings for the evolution of organelles and endosymbionts and for the discussion of genomic conflict.
Keywords: maternal inheritance, mitochondria, inbreeding, kin selection, indirect fitness effects
Matrilineal transmission, as is the general rule for mitochondria, chloroplasts, and many endo-symbiotic microbes, creates an ‘asymmetric sieve’ favoring extra-nuclear mutations advantageous for females but harmful for males (Burt and Trivers 2006; Cosmides and Tooby 1981; Frank and Hurst 1996; Zeh and Zeh 2005). Such mutations can spread because deleterious male-specific fitness effects do not affect the response to natural selection of the maternally transmitted mitochondria. This finding has become known as Mother’s Curse (Gemmell et al. 2004) and “the logic of the population genetics argument is indisputable ” (Frank and Hurst 1996). We show that a response to selection on male mitochondrial fertility and viability fitness occurs for two circumstances: (1) inbreeding; and, (2) kin selection, when males affect the fitness of sisters. Both processes facilitate a response to selection on mitochondrial mutations with direct effects on male fitness and can limit or prohibit the spread of mitochondrial mutations harmful to males. In fact, even mitochondrial mutations with deleterious effects when expressed in females but directly beneficial to males can spread under either situation.
With standard population genetic models, we illustrate how both inbreeding and kin selection allow for an adaptive response to male-specific direct effects of maternally transmitted cytoplasmic alleles. We then discuss the implications of our findings for the evolution of mitochondria and other genomes transmitted maternally as well as for their significance in discussions of genomic conflict.
Inbreeding and mitochondrial effects on male fertility
Let C1 and C2 be alternative, maternally inherited, cytoplasmic alleles, in frequencies P and Q respectively, and let f be the population’s inbreeding parameter. We allow the allele, C1 to affect male fertility, since mitochondrial effects on male sperm performance are well known in birds (Birkhead et al. 1996), insects (Rand et al 2001) and mammals (e.g. Cardullo, R. A. and J. M. Baltz 1991), including humans (Kao et al. 1995; Moore and Reijo-Pera 2000; Ruiz-Pesini et al. 2000). We assume that mating with a subfertile male (i.e. C1 cytotypes) decreases female reproductive output by the value, sFF♂. In addition to causing subfertility in males, we allow C1 to have pleiotropic effects on the fitnesses of both males and females (s♂ and s♀ respectively, Table 1).
Table 1.
Model 1: Inbreeding and male fertility effects, sFF♂
| Family | Fitness | ||||
|---|---|---|---|---|---|
| Sire | Dam | Frequency | Family | Sons | Daughters |
| C1 | C1 | P♂P♀ +Q♂P♀ f | 1 + sFF♂ | 1 + s♂ | 1 + s♀ |
| C1 | C2 | P♂Q♀ (1-f) | 1 + sFF♂ | 1 | 1 |
| C2 | C1 | Q♂P♀ (1-f) | 1 | 1 + s♂ | 1 + s♀ |
| C2 | C2 | Q♂Q♀+P♂Q♀f | 1 | 1 | 1 |
For this case, we find the general model for the change in allele frequency of C1 due to selection:
| (1) |
where the mean fitness of females is W♀ = (1+s♀P♀+sFF♂[P♂+f{Q♂P♀ − P♂Q♀}]), which reduces to W♀ = 1+P(s♀+sFF♂) when the frequency of the C1 allele is the same in both sexes. This will be approximated when selection pressure is similar in both sexes (s♀ ~ s♂).
We see from equation (1) that male fertility fitness, sFF♂, plays a direct role in gene frequency change whenever there is any level of inbreeding (f > 0). We can see this best by assuming that the C1 allele has no direct effect on female fitness (s♀ = 0). Here, equation (1) becomes ΔP♀=P♀Q♀fsFF♂/W♀ – that is the change in allele frequency is the product of the paternal effect on family fertility (sFF♂), the inbreeding rate (f), and the variance in allele frequency in females (P♀Q♀).
In the general recursion (equation [1]), ΔP will be positive whenever f sFF♂> − s♀, a result strongly suggestive of Hamilton’s rule (Hamilton 1963), where the relatedness between a male and his mate, f, times the fitness benefit to the breeding pair, sFF♂, must exceed the direct cost (s♀). Here, however, the direct cost is born by females, while the benefit derives from the fertility of their male mates.
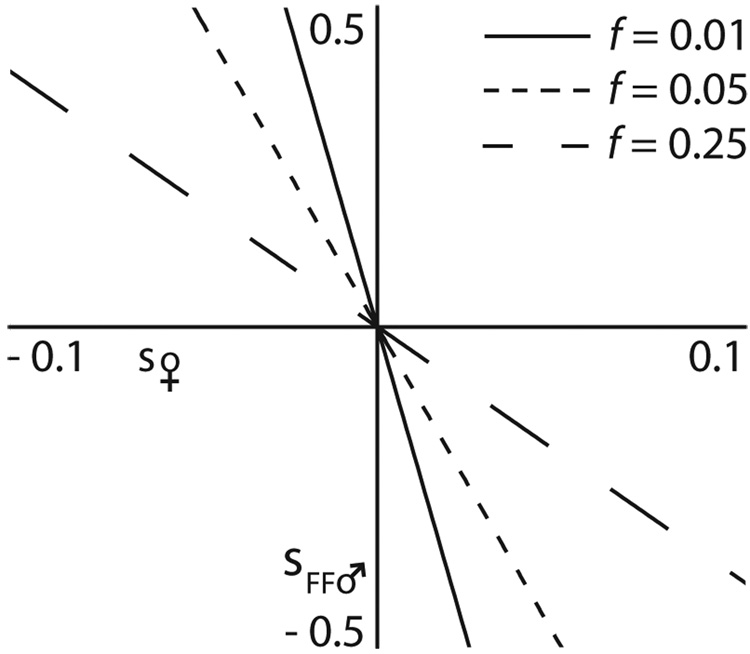
In Figure 1, we illustrate these conditions for invasion (ΔP♀ > 0) of a mitochondrial allele, C1, into a population with inbreeding rate, f, when this allele has direct effects on female viability (s♀ x-axis) and effects via males on family fertility (sFF♂ y-axis). The lines correspond to different values of the inbreeding parameter, f, and divide the plane into two regions: regions above or to the right of a line correspond to conditions that allow the spread of the C1 allele (ΔP♀ > 0) and regions to the left represent conditions where it does not spread (ΔP♀ < 0). Note that the y-axis corresponds to the case of random mating (f = 0), where all C1 alleles with a positive effect on female viability invade (s♀ > 0) and all those with a negative effect on female viability are lost (s♀ < 0) regardless of the C1 allele’s the effect on male viability. Without inbreeding (f = 0), there is no way for mitochondrial effects on male fertility fitness to influence the spread of the maternally transmitted C1 allele. These parameters represent the foundation of the ‘indisputable logic’ of Mother’s Curse, since alleles with fitness effects in the lower right quadrant of Figure 1 (s♀ > 0 and s♂ < 0) spread despite their deleterious effects on male fertility.
Figure 1.
The conditions for invasion of a mitochondrial allele, C1, into a population with inbreeding rate, f (line labels), direct effects on female viability (s♀ x-axis) and male fertility (sFF♂ y-axis). For a given inbreeding rate, f, a C1 allele for with the combination of s♀ and sFF♂ to the right of the line can invade a population composed of C2 alleles. Note that the y-axis corresponds to no inbreeding (f = 0) under this condition, all C1 alleles that increase female viability can invade regardless of the effect on male fertility, and conversely alleles that reduce female viability cannot invade even if they substantially increase male fertility. With increased rates of inbreeding, however, some C1 haplotypes that increase male fertility can invade despite their deleterious effects on female viability (upper left quadrant). Similarly, inbreeding decreases the parameter space in which an allele beneficial to female viability can invade despite its deleterious effects on male fertility (bottom right quadrant).
In the case of complete inbreeding (f = 1), the effects of C1 alleles on both sexes are equally weighted because ΔP♀ becomes P♀Q♀(s♀+sFF♂)/W♀. Thus, the C1 allele spreads if the net effect on both sexes is positive ([sFF♂ + s♀] > 0) and does not when the net effect is negative ([sFF♂ + s♀] < 0). As a result, the line for f = 1 in Figure 1 (not shown) would correspond to the case where s♀ = −sFF♂. In general, -f is the slope of the line that governs the conditions for invasion. With mild inbreeding (small f), the positive effects on male fertility have to be very large to counter even a mildly deleterious effect on female viability. In contrast, with strong inbreeding (large f), even weak positive effects on male fertility can override opposing deleterious effects on females and be important in determining the conditions and rate of spread.
Thus, inbreeding significantly changes the conditions for the spread of the C1 allele. While Gemmell et al. (2004, page 242) argue that “mating with multiple males and the ensuing sperm competition would be one approach that females could utilize to insure against the detrimental effects of mating with an infertile male,” in the long term, with random mating, this results in the opposite effect – an increase in frequency in male-sterile mitochondria. Rather, some level of inbreeding is required to maintain male-fertile mitochondria by promoting the spread of mitochondria with beneficial effects on male fertility (i.e., sFF♂ > 0) and, at the same time, limiting the spread of mutations deleterious for male fertility fitness (i.e., sFF♂ < 0). Importantly, there are conditions (in the bottom right quadrant of Fig. 1), where, despite a positive effect on female viability fitness, the C1 allele does not spread through the population because of the combination of inbreeding rates and the deleterious effect on male fertility (0 < s♀ < f sFF♂).
Although we focus here on the effects of male fertility on the reproductive output of a mating pair, other male-specific mitochondrial effects can also influence female reproductive fitness. For example, male protection of their young or other forms of paternal care influence the fitness of the matriline. Thus, additional mitochondrial defects that decrease paternal condition and consequently paternal contribution to offspring fitness are also subject to this indirect selection in inbred populations.
Kin selection: mitochondrial alleles with direct effects on male viability and indirect effects on female relatives
Here we consider a species living in matrilineal family groups where sons contribute to the viability of their sisters. Again, we let C1 and C2 be alternative, maternally inherited, cytoplasmic alleles, in frequencies P and Q respectively, and let f be the inbreeding parameter. As above, we assume that the C1 allele has two direct fitness effects, s♂ on the viability of C1 males and s♀ on that of C1 females. That is, the viability fitness of males inheriting the C1 allele from their mothers is (1 + s♂), while that of females inheriting C1 is (1 + s♀). Additionally, we let sKS♂ be the indirect effect of viable C1 males on the fitness of their sisters; thus, sKS♂ is a fitness benefit to female kin (Table 2). We assume that only surviving males influence the fitness of their sisters, so that sKS♂ is modulated by the viability effects of C1 on male fitness, s♂. These male viability effects can be either positive or negative.
Table 2.
Model 2: Kin selection where surviving brothers benefit sisters by sKS♂.
| Family | Fitness | ||||
|---|---|---|---|---|---|
| Sire | Dam | Frequency | Family | Sons | Daughters |
| C1 | C1 | P♂P♀+Q♂P♀f | 1 + sKS♂ (1 + s♂) | 1 + s♂ | 1 + s♀ |
| C1 | C2 | P♂Q♀(1-f) | 1 | 1 | 1 |
| C2 | C1 | Q♂P♀(1-f) | 1 + sKS♂ (1 + s♂) | 1 + s♂ | 1 + s♀ |
| C2 | C2 | Q♂Q♀+P♂Q♀f | 1 | 1 | 1 |
With these assumptions, the general equation describing the one-generation change in allele frequency due to selection is
| (2) |
where mean female fitness equals W♀ = (1+P♀[s♀+sKS♂{1+s♂}{1+s♀}]). Owing to strict maternal inheritance, ΔP♀ is also the total change in frequency of C1 between generations, since the frequency of the C1 allele in surviving daughters is the frequency at the start of the next generation. Note that the inbreeding parameter, f, does not appear in equation (2) because sons inherit C1 from their mothers regardless of how females obtain mates.
When there is no kin selection effect (sKS♂ = 0), ΔP♀ reduces to the classic formula P♀Q♀s♀/W♀ (Wright 1969, p. 163, equation [6.1]; Hedrick 2000, p. 105). In this instance, the direct effects of C1 on male viability, s♂, whether positive or negative, play no role in the evolutionary response. This result reflects the ‘indisputable logic’ that the absence of paternal transmission precludes an evolutionary response to male-specific selection (Burt and Trivers 2006; Frank and Hurst 1996).
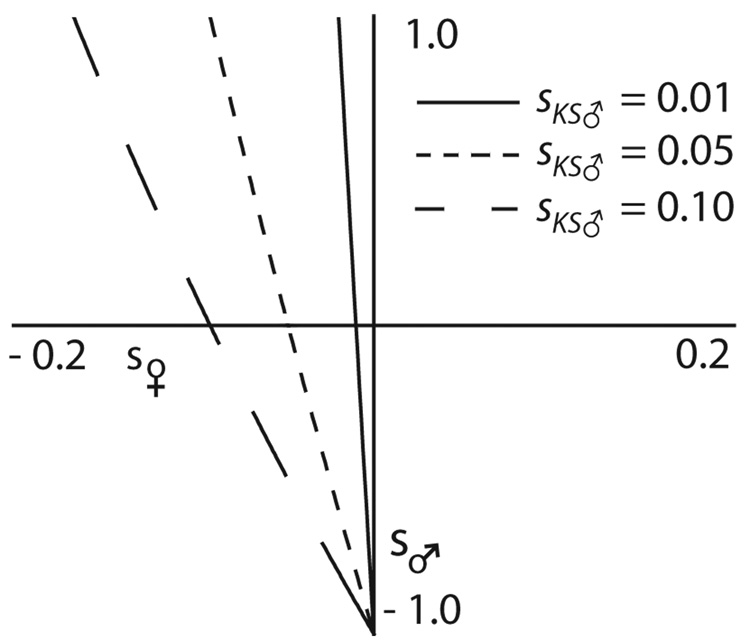
In contrast, when C1 is expressed only in males, and acts solely and indirectly to influence fitness of sisters (i.e. s♀= s♂= 0 ≠sKS♂), then we find that ΔP♀ = P♀Q♀sKS♂/W♀. With kin selection, the male-specific function of mitochondria allows a response to selection. In the general case, (s♀≠ s♂ ≠ 0 ≠ sKS♂ > 0), not only do male viability effects influence the response to selection, but also, mutations decreasing female fitness (s ♀ < 0) can be favored, so long as the term, sKS♂ (1+s♂), the net effect of sons on the fitness of their sisters, is sufficiently positive (Figure 2). In fact, a mutant cytoplasmic allele, C1, will increase in frequency whenever sKS♂ > -s♀/[(1+s♀)(1+s♂)].
Figure 2.
The conditions for invasion of a mitochondrial allele, C1, where sons increase family fitness. Allele C1 has a direct effect on male and female viability (s♂, y-axis and s♀ x-axis, respectively), and C1 sons increase mean family fitness by sKS♂ (line labels). A C1 allele can invade a population fixed for C2 so long as the combination of s♀ and s♂ is to the right of the line signifying the effect of C1 males on mean family fitness (sKS♂). Note that the y-axis corresponds to no kin selection effect (sKS♂ = 0) and, in this case, all C1 alleles that increase female viability can invade regardless of the effect on male viability, an illustration of ‘Mother’s Curse.’ Note also the cases in which the kin selection effect of C1 allows for invasion of a mitochondrial gene that increases male viability but decreases the viability of females (upper left quadrant). In fact, under some conditions, the kin-selection effect of C1 can outweigh its directly deleterious effects in both males and females (lower left quadrant).
In this model, male contribution need not be biased towards females and does not preclude sisters providing similar fitness benefit to their siblings; however, systems in which males contribute largely to family fitness will increase the efficacy of this form of selection. Strong sexual selection, in which many males are left without mates, may be one such system. In this case, the so-called ‘marriage squeeze’ can result in males becoming the helping sex, and can even result in a sex ratio skewed towards males (Pen and Weissing 2000). Moreover, while this model does not preclude female contribution to family fitness, it does not explicitly incorporate it either. If included, female contributions to family fitness would increase the importance of s♀ diminishing the ability of s♂ to overcome negative values of s♀. Even with female contribution, so long as s♀ is small, mitochondrial effects on male viability are selectable.
Discussion
Despite maternal inheritance, mitochondrial effects on male fertility and male viability become important whenever there is inbreeding, or whenever males affect the fitness of female relatives, respectively. Thus, the indisputable logic of Mother’s Curse is not an inescapable consequence of population genetic principals, but rather is a special, restrictive case resulting from neglect of the consequences of inbreeding and kin selection.
Our model of male fertility selection shares a certain similarity with models of cytoplasmic incompatibility, wherein males infected with the matrilineally transmitted microbe, Wolbachia, reduce the fertility of uninfected females (Wade and Stevens 1985; Stevens and Wade 1990). The mating activity of the infected males reduces the relative fitness of matrilines with the alternative, uninfected cytoplasm. Instead of the positive fitness effect on their own matriline (sKS♂ > 0) that we hypothesized, these males, by virtue of their maternally inherited, infected cytoplasm, have a negative fitness effect on the fertility of other, non-related and uninfected matrilines. Winther (2006) has shown how the spread of Wolbachia can be understood in this way as a model of kin selection. Thus, the difference between these cases lies in the mechanism whereby males confer a relative fitness advantage to related matrilines. For the case of cytoplasmic incompatibility, inbreeding reduces the rate of spread of Wolbachia because it reduces the proportion of uninfected females mating with infected males (Stevens and Wade 1990). Thus, the mechanism of the male fitness effects on female kin determines whether inbreeding (indirect positive effects on female kin) or outbreeding (indirect negative effects on non-kin females (Engelstädter and Charlat 2006) enhances the rate of spread of the cytoplasm in question.
Similarly, this model can be extended to the example of ‘cyto-nuclear conflict,’ cytoplasmic male sterility (CMS). CMS is a phenomenon in hermaphroditic plants, in which male-sterile cytotypes prevent the production of functional pollen and instead harness these resources for ovule production (Jacobs and Wade 2003). In self compatible species, or in subdivided metapopulations with significant FST values, pollen limitation due to CMS will have similar consequences as sperm dysfunction (sFF♂) in animals and can be selected against even when ovule production (s♀) is enhanced. This logic contradicts the argument of Mother’s Curse, but may contribute to the maintenance of polymorphism in cytoplasmic male sterility factors (McCauley et al. 2000).
Paternal leakage of mitochondria (i.e., partial paternal inheritance in the context of primarily maternal transmission) could change our model in interesting ways, but probably more so in outcrossing than in inbreeding species. Inbreeding limits the effects of paternal leakage by limiting the number of matings between males and females of different cyto-type. If only some offspring inherit mitochondria from the father, then the kin selection effect is diminished because leakage reduces the relatedness necessary for kin selection. However, paternal leakage could also increase the efficacy of selection on male specific mitochondrial effects, as paternally leaked mitochondria must have come from successful fathers. Nonetheless, this selection is probably very weak, since paternal leakage is thought to occur rarely – with a frequency of approximately 10−4 in mice (Gyllensten et al. 1991), and is orders of magnitude smaller in salmon (Wolff et al. 2008, Wolff and Gemmell 2008). Paternal leakage at these frequencies would not significantly affect the relatedness governing male fitness effects on sisters. Concerning male fertility effects, our model (equation [1]) shows that the response to selection on mitochondria-related sperm performance is proportional to the product of the rate of inbreeding, f, and the fertility effect, sFF♂. A survey of f across human populations shows a wide range of values from 3.7 × 10−2 to 5.0 × 10−4 (Bittles and Neel 1994). Assuming that paternal leakage occurs at a similar frequency in humans and mice, which is likely a conservative estimate given the ratio of mitochondrial genomes in the human sperm and egg (Reviewed in Wolff and Gemmell 2008), the strength of indirect selection on male-specific mitochondrial effects on human sperm performance via inbreeding would appear to be between five and 370 times stronger than that by direct selection of male sperm performance subsequent to paternal leakage.
Another possible evolutionary response to male specific mitochondrial defects could come from nuclear restorers of male fitness, resulting in an epistatic relationship between mitochondrial and nuclear genotypes. This has been reported for sperm function in seed beetles (Dowling et al 2007) and is well known in cases of CMS in plants (Delph et al. 2007). In species where males are the heterogametic sex, such interactions between mitochondrial effects and recessive, X-linked effects can lead to differences in s♀ and s♂. For example, Leber’s Hereditary Optic Neuropathy (LHON), is a disease causing blindness in humans that is associated with mis-sense mutations in mitochondrial DNA. It has higher penetrance in males than females, and this difference is at least partially attributable to a recessive mutation on the X-chromosome (Shankar et al. 2008). More generally, Rand et al. (2001) have shown that interactions between the mitochondria and the X-chromosome in Drosophila often have opposite fitness effects on male and female fitness. Extensions of our model to include sex differences in epistatic effects between sex-linked and mitochondrial genes as well as interactions between autosomal and mitochondrial genes will strengthen the predictive and explanatory powers of this conceptual model.
Darwin (1871) was the first to suggest that selection among tribes or matrilines was important to the evolution of human societies. Anthropologists have since postulated that early human society was divided into matrilineal groups where males contributed resources to females and offspring within the group (Hill and Kaplan 1999; Dunbar 2004). In these kinds of circumstances, it is relatively easy to imagine that the foraging actions of males are of benefit to the female members of the group. Male-specific mitochondrial diseases impairing muscle or vision function can be expected to diminish effective foraging. In general, many species are genetically subdivided into family or matrilineal groups where males perform tasks that affect group function, like the group defense, paternal care of young, and foraging and food sharing of some primates (Kappeler 2000). It is under these circumstances that we expect our kin selection model (Model 2) of mitochondrial evolution to limit the spread of male deleterious mutations and promote the spread of mutations that benefit males. While we discuss cases in which males increases the fitness of their sisters (sKS♂ > 0), our model also can explain the evolution of early acting cytoplasmic male killers. Such male-killers are thought to evolve by kin selection when males decrease the fitness of their sisters (sKS♂ < 0), presumably because of intense resource competition (Hurst 1991).
In summary, transmission mode does not dictate a gene’s evolutionary destiny because there are many ways in addition to transmission to achieve a positive regression between a gene and its fitness effects.
Supplementary Material
Acknowledgments
We thank C. Lively, J. Bever, D. E. McCauley, T. Platt, J. D. Van Dyken, P. Zee and two anonymous reviewers for their thoughtful comments. This study was supported by NIH Training Grant 1T32HD49336 and NSF Graduate Research Fellowship Program to YB and by NIH R01 GM084238-01 to MJW.
References
- Birkhead TR, Martínez JG, Burke T, Froman DP. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. Lond. 1996 doi: 10.1098/rspb.1999.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittles AH, Neel JV. The costs of human inbreeding and their implications for variations at the DNA level. Nature Genet. 1994;8:117–121. doi: 10.1038/ng1094-117. [DOI] [PubMed] [Google Scholar]
- Burt A, Trivers R. Genes in conflict. Cambridge, UK: Belknap; 2006. [Google Scholar]
- Cardullo RA, Baltz JM. Metabolic regulation in mammalian sperm: mitochondrial volume determines sperm length and flagellar beat frequency. Cell Motil. Cytoskeleton. 1991;19:180–188. doi: 10.1002/cm.970190306. [DOI] [PubMed] [Google Scholar]
- Cosmides LM, Tooby J. Cytoplasmic inheritance and intragenomic conflict. J. Theor. Biol. 1981;89:83–129. doi: 10.1016/0022-5193(81)90181-8. [DOI] [PubMed] [Google Scholar]
- Darwin CR. The descent of man and selection in relation to sex. New York, NY: Appleton; 1871. [Google Scholar]
- Delph LF, Touzet P, Bailey MF. Merging theory and mechanism in studies of gynodioecy. Trends Ecol. Evol. 2007;22:17–24. doi: 10.1016/j.tree.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Dowling DK, Nowostawski AL, Arnqvist G. Effects of cytoplasmic genes on sperm viability and sperm morphology in a seed beetle: implications for sperm competition theory? J Evol. Biol. 2007;20:358–368. doi: 10.1111/j.1420-9101.2006.01189.x. [DOI] [PubMed] [Google Scholar]
- Dunbar R. The human story: a new history of mankind’s evolution. London, UK: Faber and Faber; 2004. [Google Scholar]
- Frank SA, Hurst LD. Mitochondria and male disease. Nature. 1996;383:224. doi: 10.1038/383224a0. [DOI] [PubMed] [Google Scholar]
- Engelstädter J, Charlat S. Outbreeding selects for spiteful cytoplasmic elements. Proc. R. Soc. Lond. B. 2006;273:923–929. doi: 10.1098/rspb.2005.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell NJ, Metcalf VJ, Allendorf FW. Mother's curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 2004;19:238–244. doi: 10.1016/j.tree.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Gyllensten U, Wharton D, Josefsson A, Wilson AC. Paternal inheritance of mitochondrial DNA in mice. Nature. 1991;352:255–257. doi: 10.1038/352255a0. [DOI] [PubMed] [Google Scholar]
- Hedrick PW. Genetics of populations. Sudbury, MA: Jones and Bartlett; 2000. [Google Scholar]
- Hill K, Kaplan H. Life history traits in humans: theory and empirical studies. Annu. Rev. Anthropology. 1999;28:397–430. doi: 10.1146/annurev.anthro.28.1.397. [DOI] [PubMed] [Google Scholar]
- Hurst LD. The incidences and evolution of cytoplasmic male killers. Proc. R. Soc. Lond. B. 1991;244:91–99. [Google Scholar]
- Jacobs M, Wade MJ. A synthetic review of the theory of gynodioecy. Am. Nat. 2003;161:837–851. doi: 10.1086/375174. [DOI] [PubMed] [Google Scholar]
- Kao SH, Chao HT, Wei YH. Mitochondrial deoxyribonucleic acid 4977- bp deletion is associated with diminished fertility and motility of human sperm. Biol. Reprod. 1995;52:729–736. doi: 10.1095/biolreprod52.4.729. [DOI] [PubMed] [Google Scholar]
- Kappeler PM. Primate males: causes and consequences of variation in group composition. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- McCauley DE, Olson MS, Emery SN, Taylor DR. Population structure influences sex ratio evolution in a gynodioecious plant. Am. Nat. 2000;155:814–819. doi: 10.1086/303359. [DOI] [PubMed] [Google Scholar]
- Moore FL, Reijo-Pera RA. Male sperm motility dictated by mother's mtDNA. Am. J. Hum. Genet. 2000;67:543–548. doi: 10.1086/303061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pen I, Weissing FJ. Sex-ratio optimization with helpers at the nest. Proc. R. Soc. Lond. B. 2000;267:539–543. doi: 10.1098/rspb.2000.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand DM, Clark A, Kann LM. Sexually antagonistic cytonuclear fitness interactions in Drosophila melanogaster. Genetics. 2001;159:173–187. doi: 10.1093/genetics/159.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Lapeña AC, Díez-Sánchez C, Pérez-Martos A, Montoya J, Alvarez E, Díaz M, Urriés A, Montoro L, López-Pérez MJ, Enríquez JA. Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am. J. Hum. Genet. 2000;67:682–693. doi: 10.1086/303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SP, Fingert JH, Carelli V, Valentino ML, King TM, Daiger SP, Salomao SR, Berezovsky A, Belfort R, Braun TA, Sheffield VC, Sadun AA, Stone EM. Evidence for a novel x-linked modifier for leber hereditary optic neuropathy. Ophthalmic Genet. 2008;29:17–24. doi: 10.1080/13816810701867607. [DOI] [PubMed] [Google Scholar]
- Stevens L, Wade MJ. Cytoplasmically inherited reproductive incompatibility in Tribolium flour beetles: the rate of spread and effect on population size. Genetics. 1990;124:367–372. doi: 10.1093/genetics/124.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer R, J AJ, Breeuwer GDD, Hurst LD. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- Touzet P, Budar F. Unveiling the molecular arms race between two conflicting genomes in cytoplasmic male sterility? Trends Plant Sci. 2004;9:568–580. doi: 10.1016/j.tplants.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Wade MJ, Stevens L. Microorganism mediated reproductive isolation in flour beetles (genus Tribolium) Science. 1985;227:527–528. doi: 10.1126/science.3966160. [DOI] [PubMed] [Google Scholar]
- Winther RG. Fisherian and Wrightian perspectives in evolutionary genetics and model-mediated imposition of theoretical assumptions. J. Theor. Biol. 2006;240:218–232. doi: 10.1016/j.jtbi.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Wolff JN, Gandre S, Kalinin A, Gemmell NJ. Delimiting the Frequency of Paternal Leakage of Mitochondrial DNA in Chinook Salmon. Genetics. 2008;179:1029–1032. doi: 10.1534/genetics.107.085274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JN, Gemmell NJ. Lost in the zygote: the dilution of paternal mtDNA upon fertilization. Heredity. 2008;101:429–434. doi: 10.1038/hdy.2008.74. [DOI] [PubMed] [Google Scholar]
- Wright S. The evolution and genetics of populations: volume 2. Chicago, IL: University of Chicago Press; 1969. [Google Scholar]
- Zeh JA, Zeh DW. Maternal inheritance, sexual conflict and the maladapted male. Trends Genet. 2005;21:281–286. doi: 10.1016/j.tig.2005.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.