Abstract
Objective
Oxidative stress contributes to secondary damage after traumatic brain injury (TBI). Hypothermia decreases endogenous antioxidant consumption and lipid peroxidation after experimental cerebral injury. Our objective was to determine the effect of therapeutic hypothermia on oxidative damage after severe TBI in infants and children randomized to moderate hypothermia vs normothermia.
Design
Prospective randomized controlled study.
Setting
Pediatric ICU of Pittsburgh Children’s Hospital
Patients
The study included 28 patients
Measurements and main results
We compared the effects of hypothermia (32-33°C) vs normothermia in patients treated in a single center involved in a multi-center randomized controlled trial of hypothermia in severe pediatric TBI (GCS score ≤ 8). The patients randomized to hypothermia (n=13) were cooled to target temperature within ∼6h-24h for 48h and then re-warmed. Antioxidant status was assessed by measurements of total antioxidant reserve [AOR] and glutathione. Protein oxidation and lipid peroxidation were assessed by measurements of protein-thiols and F2-isoprostane, respectively in ventricular CSF samples (n=76) obtained on day1-3 after injury. The association between GCS score, age, gender, treatment, temperature, time after injury, and CSF AOR, glutathione, protein-thiol, F2-isoprostane levels were assessed by bivariate and multiple regression models.
Demographic and clinical characteristics were similar between the two treatment groups. Mechanism of injury included both accidental injury and nonaccidental injury. Multiple regression models revealed preservation of CSF antioxidant reserve by hypothermia (p = 0.001). Similarly, a multiple regression model showed that glutathione levels were inversely associated with patient temperature at the time of sampling (p = 0.002). F2-isoprostane levels peaked on day 1 after injury and were progressively decreased thereafter. Although F2-isoprostane levels were ∼3-fold lower in patients randomized to hypothermia vs. normothermia, this difference was not statistically significant.
Conclusion
To our knowledge this is the first study demonstrating that hypothermia attenuates oxidative stress after severe TBI in infants and children. Our data also support the concept that CSF represents a valuable tool for monitoring treatment effects on oxidative stress after TBI.
Introduction
Mild to moderate therapeutic hypothermia (32-33 °C) has been used in clinical practice in pediatric patients with severe traumatic brain injury (TBI), specifically as a second tier therapy (1, 2). Two recent studies including a multicenter trial have demonstrated safety of this treatment modality after TBI in children along with beneficial effects on intracranial hypertension (3, 4). Neuroprotective effects of hypothermia have been shown in a variety of animal species and mechanisms of injury (5-9) and clinical trials have reported efficacy in both cardiac arrest in adults (10, 11)and perinatal asphyxia in newborns (12). Several secondary injury mechanisms are favorably influenced by moderate hypothermia in experimental TBI (13, 14) (15). However, the precise mode of neuroprotective action of mild-moderate hypothermia is not known.
Moderate hypothermia has been shown to have beneficial effects on oxidative stress in experimental models of TBI. Generation of hydroxyl radicals as analyzed by salicylate-trapping method was attenuated by moderate hypothermia after fluid percussion injury in rats (17). Furthermore, treatment of rats with moderate hypothermia after controlled cortical impact increased superoxide dismutase activity relative to values in injured normothermic animals (13). Similarly, therapeutic hypothermia has been shown to attenuate consumption of endogenous antioxidants and decrease lipid peroxidation in experimental temporary focal ischemia and cardiac arrest (14, 15).
Assessment of the extent of oxidative stress in vivo is a complex task requiring employment of a battery of assays evaluating radical scavenging capacity and oxidation products of biomolecules. We previously presented evidence for free radical—mediated lipid peroxidation (by assessment of F2-isoprostanes) and protein oxidation (by assessment of protein thiol) and sustained decreases in total antioxidant reserve and glutathione (GSH) concentrations in cerebrospinal fluid (CSF) after severe TBI in infants and children. F2-isoprostanes are bioactive cyclopentanone prostaglandin-like compounds produced in vivo by free radical peroxidation of arachidonyl-containing lipids, and represent a reliable lipid biomarker of oxidative stress (16). Free radical attack on proteins results in oxidation of their sulfhydryl groups leading to decreased protein thiol concentrations. Our findings suggested that these CSF markers could be valuable to assess the effect of therapies on oxidative stress after TBI in patients.
Several studies suggest that CSF oxidation markers might be associated with outcome after TBI. Pilitsis et al., demonstrated that elevated levels of highly oxidizable polyunsaturated fatty acids in CSF were associated with worse outcome in adults with severe TBI (17). Enhanced lipid peroxidation, as assessed by CSF thiobarbituric acid reactive substances, was reported to correlate with the severity of head injury in adults with contusion (18). In a recent study, Darwish et al., showed that poor neurologic outcome was associated with increased levels of nitrotyrosine, a marker of protein damage by oxidative/nitrative stress, in CSF after TBI in adults (19). These studies indicate that CSF markers of oxidative stress may be useful in prognostication after TBI.
To date there has not been a study assessing the effects of hypothermia on oxidative stress after clinical TBI in either adults or children. Therefore, in the present study we tested the hypothesis that therapeutic hypothermia attenuates oxidative damage as assessed by markers of lipid peroxidation, protein oxidation, and antioxidant status (reduced glutathione and total antioxidant reserve [AOR]) in CSF after severe TBI in infants and children.
Methods
Patient Selection and Data Collection
We examined the effect of moderate therapeutic hypothermia on oxidative stress in CSF from subsets of patients (n=28) enrolled at our center in two concurrent randomized controlled trials assessing the effect and safety of moderate therapeutic hypothermia in severe TBI (GCS [Glasgow Coma Scale] score ≤ 8) in infants and children. The general paradigm for patients treated with hypothermia involved cooling to 32–33°C (within either 6 h or 24h following injury) for 48 h and then gradual re-warming. The details of the study protocols and results of these trials on clinical outcome have been previously reported (3). Briefly, once the patient was randomized to normothermia or hypothermia, a temperature control unit with a rectal probe was used for surface cooling or warming as needed. Temperature was maintained by means of a rectal probe at 32 to 33°C for hypothermia and at 36.5 to 37.5°C for normothermia for the 48-hour study period. To prevent shivering, which could make cooling difficult, sedation and paralysis were used before the initiation of cooling (hypothermia group) and during the study period in both groups. Patients randomized to normothermia were maintained at 36.5 to 37.5°C throughout the study period and were passively warmed if their initial presenting core temperature was less than 36°C. After 48 hours of cooling, rewarming occurred by passively warming the patient 1°C every 3 to 4 hours so as to reach normothermia (36.5°C) within 12 to 18 hours (3). The predetermined entry criteria, in addition to closed head injury and a GCS score of ≤ 8, were age of 0 – 156 months (multicenter trial) or 0 –18 years (single center trial). Patients were excluded from the study if they had a normal initial CT scan (no blood, fracture, swelling, and/or shift), GCS score of 8 with a CT scan with only minimal abnormal findings, prolonged hypotension (>15 min) defined as a mean blood pressure less than 5th percentile for age, failure to obtain informed consent within 6 h of injury (multicenter trial), failure to obtain informed consent within 24 h of admission to Children’s Hospital of Pittsburgh (single center trial), brain dead clinically, penetrating cerebral injury, coagulopathy; PT > 16 and PTT > 40, or pregnancy. This study was approved by the Institutional Review Board of the Children’s Hospital of Pittsburgh, and informed consent was obtained from parents for sample collection. CSF samples (n=76) were centrifuged for 10 min at 5000 x g and stored at -70°C until the time of analysis. Demographic and clinical parameters are seen in Table1.
Table 1.
Clinical and Demographic characteristics of patients
| Characteristic | Hypothermia (n=13) | Normothermia (n=15) |
|---|---|---|
| Age | 6.8 ± 5.1 | 5.1 ± 5.4 |
| Gender (male) | 9 (69) | 8 (53) |
| Glasgow Coma Scale Score | 6.3 ± 1.3 | 6.0 ± 1.3 |
| Mechanism of injury | ||
| Motor vehicle injury | 7 (54) | 10 (66) |
| Inflicted TBI | 2 (15) | 4 (27) |
| Fall | 3 (23) | 1 (7) |
| Other | 1 (8) | 0 (0) |
| Barbiturate use | 5 (39) | 8 (53) |
| Decrompressive craniotomy | 1 (8) | 2 (13) |
Plus-minus values are means ± SD. Percentages are shown in parenthesis.
All patients with severe TBI admitted to the Children’s Hospital of Pittsburgh were treated with ventricular catheter insertion, and CSF was drained continuously. All patients were also intubated and mechanically ventilated to PaCO2 of 35–38 mm Hg. They received sedation with narcotics (fentanyl) and neuromuscular blockade with vecuronium bromide to maintain their intracranial pressure (ICP) and cerebral perfusion pressure (CPP) in the age-appropriate target range in accordance with the guidelines for management of severe pediatric TBI (1). Barbiturates and mechanical ventilation to PaCO2 < 35 mm Hg were used as second tier therapies as needed for refractory intracranial hypertension.
Chemiluminescence measurements of total antioxidant reserve
Total AOR in CSF was assayed by chemiluminescence produced in the presence of luminol and a source of peroxyl radicals, as described by Tyurina et al. (20). A water-soluble azo-initiator, AAPH, was used to produce peroxyl radicals at a constant rate. Oxidation of luminol (400 μM) by AAPH-derived peroxyl radicals was assayed by monitoring the chemiluminescence response. The reaction was initiated by addition of AAPH. A delay in the chemiluminescence response, which is caused by interaction of endogenous antioxidants with AAPH-derived peroxyl radicals, was observed upon addition of CSF. Based on the known rate of peroxyl radical generation by AAPH, the amount of peroxyl radicals scavenged by endogenous antioxidants was determined. A Microlite ML 1000 microtiter plate luminometer (Dynatech Labs, Chantilly, VA, U.S.A.) was used for these determinations.
Fluorescence assay of protein sulfhydryls and glutathione
CSF protein sulfhydryls (Prot-SH) and glutathione concentrations were measured by fluorescent assay using using ThioGlo-1 (Convalent Associates, Inc., Woburn, MA), a maleimide reagent that produces a highly fluorescent product upon its reaction with sulfhydryl groups, as described previously (21). A Cytofluor 2350 fluorescence plate reader (Millipore Corporation, Marlborough, MA, U.S.A.) was used to detect fluorescence using excitation and emission wavelengths of 388 nm and 500 nm, respectively. The data acquired were exported from the spectrophotometer using Cytofluor software.
Determination of F2-isoprostane (8-epi-PGF2α)
CSF F2-isoprostane content was measured by a commercial enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, USA) with a detection limit: 2 pg/ml.
Statistical Analysis
Data are shown as mean ± standard error of mean. Demographic and clinical data were compared by Students t test. The association between GCS score, age, gender, treatment (randomization to hypothermia or normothermia), time after injury, and CSF F2-isoprostane, prot-SH, glutathione, AOR levels were assessed by bivariate and multiple regression models. Because there are multiple observations per person, standard regression models violated the assumption of independence of observations. Therefore, Generalized Estimating Equation models, regression models that control for the correlation within individuals, were used. Some of the study patients randomized to therapeutic hypothermia did not reach target temperature at the time of CSF sampling. Similarly, some patients randomized to normothermia were hypothermic on admission. To assess the impact of actual core temperature on CSF F2-isoprostane level, we used a second model that included the actual core temperature at the time of CSF sampling instead of randomization to hypothermia or normothermia. In each of the models, the beta-coefficient represents the average increase or decrease in CSF biochemical marker levels for a one unit increase in continuous variables (e.g., temperature) or the difference in the average CSF biochemical marker level between the two groups for dichotomous variables.
Results
Patient Demographics
Demographic and clinical parameters of TBI patients are shown in Table 1. Age ranged from 2 months to 16 years. There were 13 patients randomized to hypothermia and 15 patients randomized to normothermia. Initial GCS score ranged between 3 and 8. Mechanism of injury included both accidental injury and child abuse. Five patients randomized to hypothermia and 8 patients randomized to normothermia received barbiturates. One patient randomized to hypothermia and 2 patients randomized to normothermia underwent decrompressive craniotomy. There was no statistically significant difference between the groups for demographic and clinical characteristics.
Total antioxidant reserve
For all biochemical analysis, mean sample collection times were not different between hypothermia and normothermia groups (10.4 ± 4.8 h vs 12.4 ± 7.3 h on day 1; 42.5 ± 3.2 h vs 40.8 ± 4.4 h on day 2, 62.6 ± 5.4 h vs 66.9 ± 4.1 h on day 3, Mean ± SD). The luminol-enhanced chemiluminescence assay revealed a reduction in total antioxidant reserve in patients randomized to normothermia vs hypothermia (117.9 ± 8.34 vs 90.23 ± 5.96 on day 2 after TBI) (Fig. 1). Bivariate and multiple regression models revealed a highly significant effect of hypothermia on CSF total antioxidant reserve independent of age, gender and initial GCS score (p = 0.002 and p = 0.001) (Table 2). Sustained depletion of total antioxidant reserve was suspected because the greatest decrease was observed on day 3, compared with day 1 and 2 (Fig. 1). There was an inverse relationship between temperature and CSF total antioxidant reserve after injury (p = 0.022) (Table 3).
Figure 1.
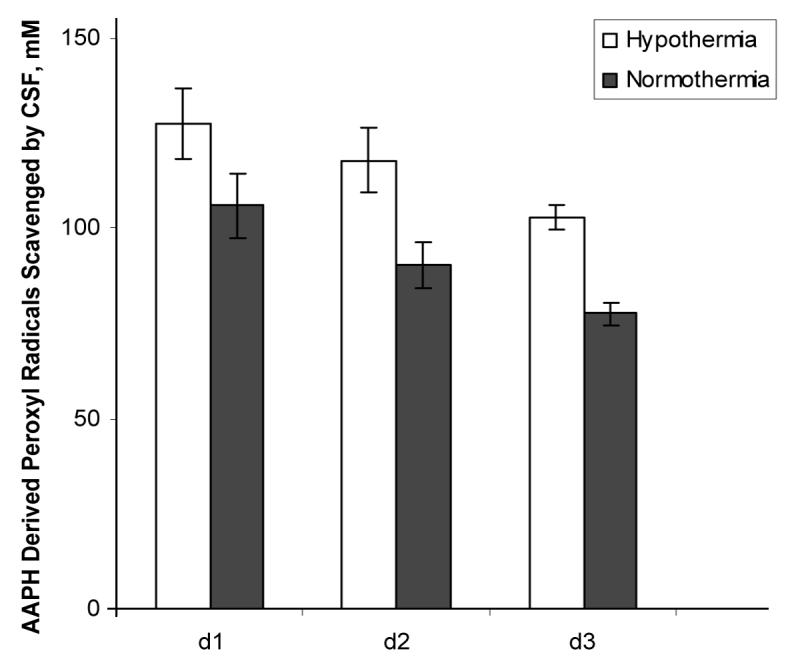
Effect hypothermia on CSF antioxidant reserves after TBI. Hypothermia preserved antioxidant reserves after TBI compared with normothermia.
Table 2.
Effect of hypothermia treatment on CSF biomarkers of oxidative stress
| CSF biomarker | Univariate model | Multivariate model | ||
|---|---|---|---|---|
| β | p value | β | p value | |
| Total Antioxidant Reserve | ||||
| Treatment (reference = NT) | -25.1 | 0.002 | -25.4 | 0.001 |
| Time (Days 1, 2, 3) | -14.5 | <.0001 | -14.3 | <.0001 |
| Age | 0 | 0.79 | 0 | 0.473 |
| Gender | -13.4 | 0.115 | -9.5 | 0.18 |
| GCS | -6.4 | 0.385 | -10.92 | 0.112 |
| Glutathione (GSH) | ||||
| Treatment (reference = NT) | -0.3 | 0.097 | -0.4 | 0.09 |
| Time (Days 1, 2, 3) | -0.3 | <.0001 | -2.9 | <.0001 |
| Age | 0 | 0.911 | 0 | 0.705 |
| Gender | -0.1 | 0.79 | 0.1 | 0.784 |
| GCS | 0.1 | 0.645 | 0.1 | 0.741 |
| Protein sulfhydryls | ||||
| Treatment (reference = NT) | -6.1 | 0.063 | -6 | 0.079 |
| Time (Days 1, 2, 3) | 0.1 | 0.969 | 0.2 | 0.873 |
| Age | 0 | 0.563 | 0 | 0.753 |
| Gender | -4.6 | 0.151 | -3.9 | 0.231 |
| GCS | -0.7 | 0.878 | -2.6 | 0.494 |
| F2 -isoprostane | ||||
| Treatment (reference = NT) | 13.3 | 0.099 | -15.6 | 0.183 |
| Time (Days 1, 2, 3) | -14.7 | 0.029 | -15 | 0.023 |
| Age | 0 | 0.913 | 0 | 0.636 |
| Gender | 3.6 | 0.668 | 4 | 0.605 |
| GCS | 5.4 | 0.381 | 11.7 | 0.188 |
GCS, Glasgow Coma Scale Score; NT, normothermia
Table 3.
Association of CSF metabolites with temperature.
| Metabolite | r | p |
|---|---|---|
| Total antioxidant reserve | -0.3 | .022 |
| Protein sulfhydryls | -0.2 | .104 |
| Glutathione | -0.3 | .002 |
| F2-Isoprostane | -0.2 | .104 |
Glutathione
GSH levels progressively decreased after the peak on day 1 similar to that previously reported (Fig. 2). Bivariate and multiple regression models revealed a tendency for higher GSH levels in CSF with hypothermia (p = 0.097 and p = 0.090) (Table 2). There was an inverse relationship between temperature and glutathione concentration in CSF after injury (p = 0.002) (Table 3).
Figure 2.
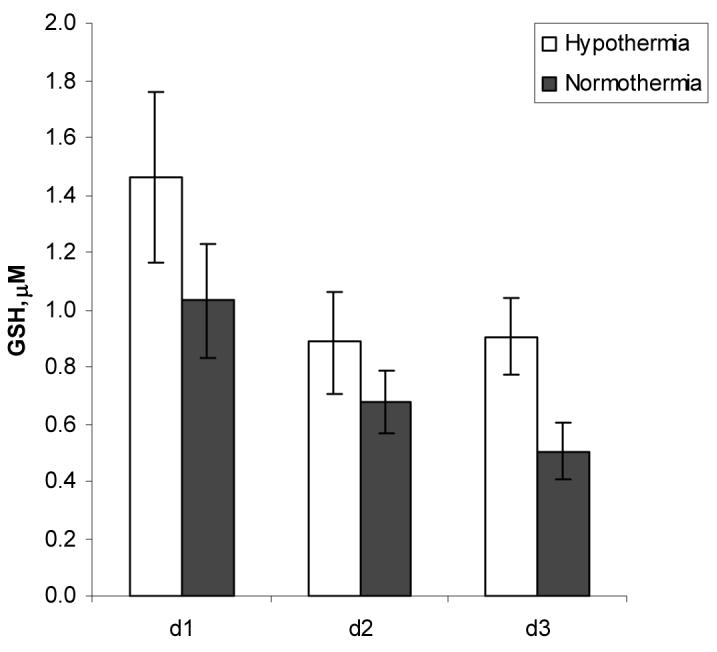
Effect of hypothermia and time on CSF GSH levels after TBI.
Protein sulfhydryl oxidation
CSF concentration of protein sulfhydryls was about 3-fold lower on d 2 in patients randomized to hypothermia than patients randomized to normothermia (9.45 ± 1.41 vs 26.59 ± 7.21 nmol/mg protein) (Fig. 3). Bivariate and multiple regression models revealed a tendency for higher CSF protein sulfhydryl levels with hypothermia (p = 0.063 and p = 0.079) (Table 2). In general there was an inverse relationship between temperature and CSF protein sulfhydryl levels at all times after injury, but this was not statistically significant (p = 0.104) (Table 3)..
Figure 3.
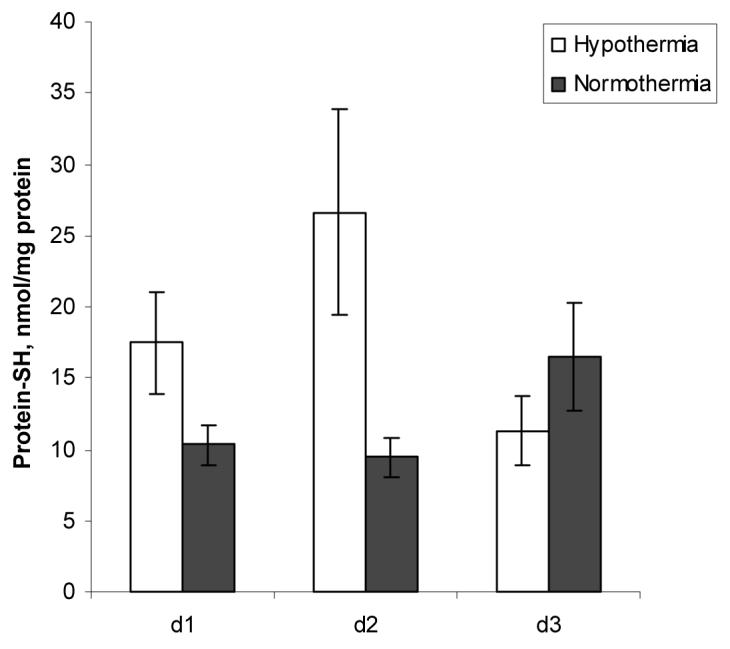
Effect of hypothermia and time on CSF Protein sulfydryl levels after TBI.
F2-isoprostane
F2-isoprostane levels progressively decreased after the peak on day 1 in patients randomized to normothermia similar to that previously reported (Fig. 4). Bivariate and multiple regression models did not reveal a significant effect of hypothermia (Table 2) despite 3.6-fold higher CSF F2-isoprostane levels in patients randomized to normothermia on d 1 after TBI (65.70 ± 24.83 pg/mL) compared with patients randomized to hypothermia (18.23 ± 1.84 pg/mL) (Fig. 4). At all times after injury, there was no significant temperature effect on CSF F2-isoprostane levels (p = 0.104) (Table 3).
Figure 4.
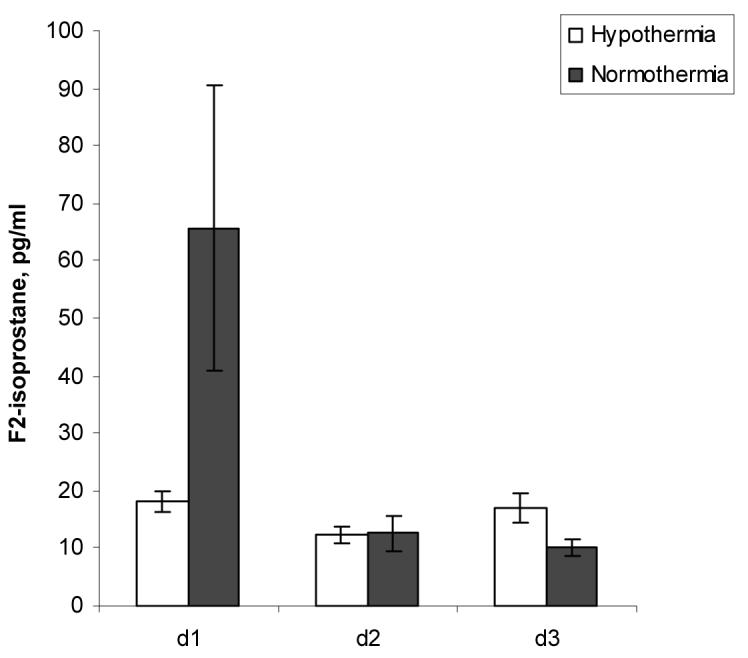
Effect of hypothermia and time on CSF F2-isoprostane levels after TBI.
Discussion
We have previously shown that severe TBI in infants and children is accompanied by marked and progressive compromise of antioxidant defenses and free radical-mediated lipid peroxidation (21). Here we present data demonstrating a beneficial effect of hypothermia on oxidative stress in the same setting. Our clinical data are consistent with results in experimental trauma models (13, 22) in that hypothermia attenuates consumption of endogenous antioxidants. In addition, we have shown for the first time that hypothermia tended to decreased protein oxidation. We observed an early peak in F2-isoprostane levels, consistent with our previous study in infants and children (21), suggesting the possible need for early application of therapies targeting some aspects of oxidative stress after TBI, such as lipid peroxidation.
Possible mechanisms of beneficial effect of hypothermia on oxidative stress after TBI
Mild-moderate hypothermia has been shown to have neuroprotective effects in experimental and clinical brain injury resulting from trauma, cardiac arrest and ischemia (10, 11, 14, 15, 22, 23). The precise mode of neuroprotective action by mild-moderate hypothermia is not known. Most likely, mild-moderate hypothermia exhibits multiple and synergistic effects on brain metabolism however it does not indiscriminately slow down all biochemical cascades. There is controversy regarding effects of mild-moderate hypothermia on cerebral energy metabolism likely due to differences in experimental insults and species studied. Mild-moderate hypothermia has been shown to decrease the global cerebral metabolic rate for glucose and oxygen but maintain a slightly better energy level by reducing ATP breakdown (24, 25). Beneficial effects of mild-moderate hypothermia on energy balance and production of reactive oxygen species in mitochondria have also been reported after experimental ischemia reperfusion injury outside the CNS and in retina (26-29). However, classic studies in cerebral ischemia failed to show an effect of mild-moderate hypothermia on energy metabolite levels (30).
Mild-moderate hypothermia reduces the increase in intracellular calcium levels, which is linked to excitotoxicity and oxidative stress, after experimental cerebral ischemia and TBI (31-33). The majority of the free radicals that are produced after brain injury are generated by enzymatically catalyzed mechanisms —such as nitric oxide synthase (NOS)- and by deregulated electron transporters in mitochondria (34-38). Therefore we speculate that beneficial effects of hypothermia on oxidative stress after TBI might be explained by prevention of mitochondrial failure and reduction in excitotoxicity. Moderate hypothermia has been shown to attenuate increases in CSF acetylcholine levels (39) and brain interstitial levels of glutamate and aspartate seen after fluid percussion injury (22). Although, the latter finding was not consistently observed across experimental TBI models (40, 41). Moderate hypothermia has also been shown to attenuate increases in CSF glutamate levels in adult severe TBI victims (23).
TBI-induced oxidative stress is importantly linked to inflammatory response. Several components of the local inflammatory response to cerebral contusion are also favorably affected by therapeutic hypothermia in experimental TBI, as evidenced by reductions in neutrophil accumulation (42-44), interleukin-1 (IL-1) mRNA upregulation (45, 46) and inducible NOS activity (47). Increases in cytokines after severe TBI, however, are not consistently attenuated by therapeutic hypothermia in adults (23, 48, 49). Similarly, macrophage accumulation as assessed by CSF quinolinic acid levels was not attenuated by moderate hypothermia in adult severe TBI victims (50).
A beneficial effect of hypothermia on oxidative stress has been shown in experimental studies outside the CNS with reduction in lipid peroxidation in ischemic kidney and liver tissue (51, 52). Antioxidant supplementation with hypothermia had additive protective effects against lipid peroxidation in these experiments. Similarly, our data shows that hypothermia only partially restored antioxidant defenses. Given that mild-moderate hypothermia has shown variable or partial beneficial effects (ICP vs outcome) in clinical TBI (4, 23, 53, 54), supplementation with antioxidants may represent a valuable adjunct to hypothermia in the treatment of TBI victims.
Clinical implications
To date, there have been no studies assessing the effect of therapeutic hypothermia on oxidative stress after TBI in either adults or infants and children. Two large clinical trials in TBI using antioxidants (PEG-SOD and tirilazad) showed largely negative results (55, 56). Although these were seminal clinical studies in this area of investigation, they focused exclusively on therapeutic effects on clinical outcome. Neither study assessed whether or not oxidative stress was favorably influenced by the treatment. Furthermore, these two agents appear to have very limited brain penetrating ability (57). Our findings suggest that quantification of biomarkers of oxidative stress and antioxidant status of CSF may provide a valuable tool for monitoring treatment effects of antioxidant strategies or other therapies such as hypothermia, anti-excitotoxic, anti-apoptotic and anti-inflammatory agents after TBI.
We have previously shown gender differences in response to beneficial effects of therapeutic hypothermia on oxidative stress in adults after severe TBI (58, 59). Current study does not show a gender difference in oxidative stress response to hypothermia in infants and children. This may suggest a powerful effect of sex steroids on this specific parameter that would manifest after puberty.
Direct assessment of relationship of biochemical markers with temperature
In this study we also carried out a separate analysis to directly assess the relationship between markers of oxidative stress and patient temperature at the time of CSF sampling. This second approach demonstrated highly significant preservation effect of hypothermia on antioxidants in CSF—confirming our initial analytical approach. Given that hypothermia took time to induce and that some patients can present with mild hypothermia, this additional statistical approach is, we believe, important to provide a more complete picture of the relationship between markers of oxidative stress and temperature.
Developmental Ramifications
Experimental studies in developmental studies of TBI support the notion that immature brain is particularly vulnerable to oxidative stress due to compromised antioxidant status (60). Our prior study supported this conclusion and showed profound antioxidant depletion lasting several days after severe TBI (21). Thus oxidative stress mechanisms might be an attractive target for therapeutic interventions in developmental CNS injury.
Limitations of the study
Despite the relatively small sample size and considerable variability in patient age, mechanism of injury, and treatment, statistical significance was achieved in a multivariate analysis between TBI patients randomized to hypothermia vs normothermia for AOR and glutathione. Although this study represents the largest biochemical study of oxidative stress markers in infants and children in a randomized trial of hypothermia after severe TBI, future studies with larger sample size are needed. Despite 3-fold lower value of F2-isoprostane in patients randomized to hypothermia vs normothermia, we were unable to show a significant attenuation of lipid peroxidation by hypothermia. Sample size estimates suggest that 36 patients per group would be needed to appropriately assess for an effect of hypothermia on this parameter accepting a power of 0.8. Similarly, the small number of patients with GCS 3-4 in both groups also limits our ability to assess the effect of hypothermia in most severely injured patients. While assessment of ventricular CSF provides great promise, it reflects changes across the entire brain and not simply in the injured tissue. It is likely that biochemical alterations seen with hypothermia may in part be from healthy brain tissue.
Barbiturates may reduce the overall cerebral metabolism and may therefore change the biochemical response. Although there was no statistically significant difference between the groups for barbiturate use (p = 0.48), a greater number of patients in the normothermia group received barbiturates vs hypothermia group. This suggests that other therapies, such as barbiturates, might be utilized more frequently for ICP control in the absence of hypothermia. In additional statistical analysis, barbiturate use did not have consistent effect on the CSF oxidative stress markers assessed in this study. Further studies with a larger sample size, including a comprehensive analysis of biochemical data as it relates to outcome, mortality, mechanism of injury (accidental trauma versus child abuse), age, gender, other therapies (such as barbiturate use and decompressive craniotomy) are needed. Asessment of the relationship between markers of oxidative stress and associated mechanisms of secondary damage such as apoptosis and excitotoxicity (61, 62) could also be revealing.
Finally although we cite these limitations, we have also reported, in the same patient population, that hypothermia failed to attenuate the marked increases in CSF cytokine levels after TBI (63). Taken together our findings mirror those observations in experimental models of TBI and support a differential effect of mild-moderate hypothermia across injury mechanisms. Future research in this area might improve our understanding of mechanisms of neuroprotection by hypothermia in TBI and enable us augment its beneficial effects by targeted combination therapies.
Conclusions
Moderate therapeutic hypothermia preserves antioxidant defenses after severe TBI in infants and children. Our data support the concept that CSF represents a valuable tool for monitoring treatment effects on oxidative stress after TBI.
Acknowledgements
This study was supported by grants from NIH (NS30318, NS38087, NS34884 NS052478, HD057587), Laerdal Foundation, CDC (University of Pittsburgh Centers for Injury Research and Control [CIRCL]) and American Heart Association (0535365N). Dr.s Paul Shore and YiChen Lai were funded by T-32 (HD40686).
References
- 1.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 8. Cerebral perfusion pressure. Pediatr Crit Care Med. 2003;4:S31–33. [PubMed] [Google Scholar]
- 2.Selden PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 14. The role of temperature control following severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S53–55. [PubMed] [Google Scholar]
- 3.Adelson PD, Ragheb J, Kanev P, Brockmeyer D, Beers SR, Brown SD, Cassidy LD, Chang Y, Levin H.Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children Neurosurgery 200556740–754.; discussion 740-754. [DOI] [PubMed] [Google Scholar]
- 4.Biswas AK, Bruce DA, Sklar FH, Bokovoy JL, Sommerauer JF. Treatment of acute traumatic brain injury in children with moderate hypothermia improves intracranial hypertension. Crit Care Med. 2002;30:2742–2751. doi: 10.1097/00003246-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Behringer W, Safar P, Kentner R, Wu X, Kagan VE, Radovsky A, Clark RS, Kochanek PM, Subramanian M, Tyurin VA, et al. Antioxidant Tempol enhances hypothermic cerebral preservation during prolonged cardiac arrest in dogs. J Cereb Blood Flow Metab. 2002;22:105–117. doi: 10.1097/00004647-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Buki A, Koizumi H, Povlishock JT. Moderate posttraumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp Neurol. 1999;159:319–328. doi: 10.1006/exnr.1999.7139. [DOI] [PubMed] [Google Scholar]
- 7.Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001;94:90–96. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- 8.Wang KK, Larner SF, Robinson G, Hayes RL. Neuroprotection targets after traumatic brain injury. Curr Opin Neurol. 2006;19:514–519. doi: 10.1097/WCO.0b013e3280102b10. [DOI] [PubMed] [Google Scholar]
- 9.Yager J, Towfighi J, Vannucci RC. Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res. 1993;34:525–529. doi: 10.1203/00006450-199310000-00029. [DOI] [PubMed] [Google Scholar]
- 10.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 11.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 12.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 13.DeKosky ST, Abrahamson EE, Taffe KM, Dixon CE, Kochanek PM, Ikonomovic MD. Effects of post-injury hypothermia and nerve growth factor infusion on antioxidant enzyme activity in the rat: implications for clinical therapies. J Neurochem. 2004;90:998–1004. doi: 10.1111/j.1471-4159.2004.02575.x. [DOI] [PubMed] [Google Scholar]
- 14.Karibe H, Chen SF, Zarow GJ, Gafni J, Graham SH, Chan PH, Weinstein PR. Mild intraischemic hypothermia suppresses consumption of endogenous antioxidants after temporary focal ischemia in rats. Brain Res. 1994;649:12–18. doi: 10.1016/0006-8993(94)91043-x. [DOI] [PubMed] [Google Scholar]
- 15.Lei B, Tan X, Cai H, Xu Q, Guo Q. Effect of moderate hypothermia on lipid peroxidation in canine brain tissue after cardiac arrest and resuscitation. Stroke. 1994;25:147–152. doi: 10.1161/01.str.25.1.147. [DOI] [PubMed] [Google Scholar]
- 16.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ., 2nd. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci USA. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilitsis JG, Coplin WM, O’Regan MH, Wellwood JM, Diaz FG, Fairfax MR, Michael DB, Phillis JW. Free fatty acids in cerebrospinal fluids from patients with traumatic brain injury. Neurosci Lett. 2003;349:136–138. doi: 10.1016/s0304-3940(03)00803-6. [DOI] [PubMed] [Google Scholar]
- 18.Kasprzak HA, Wozniak A, Drewa G, Wozniak B. Enhanced lipid peroxidation processes in patients after brain contusion. J Neurotrauma. 2001;18:793–797. doi: 10.1089/089771501316919157. [DOI] [PubMed] [Google Scholar]
- 19.Darwish RS, Amiridze N, Aarabi B. Nitrotyrosine as an oxidative stress marker: evidence for involvement in neurologic outcome in human traumatic brain injury. J Trauma. 2007;63:439–442. doi: 10.1097/TA.0b013e318069178a. [DOI] [PubMed] [Google Scholar]
- 20.Tyurin VA, Tyurina YY, Borisenko GG, Sokolova TV, Ritov VB, Quinn PJ, Rose M, Kochanek P, Graham SH, Kagan VE. Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J Neurochem. 2000;75:2178–2189. doi: 10.1046/j.1471-4159.2000.0752178.x. [DOI] [PubMed] [Google Scholar]
- 21.Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, Graham SH, Janesko K, Clark RS, Kochanek PM. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res. 2002;51:571–578. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- 23.Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 24.Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513–530. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- 25.Tokutomi T, Morimoto K, Miyagi T, Yamaguchi S, Ishikawa K, Shigemori M.Optimal temperature for the management of severe traumatic brain injury: effect of hypothermia on intracranial pressure, systemic and intracranial hemodynamics, and metabolism Neurosurgery 200352102–111.; discussion 111-102. [PubMed] [Google Scholar]
- 26.Rainio P, Kaukoranta PK, Sormunen R, Juvonen T, Peuhkurinen KJ. Ultrastructural changes in myocardium during mild hypothermic retrograde blood cardioplegia. Scand Cardiovasc J. 1998;32:353–359. doi: 10.1080/14017439850139807. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor F, Castillo-Olivares JL, Romero G, Eguaras MG, Juffe A, Gosalvez M, Figuera D. Anoxic cardiac arrest: Its effect on myocardial mitochondrial metabolism. J Cardiovasc Surg (Torino) 1975;16:493–499. [PubMed] [Google Scholar]
- 28.Simkhovich BZ, Hale SL, Kloner RA. Metabolic mechanism by which mild regional hypothermia preserves ischemic tissue. J Cardiovasc Pharmacol Ther. 2004;9:83–90. doi: 10.1177/107424840400900203. [DOI] [PubMed] [Google Scholar]
- 29.Quinones-Hinojosa A, Malek JY, Ames A, 3rd, Ogilvy CS, Maynard KI.Metabolic effects of hypothermia and its neuroprotective effects on the recovery of metabolic and electrophysiological function in the ischemic retina in vitro Neurosurgery 2003521178–1186.; discussion 1186-1177. [PubMed] [Google Scholar]
- 30.Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- 31.Takata T, Nabetani M, Okada Y. Effects of hypothermia on the neuronal activity, [Ca2+]i accumulation and ATP levels during oxygen and/or glucose deprivation in hippocampal slices of guinea pigs. Neurosci Lett. 1997;227:41–44. doi: 10.1016/s0304-3940(97)00296-6. [DOI] [PubMed] [Google Scholar]
- 32.Hu BR, Kamme F, Wieloch T. Alterations of Ca2+/calmodulin-dependent protein kinase II and its messenger RNA in the rat hippocampus following normo- and hypothermic ischemia. Neuroscience. 1995;68:1003–1016. doi: 10.1016/0306-4522(95)00213-3. [DOI] [PubMed] [Google Scholar]
- 33.Maeda T, Katayama Y, Kawamata T, Yamamoto T. Mechanisms of excitatory amino acid release in contused brain tissue: effects of hypothermia and in situ administration of Co2+ on extracellular levels of glutamate. J Neurotrauma. 1998;15:655–664. doi: 10.1089/neu.1998.15.655. [DOI] [PubMed] [Google Scholar]
- 34.Robertson CL, Soane L, Siegel ZT, Fiskum G. The potential role of mitochondria in pediatric traumatic brain injury. Dev Neurosci. 2006;28:432–446. doi: 10.1159/000094169. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan PG, Springer JE, Hall ED, Scheff SW. Mitochondrial uncoupling as a therapeutic target following neuronal injury. J Bioenerg Biomembr. 2004;36:353–356. doi: 10.1023/B:JOBB.0000041767.30992.19. [DOI] [PubMed] [Google Scholar]
- 36.Merenda A, Bullock R. Clinical treatments for mitochondrial dysfunctions after brain injury. Curr Opin Crit Care. 2006;12:90–96. doi: 10.1097/01.ccx.0000216573.26686.27. [DOI] [PubMed] [Google Scholar]
- 37.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 39.Lyeth BG, Jiang JY, Robinson SE, Guo H, Jenkins LW. Hypothermia blunts acetylcholine increase in CSF of traumatically brain injured rats. Mol Chem Neuropathol. 1993;18:247–256. doi: 10.1007/BF03160117. [DOI] [PubMed] [Google Scholar]
- 40.Palmer AM, Marion DW, Botscheller ML, Redd EE. Therapeutic hypothermia is cytoprotective without attenuating the traumatic brain injury-induced elevations in interstitial concentrations of aspartate and glutamate. J Neurotrauma. 1993;10:363–372. doi: 10.1089/neu.1993.10.363. [DOI] [PubMed] [Google Scholar]
- 41.Koizumi H, Fujisawa H, Ito H, Maekawa T, Di X, Bullock R. Effects of mild hypothermia on cerebral blood flow-independent changes in cortical extracellular levels of amino acids following contusion trauma in the rat. Brain Res. 1997;747:304–312. doi: 10.1016/s0006-8993(96)01240-1. [DOI] [PubMed] [Google Scholar]
- 42.Whalen MJ, Carlos TM, Clark RS, Marion DW, DeKosky ST, Heineman S, Schiding JK, Memarzadeh F, Kochanek PM. The effect of brain temperature on acute inflammation after traumatic brain injury in rats. J Neurotrauma. 1997;14:561–572. doi: 10.1089/neu.1997.14.561. [DOI] [PubMed] [Google Scholar]
- 43.Rosomoff HL, Clasen RA, Hartstock R, Bebin J. Brain reaction to experimental injury after hypothermia. Arch Neurol. 1965;13:337–345. doi: 10.1001/archneur.1965.00470040003001. [DOI] [PubMed] [Google Scholar]
- 44.Chatzipanteli K, Alonso OF, Kraydieh S, Dietrich WD. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J Cereb Blood Flow Metab. 2000;20:531–542. doi: 10.1097/00004647-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Goss JR, Styren SD, Miller PD, Kochanek PM, Palmer AM, Marion DW, DeKosky ST. Hypothermia attenuates the normal increase in interleukin 1 beta RNA and nerve growth factor following traumatic brain injury in the rat. J Neurotrauma. 1995;12:159–167. doi: 10.1089/neu.1995.12.159. [DOI] [PubMed] [Google Scholar]
- 46.Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD.Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature Neurosurgery 200251195–203.; discussion 203. [DOI] [PubMed] [Google Scholar]
- 47.Chatzipanteli K, Wada K, Busto R, Dietrich WD. Effects of moderate hypothermia on constitutive and inducible nitric oxide synthase activities after traumatic brain injury in the rat. J Neurochem. 1999;72:2047–2052. doi: 10.1046/j.1471-4159.1999.0722047.x. [DOI] [PubMed] [Google Scholar]
- 48.Aibiki M, Maekawa S, Ogura S, Kinoshita Y, Kawai N, Yokono S. Effect of moderate hypothermia on systemic and internal jugular plasma IL-6 levels after traumatic brain injury in humans. J Neurotrauma. 1999;16:225–232. doi: 10.1089/neu.1999.16.225. [DOI] [PubMed] [Google Scholar]
- 49.Shiozaki T, Kato A, Taneda M, Hayakata T, Hashiguchi N, Tanaka H, Shimazu T, Sugimoto H. Little benefit from mild hypothermia therapy for severely head injured patients with low intracranial pressure. J Neurosurg. 1999;91:185–191. doi: 10.3171/jns.1999.91.2.0185. [DOI] [PubMed] [Google Scholar]
- 50.Sinz EH, Kochanek PM, Heyes MP, Wisniewski SR, Bell MJ, Clark RS, DeKosky ST, Blight AR, Marion DW. Quinolinic acid is increased in CSF and associated with mortality after traumatic brain injury in humans. J Cereb Blood Flow Metab. 1998;18:610–615. doi: 10.1097/00004647-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 51.McAnulty JF, Huang XQ. The efficacy of antioxidants administered during low temperature storage of warm ischemic kidney tissue slices. Cryobiology. 1997;34:406–415. doi: 10.1006/cryo.1997.2011. [DOI] [PubMed] [Google Scholar]
- 52.Lee KT, Tsai LY, Sheen PC. Effect of vitamin E, topical hypothermia and steroid on ischemic liver in rats. Kaohsiung J Med Sci. 1998;14:6–12. [PubMed] [Google Scholar]
- 53.Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, Jr., Muizelaar JP, Wagner FC, Jr., Marion DW, Luerssen TG, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 54.Gupta AK, Al-Rawi PG, Hutchinson PJ, Kirkpatrick PJ. Effect of hypothermia on brain tissue oxygenation in patients with severe head injury. Br J Anaesth. 2002;88:188–192. doi: 10.1093/bja/88.2.188. [DOI] [PubMed] [Google Scholar]
- 55.Young B, Runge JW, Waxman KS, Harrington T, Wilberger J, Muizelaar JP, Boddy A, Kupiec JW. Effects of pegorgotein on neurologic outcome of patients with severe head injury. A multicenter, randomized controlled trial. Jama. 1996;276:538–543. [PubMed] [Google Scholar]
- 56.Marshall LF, Maas AI, Marshall SB, Bricolo A, Fearnside M, Iannotti F, Klauber MR, Lagarrigue J, Lobato R, Persson L, et al. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J Neurosurg. 1998;89:519–525. doi: 10.3171/jns.1998.89.4.0519. [DOI] [PubMed] [Google Scholar]
- 57.Hall ED, Andrus PK, Smith SL, Fleck TJ, Scherch HM, Lutzke BS, Sawada GA, Althaus JS, Vonvoigtlander PF, Padbury GE, et al. Pyrrolopyrimidines: novel brain-penetrating antioxidants with neuroprotective activity in brain injury and ischemia models. J Pharmacol Exp Ther. 1997;281:895–904. [PubMed] [Google Scholar]
- 58.Bayir H, Marion DW, Puccio AM, Wisniewski SR, Janesko KL, Clark RS, Kochanek PM. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- 59.Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J Neurotrauma. 2004;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- 60.Fan P, Yamauchi T, Noble LJ, Ferriero DM. Age-dependent differences in glutathione peroxidase activity after traumatic brain injury. J Neurotrauma. 2003;20:437–445. doi: 10.1089/089771503765355513. [DOI] [PubMed] [Google Scholar]
- 61.Kochanek PM, Clark RS, Ruppel RA, Adelson PD, Bell MJ, Whalen MJ, Robertson CL, Satchell MA, Seidberg NA, Marion DW, et al. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside. Pediatr Crit Care Med. 2000;1:4–19. doi: 10.1097/00130478-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Satchell MA, Lai Y, Kochanek PM, Wisniewski SR, Fink EL, Siedberg NA, Berger RP, DeKosky ST, Adelson PD, Clark RS. Cytochrome c, a biomarker of apoptosis, is increased in cerebrospinal fluid from infants with inflicted brain injury from child abuse. J Cereb Blood Flow Metab. 2005;25:919–927. doi: 10.1038/sj.jcbfm.9600088. [DOI] [PubMed] [Google Scholar]
- 63.Buttram SD, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, Berger RP, Clark RS, Kochanek PM. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J Neurotrauma. 2007;24:1707–1717. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]


