Abstract
OBJECTIVE
Hutchinson-Gilford progeria syndrome (HGPS) is a rare early-onset accelerated senescence syndrome. In HGPS, a recently identified de novo dominant mutation of the lamin A gene (LMNA) produces abnormal lamin A, resulting in compromised nuclear membrane integrity. Clinical features include sclerotic skin, cardiovascular and bone abnormalities, and marked growth retardation. Craniofacial features include “bird-like” facies, alopecia, craniofacial disproportion and dental crowding. Our prospective study describes dental, oral soft tissue, and craniofacial bone features in HGPS.
METHODS
Fifteen patients with confirmed p.G608G LMNA mutation (1–17 years, 7 males, 8 females) received comprehensive oral evaluations. Anomalies of oral soft tissue, gnathic bones and dentition were identified.
RESULTS
Radiographic findings included hypodontia (n=7), dysmorphic teeth (n=5), steep mandibular angles (n=11), and thin basal bone (n=11). Soft tissue findings included ogival palatal arch (n=8), median sagittal palatal fissure (n=7), and ankyloglossia (n=7). Calculated dental ages (9months–11y2m) were significantly lower than chronological ages (1y6m–17y8m) (p=0.002). Eleven children manifested a shorter mandibular body, anterior/posterior cranial base and ramus, but a larger gonial angle, compared to age/gender/race norms.
CONCLUSION
Novel oral-craniofacial phenotypes and quantification of previously reported features are presented. Our findings expand the HGPS phenotype and provide additional insight into the complex pathogenesis of HGPS.
Keywords: Progeria, craniofacial, dentition, oral, phenotype
INTRODUCTION
Originally described more than 100 years ago, Hutchinson-Gilford progeria syndrome (HGPS, Online Mendelian Inheritance in Man #176670) is a rare (1 in 4–8 million births) genetic disorder characterized by a distinct aged appearance very early in life (Martin 2005; Pollex et al, 2004). Since 1886 fewer than100 cases of HGPS have been reported, with approximately 40 cases currently diagnosed (Pollex et al, 2004). From the Greek geras, meaning “old age,” progeria is a human disease model of accelerated senescence. Affected children typically appear normal at birth but begin to demonstrate features of accelerated aging within the first year of life. Mean age at diagnosis is 2.9 years (Hennekam 2006).
The inheritance of HGPS was once proposed to be autosomal recessive on account of affected individuals found in consanguineous families (Pollex et al, 2004). However, cases of sporadic autosomal dominant inheritance were documented and eventually lead to the isolation of the HGPS gene on chromosome 1q (Pollex et al, 2004). Most cases of HGPS are caused by a de novo single-nucleotide substitution in codon 608 of prelamin A (p. G608G (GGC>GGT), p. G608S (GGC>AGC)), leading to the mutated HGPS gene product lamin A (LMNA), a structural component of the nuclear membrane (Fong et al, 2004, Goldman et al, 2004). Lamin A contributes to nuclear structural integrity and chromatin regulatory mechanisms (Martin 2005). The mutant form of lamin A, called progerin, acts in a dominant negative fashion (Goldman et al, 2004). While progerin is expressed at very low levels normally, it is expressed at much greater levels in HGPS (McClintock et al, 2007). Progerin accumulation in cells has been associated with instability of the nuclear membrane, progressive nuclear damage and premature cell death. Studies have shown structural nuclear abnormalities in 48% of HGPS fibroblast nuclei compared with less than 6% of normal control cells (Pollex et al, 2004; Paradisi et al 2005). Furthermore, HGPS fibroblasts undergo hyperproliferation followed by rapid apoptosis (Pollex et al, 2004), demonstrating the cellular processes in premature aging from this abnormal gene product.
Clinically, the striking features of HGPS involve alterations in skin, bone and cardiovascular tissues; affected children exhibit respiratory and cardiovascular capacities of senior citizens (Pollex et al, 2004). Marked retardation of growth, loss of subcutaneous fat, and distinctive bone changes (i.e., resorption of the clavicles and replacement by fibrous tissue; osteolysis of the distal phalanges; and joint dislocations) are hallmark characteristics (Sarkar et al, 2001). On average, death occurs at 13 years of age, most commonly from progressive coronary and cardiovascular atherosclerosis (Pollex et al, 2004, Merideth et al, 2008)
The facial features are distinct and include prominent eyes and a beaked nose, resulting in the characteristic “bird-like” facies which are often missing skin appendages such as eye lashes, eyebrows, and earlobes (Figure 1). Typical craniofacial features include alopecia with easy mapping of the scalp veins, micrognathia and craniofacial disproportion (Pollex et al, 2004). Deficiencies in the growth of both the maxilla and mandible have been described, as well as dental crowding, irregular teeth eruption and localized areas of enamel hypoplasia (Gordon et al, 2007; Hasty et al, 1988; Wesley et al, 1979; Yu et al, 1991). Significant delay in dental eruption has been noted, although data on permanent teeth eruption remain scarce (Hennekam 2006). To date, a systematic assessment of oral, dental and craniofacial phenotypes has not been conducted (Hennekam 2006).
Figure 1.
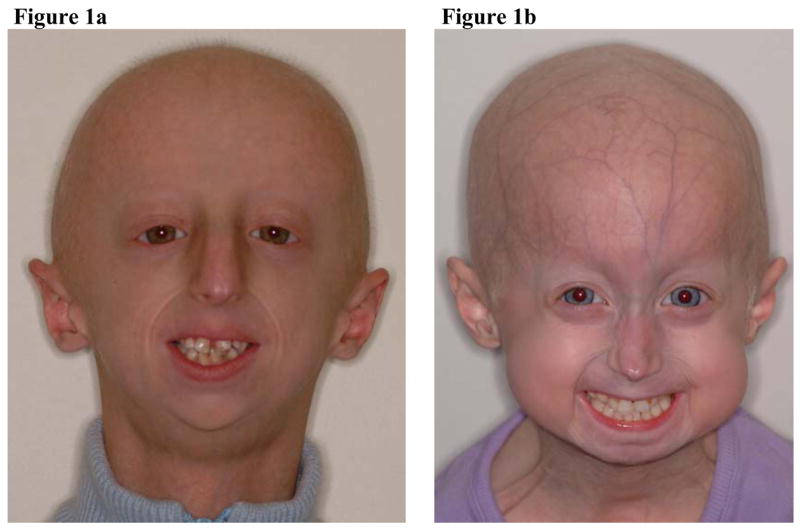
10-year old Caucasian male (a) and 5–year old Caucasian female (b) with typical HGPS facial features (i.e., loss of subcutaneous fat, beaked nose, missing eye lashes and eyebrows, alopecia with easy mapping of the scalp veins, absence of earlobes and micrognathia).
The objective of the present study is to further characterize the intraoral, dental and craniofacial features in HGPS. We conducted a prospective examination of 15 HGPS patients, and now present detailed characteristics of dentition, oral soft tissues and occlusal development, as well as of craniofacial features using cephalometric analyses. General characteristics of the 15 patients described here have been previously reported (Merideth et al, 2008).
METHODS
Fifteen unrelated patients with confirmed p. G608G LMNA mutation, representing nearly half of all children currently diagnosed with HGPS (age range: 1 year 6months to 17 years, 8months; mean: 7y1m±4.29; median: 6y11m; 7 males, 8 females), were referred for consultation to the dental clinic at the National Institute of Dental and Craniofacial Research, Bethesda, MD, as part of their participation in an IRB-approved natural history study at the National Human Genome Research Institute, Bethesda, MD. Parental-informed consent was obtained.
Oral mucosal and gingival tissues were evaluated for irregularities and presence of lesions, and significant findings were photographed. Symptomatic complaints involving oral structures were addressed.
Radiographic evaluations of teeth, gnathic bones and surrounding structures were conducted using panoramic and lateral cephalometric radiographs (Planmeca® PM 2002 CC, Helsinki, Finland) as well as intraoral radiographs, as indicated. Due to difficulties in compliance, radiographic images were not obtained from patients younger than 3 years (n=4).
Patients’ dental charts and radiographic records were compared to standardized age ranges for the exfoliation of primary teeth and eruption of permanent teeth (Woelfel 1990a). Tooth emergence was ascertained by direct inspection. A tooth was considered erupted if any portion of the crown was clinically visible. Chairside parental interviews were also conducted to assess the timing of primary and permanent teeth eruption, exfoliation of primary teeth, and history of primary teeth extraction. Each patient’s chronological age was compared to the calculated dental age based on the number of clinically visible erupted teeth, rounded to the nearest month, as described by Hagg et al, 1985. The presence of other dental anomalies (e.g., hypodontia, hyperdontia, taurodontism) was also noted.
In order to assess the role of tooth size in reported arch length insufficiencies, the mesiodistal crown widths (CW) of the mandibular permanent 1st molars were measured from panoramic radiographs. CW was determined as the maximum length of the line paralleling the cervical margins of teeth #19 and 30. CW dimensions of maxillary molars, mandibular 2nd molars, and anterior teeth were not measured due to the difficulties in visualizing the mesiodistal crown margins from dental crowding and radiographic overlap of surrounding structures. Although a certain amount of magnification is inherent in panoramic radiographs, the focal trough – the three-dimensional horse-shoe-shaped image layer in which structures are displayed most sharply – is characteristically wider in the posterior segments than in the anterior portions of the jaws (White et al, 2000). This allows for a greater range of variables that influence image definition in the posterior jaws, leading to less horizontal distortion of the posterior teeth. In addition, the brand of equipment used in this study allowed further adjustments of the focal trough to accommodate the jaw size and shape of HGPS patients; the same setting was used for all HGPS patients in this study.
Cephalometric radiographs were processed with digital tracing software (Dolphin® Imaging 9, Dolphin Imaging & Management Solutions, Chatsworth, CA). Craniofacial measurements were analyzed with standardized cephalometric analyses (Scott:Rickets and Scott:Jarabak, Dolphin Imaging & Management Solutions) and compared with age, gender and race norms. Due to severe dental crowding and delayed teeth eruption in the majority of patients, which often precluded proper visualization of dental landmarks, occlusal relationships as defined by Angle’s classification categories were not assessed.
RESULTS
ALTERATIONS IN DENTAL DEVELOPMENT
Hypodontia, denoting the lack of development of one or more teeth excluding third molars, was present in the majority of patients. Among subjects with available radiographs (n=11, age: 5y to16 y8m), seven (64%) exhibited congenitally missing teeth, with the second permanent premolars (teeth #4, 13, 20, 29) most often missing (Table 1). The number of missing second premolars ranged from two to four. In addition, one patient (9y10m Caucasian male) exhibited congenitally missing permanent maxillary lateral incisors (#7, 10) and a permanent maxillary second molar (#15). With reported normal prevalences of 3.5% to 6.5% (Nunn et al, 2003; Zhu et al, 1996; Caldo-Teixeira et al, 2003; Stephen et al, 2003), a significantly higher occurrence of hypodontia was found in our HGPS patients (binomial proportion test, exact two-sided p = 2.56 × 10−6 ).
Table 1.
Dental development and occlusion in HGPS.
| Pt | Gender | Race | Chron-ological Age | Dental Age | Congenitally Missing Teeth | Dysmorphic Teeth | Retained Deciduous Teeth | Delayed Decidous Teeth | Delayed Permanent Teeth | Double Row |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Hisp | 17y8m | 11y2m | #2,15 | Y (1) | Y (11) | |||
| 2 | F | Hisp | 12y2m | 2y4m | #2,15 | Y (20) | Y (24) | |||
| 3 | M | Cauc | 10y1m | 6y11m | #2,15 | Y (4) | Y (7) | Y | ||
| 4 | M | Cauc | 9y10m | 6y11m | #7,10,15,20,29 | Y (3) | Y (6) | Y | ||
| 5 | M | Cauc | 9y5m | 1y11m | #4,13,20,29 | #2,3,14 | Y (8) | Y (4) | Y (12) | |
| 6 | F | Cauc | 8y7m | 2y4m | #20, 29 | Y (8) | Y (8) | |||
| 7 | M | Cauc | 8y | 2y4m | #20,29 | #2,15 | Y (5) | Y (8) | Y | |
| 8 | F | Hisp | 6y11m | 2y4m | #4, 13, 29 | |||||
| 9 | M | Hisp | 6y10m | Unknown | ** | ** | ** | Y (2) | ** | |
| 10 | F | Cauc | 5y8m | 2y4m | #4,13,20,29 | |||||
| 11 | F | Cauc | 5y | 2y4m | #4,13,20,29 | |||||
| 12 | F | Cauc | 2y3m | 1y7m | * | Y (7) | ||||
| 13 | F | Cauc | 2y1m | 1y7m | * | Y (6) | ||||
| 14 | M | Cauc | 1y9m | 9m | * | Y (19) | ||||
| 15 | F | Cauc | 1y6m | 9m | * | Y (5) |
F, female; M, male; Hisp, Hispanic; Cauc, Caucasian.
Y, positive finding (numbers in parentheses indicate known quantities).
No radiographs available
Premature extraction of deciduous teeth due to decay
The following normative CW measurements for mandibular first molars were reported by Woelfel: range = 9.8 mm to 14.5 mm, combined mean for teeth #19 & 30 = 11.4 mm (Woelfel 1990b). Although most of the HGPS CW’s for mandibular permanent first molars measured within published normal ranges (Table 2), the HGPS CW means (12.6 mm for tooth #19, 12.5 mm for tooth #30) were statistically larger (t-test p=0.0169 & 0.0043 for tooth #19 & #30, respectively) than controls.
Table 2.
Mesiodistal crown widths (CW) of mandibular permanent 1st molars (millimeters)
| Patient | Age | Gender | Mandibula1st Molars | |
|---|---|---|---|---|
| #19 | #30 | |||
|
|
||||
| 1 | 17y8m | M | 16 | 13 |
| 2 | 12y2m | F | 11 | 13 |
| 3 | 10y1m | M | 13 | 14 |
| 4 | 9y10m | M | 12 | 12 |
| 5 | 9y5m | M | 12 | 12 |
| 6 | 8y7m | F | 12 | 13 |
| 7 | 8y | M | 13 | 14 |
| 8 | 6y11m | F | 14 | 12 |
| 9 | 6y10m | M | 13 | 11 |
| 10 | 5y8m | F | 11 | 13 |
| 11 | 5y | F | 12 | 11 |
| Mean | 12.6 | 12.5 | ||
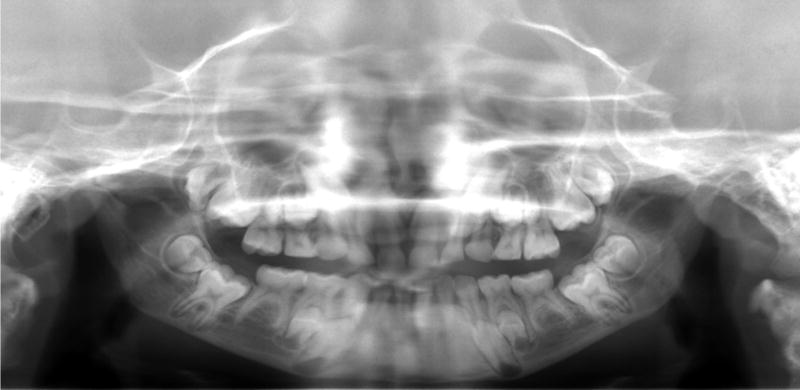
Aberrant tooth morphologies were noted radiographically. Constriction of the cervical portion of the maxillary and mandibular molars, resulting in bulbous crowns and relatively slender roots, were observed in some of the older patients (i.e., patients with visible root development; n = 5, 3 males, 2 females, age: 8y to 17y8m) (Figure 2, 3). Dysmorphic maxillary permanent second molars, characterized by an asymmetrical crown with a pronounced mesial cervical curvature and marked protrusion of the mesial cusp, were found in five older patients (4 males, 1 female, age: 8y to 17y8m) (Figure 2, 3). Although much variation had been found in the normal morphology of maxillary second molars, the mesial cusps of HGPS second molars were recognizably longer than the distal cusps, giving the occlusal surface a dramatic mesial-to-distant slant and an asymmetrical pulp chamber. One patient (9y5m-old Caucasian male) exhibited similarly dysmorphic maxillary permanent 1st molars. Of note is the pronounced distal tilting of most maxillary molars, with several in near-horizontal displacement (Figure 3).
Figure 2.
Eight-year old Caucasian male with HGPS demonstrating a hypoplastic mandible with a steep mandibular angle, delayed dental eruption, delayed root resorption of deciduous anteriors, congenitally missing mandibular permanent 2nd premolars, dysmorphic maxillary permanent molars, and bulbous mandibular molar crowns.
Figure 3.
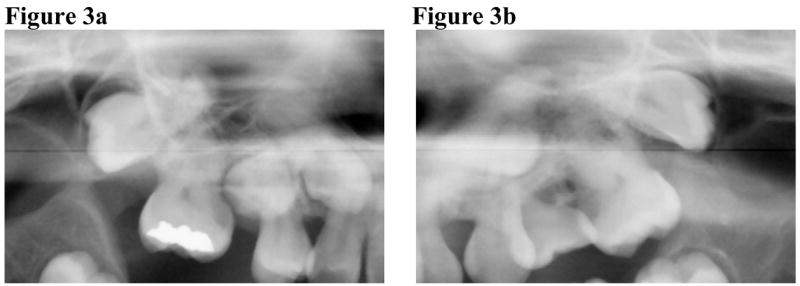
Seventeen-year old Hispanic HGPS male demonstrating dysmorphic maxillary molars with pronounced distal tilting of the 2nd molars (a, b).
ALTERATIONS IN DENTAL ERUPTION
Based on the number of clinically emerged teeth, dental age values were calculated for all but one patient (6y10m-old Hispanic male with premature extractions of deciduous teeth due to decay) (Table 1). All calculated dental ages (range: 9 months to 11y2m, mean: 4y5m, median: 2y4m) were significantly lower (paired t-test p=0.0002) than their corresponding chronological ages (range: 1y6m to 17y8m, mean: 7y2m, median: 6y11m) (Table 1).
All patients were at least partially dentulous. Among those between 6 to12 years – the mixed dentition period (n=8) - six (75%) remained in deciduous dentition. Even the study’s oldest patient (17y8m-old Hispanic male) remained in mixed dentition. Three patients exhibited “double rows” of teeth where succedaneous teeth erupted lingual of the deciduous teeth (Figure 4a). Radiographically, four other patients exhibited absence of root resorption in deciduous anterior teeth, subsequently delaying the eruption of succedaneous teeth (Figure 2).
Figure 4. Clinical oral findings in HGPS.
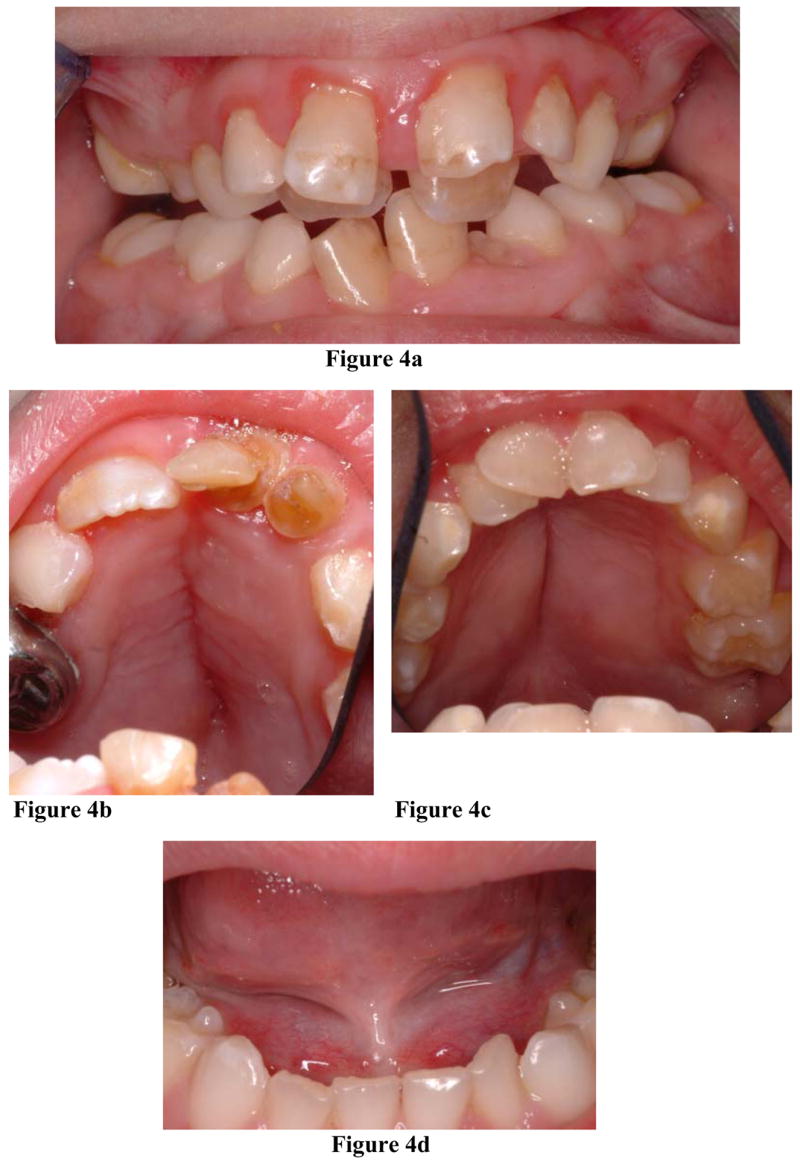
a. Ten-year old HGPS male exhibiting “double rows” of maxillary teeth. b, c. HGPS patients manifesting an ogival palatal vault and a midline sagittal palatal fissure.
d. Ankyloglossia in an 8-year old HGPS male.
In all patients over age 2 (n=13), dental crowding was observed in both deciduous and permanent teeth, exhibiting lingual rotation and lingual displacement of maxillary and mandibular anterior teeth.
ORAL SOFT TISSUE FINDINGS
Variations from normal palatal morphology were prevalent. Due to difficulties in visualization, palatal forms were not addressed in three patients (Table 3). Among those examined, eight patients (67%, age range: 2y3m to 12y2m, 4 males, 4 females) demonstrated an ogival palatal vault (Figure 4b, 4c). Seven of these patients (88%) developed a median surface fissure that spanned the hard palate sagitally (Figure 4b, 4c). In four of these patients, the maxillary teeth formed a triangular arch (Figure 4). Only the youngest of these patients (2y3m-old Caucasian female) did not manifest a median sagittal palatal fissure.
Table 3.
Palatal and other oral soft tissue findings in HGPS.
| Patient | Gender | Race | Chronological Age | Palatal Vault Form | Maxillary Arch Form | Median Palatal Fissure | Ankyloglossia |
|---|---|---|---|---|---|---|---|
| 1 | M | Hisp | 17y8m | Unknown | Unknown | Unknown | Unknown |
| 2 | F | Hisp | 12y2m | Ogival | Curved | Y | |
| 3 | M | Cauc | 10y1m | Ogival | Triangular | Y | Y |
| 4 | M | Cauc | 9y10m | Ogival | Curved | Y | Y |
| 5 | M | Cauc | 9y5m | Ogival | Triangular | Y | Y |
| 6 | F | Cauc | 8y7m | Ogival | Triangular | Y | |
| 7 | M | Cauc | 8y | Dome-shaped | Curved | Y | |
| 8 | F | Hisp | 6y11m | Ogival | Curved | Y | |
| 9 | M | Hisp | 6y10m | Ogival | ** | Y | Y |
| 10 | F | Cauc | 5y8m | Dome-shaped | Curved | ||
| 11 | F | Cauc | 5y | Dome-shaped | Curved | ||
| 12 | F | Cauc | 2y3m | Ogival | Triangular | Y | |
| 13 | F | Cauc | 2y1m | Unknown | Unknown | ||
| 14 | M | Cauc | 1y9m | Dome-shaped | *** | Y | |
| 15 | F | Cauc | 1y6m | Unknown | *** |
F, female; M, male; Hisp, Hispanic; Cauc, Caucasian; Y, positive finding
Premature extraction of deciduous teeth due to decay
Only 3 deciduous teeth erupted
Ankyloglossia, a developmental anomaly of the tongue characterized by a short, thick lingual frenum that limits tongue mobility, was found in seven of the fourteen examined patients (50%; age range: 10y1m to 1y9m; 6 males, 1 female) (Table 3, Figure 4d). With reported normal population prevalences ranging from 1.7% to 2.4% (Neville et al, 2002a), ankyloglossia was exceedingly more prevalent in our HGPS group (exact test (two-sided) p=2.71 × 10−8). Reported to be four times more common in males than female, ankyloglossia was also more prevalent in HGPS males (86%) than females (14%).
RADIOGRAPHIC FINDINGS
PANOGRAPHIC FINDINGS
In addition to dental dysmorphisms, panographic evaluation yielded universally hypoplastic mandibles with a steep mandibular angle (Figure 2). The condylar processes were positioned inferiorly and posteriorly, creating the impression that the mandible was bowing in the inferior-posterior plane. The permanent teeth appeared enlarged in proportion to the gnathic bones, with the mandibular molars encased deep inside the rami and high in the coronoid processes. Basal bone height (i.e., bone inferior to the mandibular canal) appeared universally thin, with the developing root apices in near contact with the inferior cortical border. Pronounced curvature of the maxilla also was observed. The distally tilted permanent molars overlapped the posterior maxillary border, often obliterating this anatomic landmark.
CEPHALOMETRIC ANALYSES (Figure 5)
Figure 5.
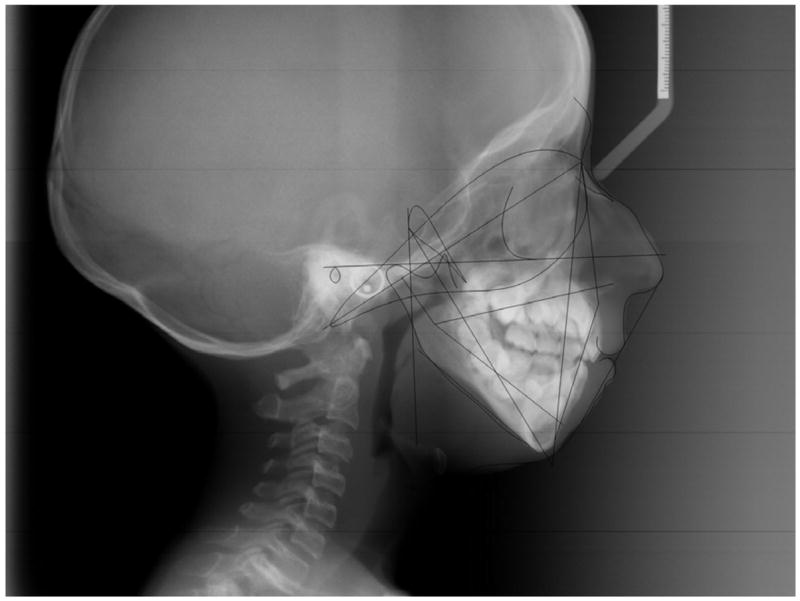
Cephalometric measurements on 12–year old Hispanic HGPS female.
Measurements of mandibular body length (Go-Gn) were consistently smaller than the age, gender and race norms, with standard deviations (SD) ranging from −5.6 to −13.3. For ramus height (Ar-Go), SD ranged from −5.6 to −9.5. The gonial (jaw) angle (Ar-Go-Me) was increased in all HGPS patients (SD range: 0.8 to 5.9) (Table 4).
Table 4.
Cephalometric values and analyses of eleven HPGS patients.
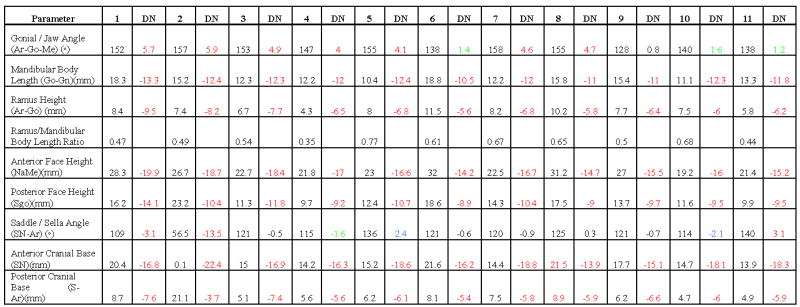
|
, patient number; DN, deviation from norm (Black SD ≤1, Green SD 1 ≤2, Blue SD >2 ≤3, Red SD >3); Ar (articulare), posterior border of neck of condyle; Go (gonion), most convex point along the inferior border of the ramus; Me (menton), most inferior point of the symphysis; Gn (gnathion), most inferior midpoint on soft tissue contour of the chin located at the level of hard tissue menton; Na (nasion), intersection of the internasal suture with the nasofrontal suture in the midsagittal plane; S (sella), center of the pituitary fossa of the sphenoid bone; SN, line that connects the midpoint of sella turcica with nasion.
Measurement of the cranial base found a smaller saddle/sella angle (SN-Ar) in 8 of 11 patients (73%; SD range: −0.5 to −13.5) and increased in 3 patients (27%; range: 0.3 to 3.1). Measurement of the anterior and posterior bases yielded universally shorter lengths in HGPS compared to norms (Table 4).
The proportion between ramal height and mandibular body length was also calculated ([Ar-Go/Go-Gn] × 100). As shown in Table 4, the proportions (0.61 to 0.68) in four patients (1 male, 3 females, age: 5y8m to 8y7m) were similar to the 2:3 ratio found in normal populations (Costaras-Volarich, et al 1984); however in five patients (3 males, 2 females, age: 5y to 16y8m) the proportion approached a 1:2 ratio (0.44 to 0.54). In addition, one patient (9y10m-old Caucasian male) demonstrated a dramatically shorter ramus than mandibular body (0.35 ratio) whereas another (9y5m-old Caucasian male) had a higher-than-normal ramal height:mandibular body length ratio (0.77).
DISCUSSION
This prospective study confirmed several craniofacial and dental findings previously noted in HGPS, and also identified novel findings. Cephalometric analyses quantified the marked retardation of gnathic bone growth universally present in our patients, as evidenced by the lack of anteroposterior and vertical development of both maxilla and mandible. The retrognathic facial appearance seemed to have resulted from the invariably obtuse gonial (jaw) angle, the steep mandibular plane angle, and the lack of development of the pogonion (boney chin). Anterior-posteriorly, the enlarged jaw angles rendered an inferior-posterior bowing appearance to the mandible. Disproportions in the lengths of the ramus and mandibular body were also calculated. Although the proportions in four patients (0.61 to 0.68) were similar to the 2:3 ratio reported as normative values, in five patients these approached a 1:2 ratio (0.44 to 0.54). These normal to smaller-than-normal ratios suggest that in some, retardation of the ramal segments may be even more pronounced than retardation of the anterior mandible. These measurements followed no age correlation.
Our cephalometric measurements provided a basis for interpreting several dental findings. As dental crowding and malocclusion are commonly associated with tooth size and arch-length discrepancies, the insufficient jaw sizes observed in HGPS – illustrated by minimal basal bone, dislocation of mandibular teeth in the coronoids and rami, and distal tilting of maxillary molars - may have predisposed to delayed dental eruption, dental crowding and malocclusion. Accordingly, calculated dental ages were significantly lower than chronological ages (paired t-test p=0.0002), with the mean HGPS dental age 33 months younger than the mean chronological age.
The significantly wider mandibular first molar crowns may have further compounded the tooth size-arch length discrepancy. The images of these wider-than-normal mandibular crowns, as well as the constriction at the cervical portions in both maxillary and mandibular molars, rendered these crowns a bulbous appearance (Figure 2) resembling those seen in dentinogenesis imperfecta (DI) (Neville et al, 2003, White et al, 2000). Unlike DI, however, obliteration of pulp chambers and canals was not observed in HGPS. Since the buccolingual dimension of these teeth could not be assessed radiographically, it remains to be seen whether a positive correlation exists between the mesiolingual and buccolingual diameters, which may further contribute to dental crowding.
With varying strengths of correlation, numerous hereditary syndromes have been associated with hypodontia (e.g., ectodermal dysplasia, Down, Crouzon, Turner, cranio-oculo-dental), with genetic, environmental and epigenetic factors exerting strong influences on tooth development (Neville et al, 2002b). Hypodontia correlates with the absence of appropriate dental lamina, a condition that is genetically controlled as well as highly sensitive to external stimuli (e.g., trauma, infection, chemotherapeutics and endocrine disturbances) (Neville et al, 2002b). After the third molars, the most frequently missing permanent teeth are the second premolars and lateral incisors (Nunn et al, 2003; Zhu et al, 1996; Caldo-Teixeira et al, 2003; Stephen et al, 2003). In agreement with previous reports on HGPS, a significantly higher prevalence of congenitally missing permanent second premolars (64% versus 3.5%–6.5% in normal population) was found in our study. Classified according to severity, all HGPS patients with hypodontia manifested the “mild-to-moderate” form (denoting the non-development of two-to-five teeth, with “severe hypodontia” indicating the absence of six or more teeth) (Dharajani, 2002). It should be noted that overlap of radiographic structures in the maxillary arch often precluded visualization of teeth or developing tooth buds, suggesting that the true prevalence of hypodontia in this group may have been underestimated.
Similarly, our assessment of tooth eruption based on the presence of any portion of clinical crowns, either partial or complete, may have underestimated the degree of delay in dental eruption. Radiographic findings indicate irregularities in both crown and root development (Figures 2 & 3), suggesting that, in addition to insufficient jaw growth, abnormal tooth development may play a direct role in the dental anomalies noted clinically. Although the factors controlling tooth development remain incompletely understood, we have determined that LMNA is expressed in developing mice molars (unpublished data). Additionally, LMNA is expressed in cDNA libraries from developing human teeth and from human palatal tissues (unpublished data). Characterization of LMNA expression in specific stages of dental crown and root development may further elucidate the direct role of LMNA in root formation and dental eruption, and subsequently determine how LMNA mutations contribute to the observed dental anomalies. Studies of other laminopathies (i.e. restrictive dermatopathy, mandibuloacral dysplasia, Werner syndrome) have also indicated the presence of dental abnormalities (Mau et al 1997; Uhrhammer et al 2006). Characterization of these dental anomalies may add to the knowledge of oral involvement in laminopathies, and help distinguish HPGS from other genetic disorders associated with this gene.
Several oral soft tissue characteristics were noted. Ogival palatal vaults were twice as prevalent in HGPS as the normative dome-shaped palate. Except for the youngest patient, all with ogival vaults developed a median sagittal fissure along the palatal gingiva, suggesting that maxillary growth constriction may have predisposed to this soft tissue alteration. Similar to dental crowding, the cephalometric measurements provided a framework for interpreting some oral soft tissue characteristics. Constriction of maxillary growth seemed consistent with previously reported craniofacial alterations, such as the viscerocranium becoming small relative to the neurocranium over time, often resulting in prominently flared ears (Hennekam 2006).
Another novel finding was the presence of ankyloglossia (tongue-tie) in these HGPS patients. In the general population, the severities of ankyloglossia range from mild cases with limited significance to rare examples of complete fusion of the tongue to the floor of the mouth. The prevalence of ankyloglossia in our HGPS group – all manifesting the mild form - was significantly higher than the normal population (exact test (two-sided) p=2.71 × 10−8) and was found to have no age correlation. In the general population, reported clinical consequences include the development of anterior open bite, difficulties during infant feeding and swallowing, and speech problems (Ruffoli et al, 2005).
SUMMARY
Hypodontia, delayed dental eruption and craniofacial alterations in HGPS had been reported from case studies and through empirical observations. Our study provided quantitative details to these observations and suggested mechanisms explaining these characteristics. In addition, novel phenotypic features (i.e., dental dysmorphologies, oral soft tissue anomalies) were described in detail. By contributing to the HGPS phenotype database, our observations may allow anticipation of problems related to oral and craniofacial structures (e.g., increased caries rate, severe malocclusion), hasten the implementation of treatment, and shed additional light on the complex pathogenesis of this disorder.
Acknowledgments
This work was supported by the intramural programs of NIDCR and NHGRI, NIH.
We thank the children with progeria, their families, and The Progeria Research Foundation Diagnostics Program (Peabody, MA) for their participation in this study.
References
- 1.Caldo-Teixeira AS, Puppin-Rontani RM. Management of severe partial hypodontia: case report. J Clin Pediatr Dent. 2003;27:133–136. doi: 10.17796/jcpd.27.2.791p033383312768. [DOI] [PubMed] [Google Scholar]
- 2.Costaras-Volarich M, Pruzansky S. Is the mandible intrinsically different in Apert and Crouzon syndromes? Am J Orthod. 1984;85:475–487. doi: 10.1016/0002-9416(84)90087-3. [DOI] [PubMed] [Google Scholar]
- 3.Dharajani PJ. Hypodontia: etiology, clinical features, and management. Quintessence Int. 2002;33:294–302. [PubMed] [Google Scholar]
- 4.Fong LG, Ng JK, Meta M, et al. Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc Natl Acad Sci USA. 2004;101(52):18111–18116. doi: 10.1073/pnas.0408558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman RD, Shumaker DK, Erdos MR, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon LB, McCarten KM, Giobbie-Hurder A, et al. Disease progression in Hutchinson-Gilford progeria Syndrome: Impact on growth and development. Pediatrics. 2007;120(4):824–33. doi: 10.1542/peds.2007-1357. [DOI] [PubMed] [Google Scholar]
- 7.Hagg U, Taranger J. Dental development, dental age and tooth counts. Angle Orthod. 1985;55(2):93–107. doi: 10.1043/0003-3219(1985)055<0093:DDDAAT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Hasty MF, Vann WF. Progeria in a pediatric dental patient: literature review and case report. Pediatr Dent. 1988;10:314–319. [PubMed] [Google Scholar]
- 9.Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006 doi: 10.1002/ajmg.a.31346. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Martin GM. Genetic modulation of senescent phenotypes in Homo sapiens. Cell. 2005;120:523–532. doi: 10.1016/j.cell.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Mau U, Kendziorra H, Kaiser P. Restrictive dermopathy: report and review. Am J Med Genet. 1997;71(2):179–185. doi: 10.1002/(sici)1096-8628(19970808)71:2<179::aid-ajmg11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.McClintock D, Ratner D, Lokuge M, et al. The mutant form of lamin A that Causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS ONE. 2007;2(12):e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merideth MA, Gordon LB, Clauss S, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral & Maxillofacial Pathology. 2. WB Saunders Co; Philadelphia: 2002a. Developmental defects of the oral and maxillofacial region; pp. 10–11. [Google Scholar]
- 15.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral & Maxillofacial Pathology. 2. WB Saunders Co; Philadelphia: 2002b. Abnormalities of teeth; p. 70. [Google Scholar]
- 16.Neville BW, Damm DD, White DK. Color Atlas of Clinical Oral Pathology. 2. BC Decker Inc; Hamilton, Ontario: 2003. Dentinogenesis imperfecta (hereditary opalescent dentin, capdepont teeth) pp. 78–79. [Google Scholar]
- 17.Nunn JH, Carter NE, Gillgrass TJ, et al. The interdisciplinary management of hypodontia: background and role of paediatric dentistry. Br Dent J. 2003;194:245–251. doi: 10.1038/sj.bdj.4809925. [DOI] [PubMed] [Google Scholar]
- 18.Paradisi M, McClintock, Boguslavsky RL, et al. Dermal fibroblasts in Hutchinson-Gilford progeria syndrome with the lamin A G608G mutation have dysmorphic nuclei and are hypersensitive to heat stress. BMC Cell Biol. 2005;6:27. doi: 10.1186/1471-2121-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollex RL, Hegele RA. Hutchinson-Gilford progeria syndrome. Clin Genet. 2004;66:375–381. doi: 10.1111/j.1399-0004.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 20.Ruffoli R, Giambelluca MA, Scavuzzo MC, et al. Ankyloglossia: a morphofunctional investigation in children. Oral Dis. 2005;11:170–174. doi: 10.1111/j.1601-0825.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar PK, Shinton RA. Hutchinson-Guilford progeria syndrome. Postgrad Med J. 2001;77:312–317. doi: 10.1136/pmj.77.907.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephen A, Cengiz SB. The use of overdentures in the management of severe hypodontia associated with microdontia: a case report. J Clin Pediatr Dent. 2003;27:219–222. doi: 10.17796/jcpd.27.3.v433g54716885h22. [DOI] [PubMed] [Google Scholar]
- 23.Uhrhammer NA, Lafarge L, Dos Santos L, et al. Werner syndrome and mutations of the WRN and LMNA genes in France. Hum Mutat. 2006;27(7):718–719. doi: 10.1002/humu.9435. [DOI] [PubMed] [Google Scholar]
- 24.Wesley RK, Delaney JR, Litt R. Progeria: Clinical consideration of an isolated case. J Dent Child. 1979;46(6):487–92. [PubMed] [Google Scholar]
- 25.White SC, Pharoah MJ. Oral Radiology Principles and Interpretation. 4. Mosby Inc; St. Louis: 2000. Radiographic Interpretation of Pathology; pp. 319–320. [Google Scholar]
- 26.Woelfel JB. Dental Anatomy: Its Relevance to Dentistry. 4. Lea & Febiger; Philadelphia: 1990a. Terminology; p. 29. [Google Scholar]
- 27.Woelfel JB. Dental Anatomy: Its Relevance to Dentistry. 4. Lea & Febiger; Philadelphia: 1990b. Permanent Mandibular Molars; p. 157. [Google Scholar]
- 28.Yu QX, Zeng LH. Progeria; report of a case and review of the literature. J Oral Pathol Med. 1991;20:86–88. doi: 10.1111/j.1600-0714.1991.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhu JF, Marcushamer M, King DL, Henry RJ. Supernumerary and congenitally absent teeth: a literature review. J Clin Pediatr Dent. 1996;20:87–95. [PubMed] [Google Scholar]