Abstract
The purpose of this study was to determine the effect of transplanted human mesenchymal stem cells (hMSCs) on wound healing. In this model, full-thickness cutaneous wounds were created by incision in the skin of adult New Zealand white rabbits and treated by transplanted human MSCs into the wounds. Wound healing was evaluated by histologic analysis and tensiometry over time. A total of 15 New Zealand white rabbits with 10 wounds per animal were examined in this study. Animals were treated with human MSCs and euthanized at 3, 7, 14, 21 and 80 days after manipulation. The hMSCs were labeled with a fluorescent dye (CM-DiI), suspended in PBS, and used to treat full-thickness incisional wounds in rabbit skin. Tensiometry and histology was used to characterize the wound-healing rate of the incisional wounds. These results showed that transplanted hMSCs significantly inhibited scar formation and increased the tensile strength of the wounds. Importantly, MSCs from genetically unrelated donors did not appear to induce an immunologic response. In conclusion, human mesenchymal stem cell therapy is a viable approach to significantly affect the course of normal cutaneous wound healing and significantly increase the tensile strength.
Introduction
Cutaneous scarring has been a major challenge to plastic surgeons for many years owing to the lack of an effective treatment. Even incisional wound scarring encountered by plastic surgeons in daily practice remains a frustrating clinical problem. Although the mechanism of scar formation in adult wounds remains incompletely understood, the most recent evidence suggests the process of cutaneous wound repair involves recruitment of non-resident undifferentiated cells from distant sources, such as the bone marrow [1-3]. This evidence has raised the concept that interventional strategies can be designed to exploit reparative cells for wound repair.
Among the candidates for the reparative cells are the adult stem cells from bone marrow referred to either as mesenchymal stem cells or as marrow stromal cells (MSCs) [4]. Little information is currently available about the biology of endogenous stem cell population in adults on their precise role in tissue repair and regeneration. This may be due partly to the lack of useful cell specific markers. However, it is now clear that MSCs can be easily isolated and expanded in culture through many generations while retaining the capacity to differentiate [3]. Although MSCs lack tissue specific characteristics, they can differentiate under the influence of appropriate signals into specialized cells with a phenotype distinct from that of the precursor [3].
Based on these considerations, it has been hypothesized the stem cells in adult tissues are reservoirs of reparative cells, ready to mobilize and differentiate in response to wound signals or disease conditions [3]. Three basic mechanisms have been proposed to explain how MSCs could repair tissue injury: a) creation of a milieu that enhances regeneration of endogenous cells, b) transdifferentiation, or c) cell fusion [4-6]. Because of their potential use in regenerative medicine and tissue engineering, mesenchymal stem cells have been explored for their therapeutic use, involving of autologous or allogenic transplantation into patients through local delivery or systemic infusion [3]. Of note, some striking preclinical examples of mesenchymal stem cell therapy have been reported recently involving cardiovascular repair [7], treatment of lung fibrosis [8], spinal cord injury [9] and bone and cartilage repair [3].
From these findings that illustrate the therapeutic value of mesenchymal stem cells in regenerative medicine, we hypothesized the transplantation of human MSCs to the site of the lesion may enhance cutaneous wound healing in incisional wounds. In the present study, we tested this therapy by transplanting human MSCs into a rabbit model of incisional wounds and evaluated wound healing by measurement of tensile strength and histological characteristics. To our knowledge, there have been no prior reports that correlate the effect of transplanted human MSCs on the tensile strength in dermal wounds. Our results demonstrate that human MSC therapy is a viable approach to significantly affect the course of normal cutaneous wound healing and dramatically increase the tensile strength.
Material and Methods
Human Mesenchymal Stem Cells and Labeling
Early passage human mesenchymal stem cells (hMSCs) isolated from normal healthy donors [10] were prepared and extensively characterized at the Tulane Center for the Preparation and Distribution of Adult Stem Cells (www.som.tulane.edu/gene_therapy/distribute.shtml). Frozen vials were obtained under an IRB approved protocol of the University of Alabama at Birmingham and cultured according to the procedures provided. These cells are early progenitors that can differentiate into multiple lineages including osteoblasts, adipocytes, myotubes, and neural cells [11]. Carbocyanine dye (CellTracker CM-DiI; Molecular Probes Inc, Eugene, Ore) was used to label the human mesenchymal stem cells according to the manufacturer's standard protocol as previously described [12]. The cells were counted (1.5 × 106) and suspended in 100 μl of PBS.
Animal Model
A total of 15 New Zealand white adult rabbits, weighing 3 to 4 kg (Myrtles Rabbitry; Thompson Station, TN, USA) were used in these experiments. All experimental protocols were approved, and the procedures followed were in accordance with the ethical standards of the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Full-thickness 3-cm-long incisional wounds up to the fascia, were created in the dorsal skin of the New Zealand rabbit at intervals of 2 cm. Each animal received 10 wounds, with 5 wounds assigned as human mesenchymal treated wounds and 5 wounds as control. Of note, preliminary results (data not shown) suggested no histomorphologic difference in untreated wounds versus PBS treated wounds. We thus utilized the untreated wounds as the control group.
After creating the incisional wound, the wounds were sutured with 5-0 Prolene thread (Ethicon Inc., Johnson & Johnson Company, Somerville, NJ, USA) which was removed on day 7 after surgery. Suturing was followed by intradermal injection of 1.5 × 106 human mesenchymal stem cells resuspended in 100 μl phosphate-(27-gauge needles fastened to 1-ml syringes) buffered saline or left without treatment. No antibiotics were used. For all procedures, the animals were shaved and the skin was examined to ensure integrity and lack of any infection. The experimental area was thoroughly cleansed with 10% povidone-iodine and 70% alcohol swabs before manipulation. Aseptic technique was maintained throughout all procedures. The animals were fully anesthetized throughout the procedure and were observed every 8 hours for the next 48 hours and daily throughout the rest of the 80-day experimental period. Post-procedure behavior patterns did not change from the pre-procedure patterns. No changes in appetite or behavior were observed at any point in the study. All animals were healthy and alive at the end of the experimental period, without weight loss.
Wound Preparation
The 15 animals were euthanized in groups of three, at days 3, 7, 14, 21 and 80 after incision. The rabbits were heavily anesthetized using a ketamine-xylazine mixture, followed by intracardiac administration of euthanasia solution (pentobarbital sodium and phenytoin sodium, 1.5 mL). The dorsal skin was removed using aseptic technique, followed by dissection of each individual wound. Each wound was cut in three pieces. One was placed into buffered formalin solution for histopathological examination, one was used for frozen section, and the last was used for the tensile strength test as described below.
Wound Histology and Evaluation
Serial 5 μm sections were obtained from the paraffin embedded wounds using a 30/50 microtome (Leica; Heidelberg, Germany) and stained with hematoxylin and eosin as previously described (Culling, 1974). In addition, dermal wounds for each group of mice were embedded in Tissue TEK OTC compound (Miles, Elkhart, IN), snap-frozen in liquid nitrogen, and stored at -80°C. The epithelial gap was defined as the distance between encroaching epidermal elements. The boundaries of the granulation tissue were defined superior to the transition zone extending from normal epidermis laterally to the hypertrophic epidermis, superior to the muscle, and deep to the epithelial basement membrane or the open wound surface [13]. The histological sections were assessed using the histological scale of Singer et al. [14]. The wounds were evaluated for the presence of hyperkeratosis, epidermal hyperplasia, presence and depth of scar, fibroplasia, vascular proliferation, and absence of adnexa, including hair follicles, apocrine glands and smooth muscle. Evaluation was performed by two different investigators blinded to the treatment protocol.
Immunofluorescence
Carbocyanine dye labeled hMSCs were identified as following: for CellTracker CM-DiI histochemistry, frozen tissue sections were air dried, fixed in 4% paraformaldehyde for 1 hour, permeabilized in PBS with 0.2% Triton X-100, washed 5 min in PBS and blocked in 25% goat serum for 30 min. Then the tissue sections were treated overnight with rabbit anti-GFP antibody (ab6567, Abcam) at 4 degree C. The sections were washed in PBS (3 × 5 min.) and treated with Alexa 485 labeled anti-rabbit secondary antibody (Alexa, Molecular Probes, Invitrogen). They were finally washed as before in PBS. All sections were mounted with Vectashield mounting medium with 4′, 6-diamidino-2-phenylindole (H-1200; Vector Laboratories) and mounted in DAPI containing mounting medium (VectaSheild, Vector laboratories). The images were captured with a FITC filter in an Olympus AX70 fluorescence microscope equipped with a Zeiss Axiocam camera (Carl Zeiss, Oberkochem, Germany). Individual images were processed and merged using Adobe Photoshop 5.5 application software.
Measurement of tensile strength
At necropsy, the middle third of the excised wounds were cut into sections of 5 to 10 mm in width. Since wounds were cut to the panniculus, tissue up to this level was removed from the sections. Specimen width was measured (mm) using digital calipers transversely across the wound. After necropsy, each specimen was frozen to prevent deterioration. Before mechanical testing, each specimen was thawed and balsa wood affixed with methyl cyanoacrylate (Loctite®; Avon, OH) to reduce grip slippage. An 858 Mini Bionix Material Testing System (MTS Systems; Eden Prairie, MN) was used to perform tensile tests at 1 mm/s until failure. Tensile strength data was recorded as maximum force (N) and force divided by wound width (N/m). The results for individual sections from one wound were combined for each wound specimen to determine an average tensile strength per wound. The tensile strength per wound was tabulated for each group at each time point, and the means and SDs were determined using database software.
Statistical Analysis
Differences between groups were analyzed using the Student-Fisher t-test, differences were considered significant when P < 0.05.
Results
Transplantation of Human Mesenchymal Stem Cells Reduces Wound Scarring in Full-thickness Incisional Wounds
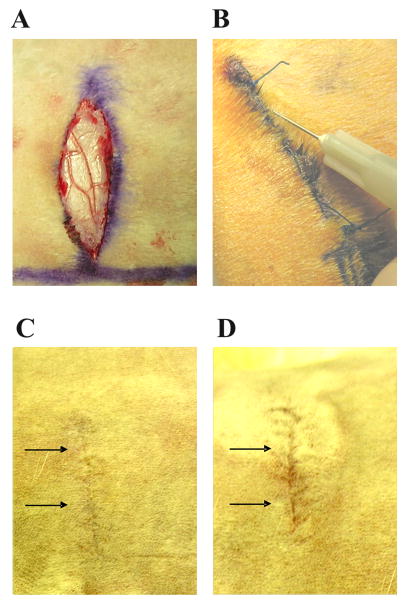
As represented in Figure 1, full-thickness incisional wounds were created in the rabbit model (Figure 1A) and after suturing, hMSCs were transplanted around the wound (Figure 1B). The progress of wound repair was followed, and at day 80, the overall macroscopic appearance of scar formation was examined; in incisional wounds treated with hMSCs scarring was less evident (Figure 1C) than in non-treated incisional wounds (Figure 1D).
FIGURE 1. Full-thickness 3-cm-lomg incisional wounds in New Zealand rabbits.
Wounds were created in the dorsal skin up to the fascia (A) and 1.5 × 106 hMSCs suspended in 100 μl of PBS were injected around the wounds (B). At day 80 the macroscopic appearance of the scarring in incisional wounds treated with hMSCs (C) was less detectable compared with untreated wounds (D).
We examined the effect of transplanted human mesenchymal stem cells on wound healing by histopathological examination. In this analysis, rabbits were subjected to full-thickness wounds and samples of wounded skin were subsequently harvested over 80 days. At the early stage of wound repair (days 3 and 7 after surgery and treatment) we did not observe any differences in epithelial gap and in granulation tissue area between hMSC treated and non-treated wounds (Figure 2). At day 14 (Figure 2) and 21 (Figure 3) after surgery a larger amount of granulation tissue was observed in the non-treated wounds than in the hMSC treated wounds. At day 80 after surgery (Figure 3), the width of the scar was much narrower in hMSC treated wounds compared with non-treated wounds. Thus, the histopathological examination of the hMSC treated incisional wounds showed less evidence of scar formation than those of the control group.
FIGURE 2. Histopathological examination of full-thickness incisional wounds at days 3, 7 and 14 after surgery and treatment.
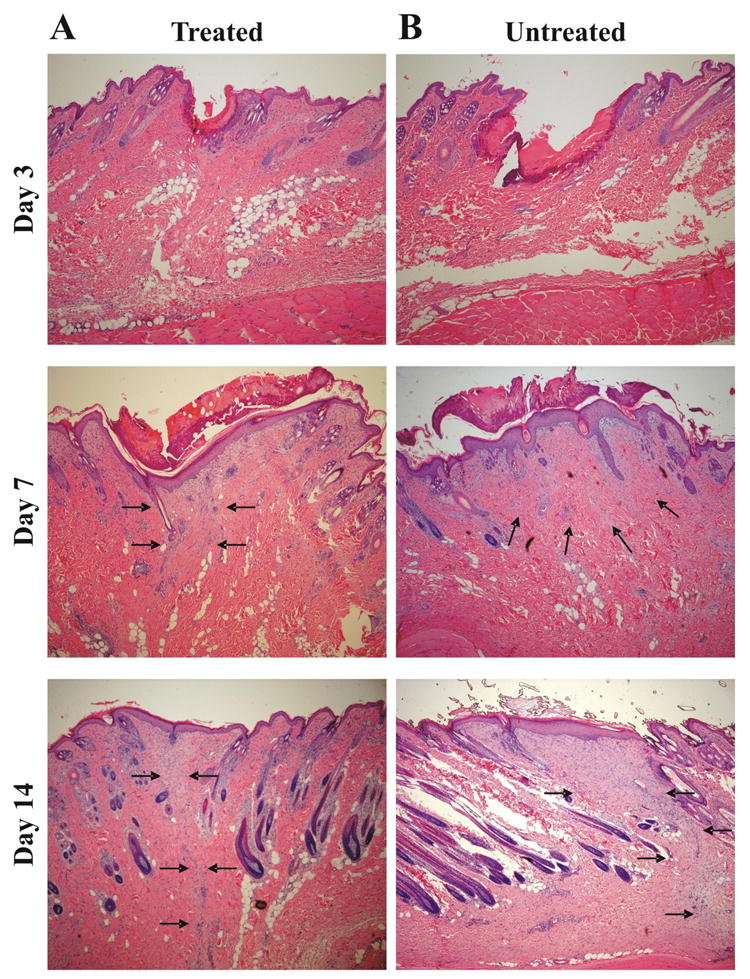
Histopathology of the (A) hMSC treated group and (B) control group at 3, 7 and 14 days after the operation (H&E stain, 40×). Tissue sections were stained with hematoxylin and eosin and examined for the differences in epithelial gap and granulation tissue by a Pathologist blinded to the treatment groups. No differences in epithelial gap could be distinguished among the three treatment groups at day 3 and 7 after surgery. At day 14 after surgery and treatment the granulation tissue area was smaller in the hMSC treated wounds (A) compared with untreated wounds (B). In none of the groups signs of inflammation could be detected. Arrows indicate to granulation tissue.
FIGURE 3. Histopathological examination of full-thickness incisional wounds at days 21and 80 after surgery and treatment.
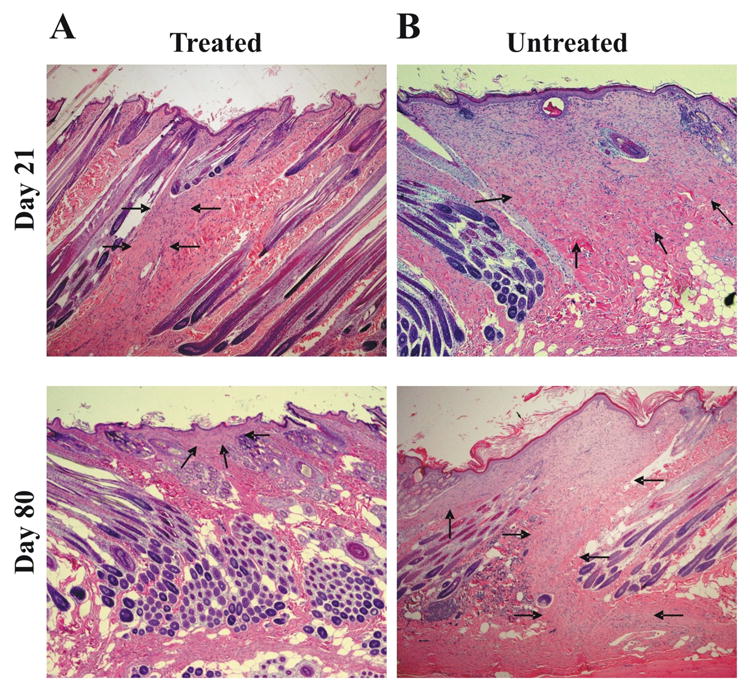
Histopathology of the (A) hMSC treated group and (B) control group at 21 and 80 days after the operation (H&E stain, 40×). Tissue sections were stained with hematoxylin and eosin and examined for the granulation tissue by a pathologist blinded to the treatment. The width of the granulation tissue is narrower in the hMSC treated group (A) than the control group (B) at days 21 and 80 after surgery. In none of the groups signs of inflammation could be detected. Arrows indicate to granulation tissue.
Histomorphologic Evaluation of Wounds by Singer classification
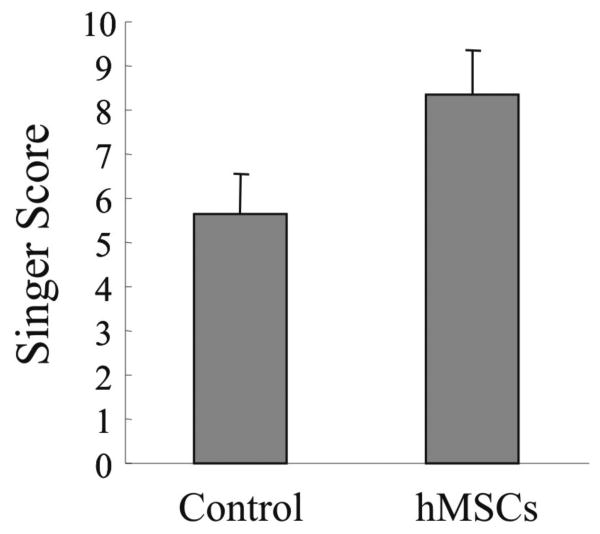
Next, we used the Singer classification [14] for the histological evaluations to quantify the cutaneous wound healing in incisional wounds after hMSC transplantation. Figure 4 shows the histomorphologic scales at day 80 after surgery and treatment. The scores range from 0 (worst scarring) to 10 (absence of scarring). The hMSC treated wounds displayed a significant higher score compared with non-treated wounds. Thus, the evaluation of histomorphology by Singer indicated better wound healing in hMSC treated incisional wounds.
FIGURE 4. Histomorphologic evaluation of the incisional wounds by Singer classification at day 80 after surgery and treatment.
Incisional wounds treated with hMSCs or untreated wounds (control) were evaluated for the presence of hyperkeratosis, epidermal hyperplasia, presence and depth of collagen disorganization, fibroplasia, vascular proliferation, absence of adnexa, including hair follicles, apocrine glands, and smooth muscle according to Singer [14] at day 80 after treatment. The scores ranged from 0 (worst scarring) to 10 (absence of scarring). Results represent the mean ± SD; n = 3 rabbits at day 80. * p < 0.05 hMSC treated group versus control group.
Imaging of Human Mesenchymal Stem Cells Transplanted in Incisional Wounds
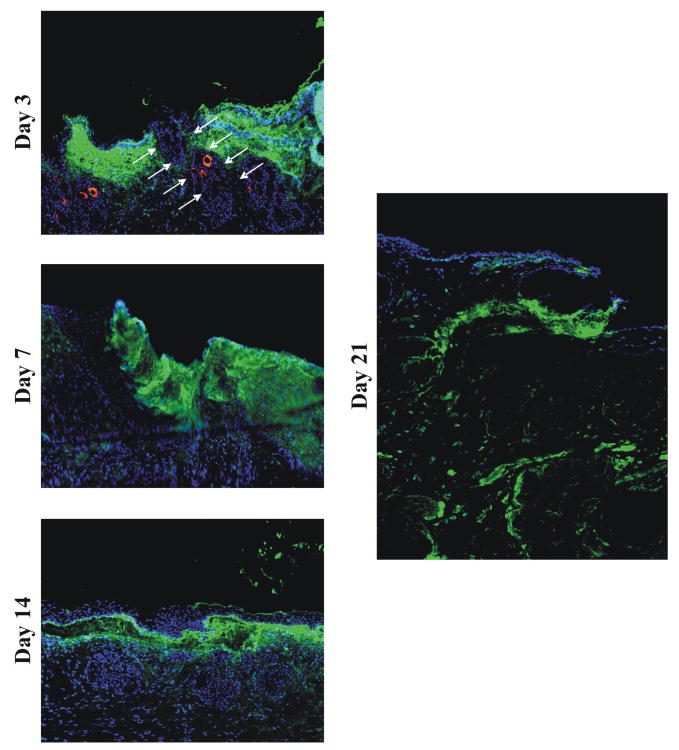
We followed the migration pattern of transplanted human mesenchymal stem cells after injection into incisional wounds by labeling the cells with a fluorescent (CM-Dil) dye. Previous in vivo experiments have shown that dye-labeled cells such as fibroblasts are still present and detectable in unwounded skin at 28 days [12]. Incisional wounds treated with labeled hMSC were imaged at day 3, 7, 14, 21 and 80. As shown by fluorescence microscopy in Figure 5, the fluorescence of hMSCs persisted through day 21 (as indicated in bright green), although the intensity was reduced due to dilution of the label from multiple cell divisions. Fluorescent label was not detected in control wounds. In wounds collected at days 3 and 7, a diffuse dispersion pattern was observed for the hMSCs in the immediate wound area. At day 14, the labeled hMSCs were concentrated at the epidermal-dermal interface. However, by day 21 transplanted hMSCs were observed migrating towards the border of the dermis and the underlying fascia. Thus, hMSC in incisional wounds could be identified up to 21 days after transplantation showing a specific migration pattern for the hMSC from the epidermal-dermal interface towards the border of the dermis.
FIGURE 5. Imaging of hMSCs transplanted in incisional wounds.
Shown are representative fluorescence microscopy images of hMSCs labeled by CM-Dil dye in incisional wounds at day 3, 7, 14, 21. The hMSC are indicated in green and DAPI positive nuclear signals are indicated in blue.
Human Mesenchymal Stem Cells Increase Tensile Strength in Incisional Wounds
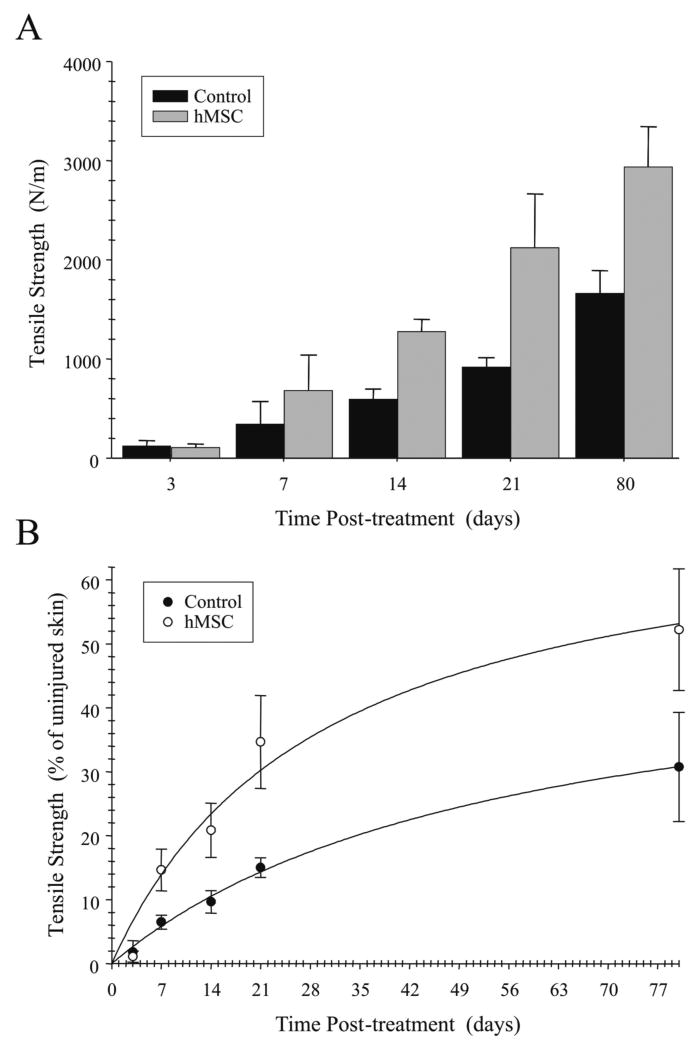
The ultimate goal of this study was to determine whether the transplantation of hMSCs could reduce scar formation in vivo. Encouraged by the positive results of the hMSC treatment in the histopathological examinations, we investigated the effect of hMSCs in incisional wounds on tensile strength. The tensile strength of incision wounds is an objective means to describe the wound healing rate [15]. The mean levels of tensile strength in wounded skin tissues on days 3, 7, 14, 21 and 80 after surgery for each treatment group are shown in Figure 6A. While there was no significant difference in tensile strength between the hMSCs and non-treated group at days 3 and 7, the tensile strength was significantly higher at days 14, 21 and 80 in the hMSC treated group compared with the non-treated group.
FIGURE 6. Transplantation of hMSCs increases tensile strength in incisional wounds.
The mean tensile strength in N/m at day 3, 7, 21 and 80 after surgery in hMSC treated and non-treated wounds (A). Comparison of the percentage of tensile strength of the incisional wounds treated with either hMSC or untreated to uninjured skin day 3, 7, 21 and 80 after surgery (B). Results represent the mean ± SD; n = 3 rabbits *p < 0.05 hMSC treated wounds versus untreated wounds.
Finally, we compared the percentage of tensile strength of the incisional wounds treated with either hMSC or untreated to uninjured skin over a postoperative period of 80 days. As shown in Figure 6B wounds treated with hMSC revealed a significantly higher regain in tensile strength (p < 0.05) compared with untreated wounds over a time course of 80 days. Incisional wounds treated with hMSCs regained 52% while untreated wounds regained only 31% of the tensile strength of unwounded tissue by 80 days after initial wounding. Thus, incisional wounds treated with hMSCs regained tensile strength at a significantly higher level than untreated wounds. In the aggregate, these data of tensile measurements correlate with the histopathological findings suggesting the treatment with hMSC can enhance wound healing.
Discussion
The major goal of this study was to investigate the impact of transplanted human mesenchymal stem cell on the wound healing process in incisional dermal wounds. To this end, we demonstrated that transplantation of human mesenchymal stem cells in a rabbit model at the time of wound closure significantly increased the tensile strength and dramatically improved the condition of the scar by histological evaluation as well as by surface inspection. Thus, our findings provide feasibility for using non-autogenic transplanted mesenchymal stem cells in developing cell therapies of regulated wound healing, to produce a more regenerative cutaneous healing response.
Mesenchymal stem cells possess the capacity for site-specific differentiation of cell types in response to cues provided by different organs. Mesenchymal stem cells can differentiate not only into mesenchymal lineage cells but also endothelium and endoderm in vitro [16]. This phenomenon suggests that mesenchymal stem cells are involved in cutaneous wound healing. The process of cutaneous wound healing has been divided into three distinct phases: 1) the initial lag phase (days 0 to 5), in which there is no gain in wound strength, 2) the fibroplasias phase (days 5 to 14) in which a rapid increase in wound strength occurs, and 3) the final maturation phase (day 14 until final healing) in which further connective tissue remodeling occurs [17].
In cutaneous wound healing, scarring is the mechanism by which the integrity of adult wounds is restored. While there is no consensus regarding the exact definition of a scar, a cutaneous scar is commonly classified as a disorganization of dermal collagen that results from tissue injury. While histological categorization of scars into “good” or “bad” is subjective, few studies have attempted to develop a method by which this distinction can be made other than at the extreme end of the spectrum of healing (i.e., keloids and hypertrophic scars) [18]. Therefore we used the Singer classification for the histological evaluations of the wounds to quantify the cutaneous scars after transplantation of human mesenchymal stem cells [18]. In this study, incisional wounds treated with human mesenchymal stem cells showed significantly less scarring than the control group.
In the present study, we tested the therapeutic value of mesenchymal stem cell therapy to enhance cutaneous wound healing. We used human cells transplanted into a rabbit xenogeneic host model of incisional lesions, based on the accumulating evidence showing the potential in vivo immune modulation and immune privileged properties of mesenchymal stem cells [19-22]. Additional experiments suggest that neither human nor animal MSCs express co-stimulatory antigens and thus appear to be immuno-privileged [5, 23]. However, human mesenchymal stem cells may not be sufficiently immune tolerant that they can be transplanted into xenogeneic hosts without immune suppression [24-26]. Since the rabbits in this study were not immunosuppressed, the positive results may have been due to the skin as an immune privileged site. It is noteworthy that histologic evaluation showed no signs of an inflammatory response in incisional wounds of rabbits treated with human mesenchymal stem cells. In future studies we will quantify the length of survival of human mesenchymal stem cell transplants in rabbit incisional lesions.
The incisional wound repair model fulfills many of the criteria for a good animal model of wound repair because it can be accurately reproduced and is available for multiple treatments on the same animal. While several animal species such as mouse, rat, hamster, rabbit, and pig have been used to model wound repair, consistent results are dependent on correlative outcome measurements that include wound strength and histology. Encouraged by the positive results of the histological evaluation in the group treated with human mesenchymal stem cells, we investigated the impact of transplanted human mesenchymal stem cells on the tensile strength.
An optimal scar is one that most resembles the collagen structure, architecture of normal skin and tensile strength. Thus, the biological status of the repaired wound depends on the proper chemical reorganization of the collagen fibers during the remodeling stage, which affects the tensile strength. The tensile strength which is the breaking strength per unit thickness of tissue is used to describe the healing rate of wounds and a useful comparative measurement for wounds [15]. The plot of the tensile strength as a function of time has the characteristic of an exponential curve [27]. It is known that during the first days of the wound reparative process, the tensile strength increases moderately. Then an acceleration of the healing process occurs with a rapid increase in the tensile strength, which reaches a steady state at about 8 weeks and a maximum peak after more than one year post injury [27]. Any interference with the collagen synthesis process will reflect on the biomechanical properties of the tissue and on the wound recuperation period [27]. In this study, the tensile strength was significantly greater after the surgery in the group treated with hMSCs compared with the control group. Although the strength of repaired skin incision never reached that of uninjured skin, it has been previously demonstrated that rabbit incisional skin wounds regain only 40% of the tensile strength of unwounded tissue 120 days after wounding. Incisional wounds treated with hMSCs regained 52% while untreated wounds regained only 31% of the tensile strength of uninjured skin at day 80 after surgery and treatment.
In conclusion, we have demonstrated the principle of human mesenchymal stem cells to ameliorate wound healing in acute incisional wounds by expediting the regain of tensile strength. To our knowledge, this study is the first to examine the mechanistic effect of transplanted mesenchymal stem cells on the tensile strength in cutaneous wound healing. One recent report describing that human mesenchymal stem cells transferred in vivo in a three-dimensional matrix consisting of fibrin gel, may improve skin injuries in mice was published [5]. However, no method of scar analysis was described and no histological evaluation was presented. Most importantly, local transplantation of the fibrin gel or hMSC alone as a control group was not performed. This is not unimportant, based on the observation that fibrin itself can support the wound healing process of the epidermis through the TGF-alpha/EGF-R pathway [28]. Another report used an excisional wound model in nude rats to examine wound healing in a human mesenchymal stem cell-populated porcine skin substitute [29]. In this model, the wound size was significantly smaller in the human mesenchymal stem cell-treated groups compared with control groups. However, this analysis was based on wound size alone. Thus, these studies support our studies and highlight the promise embodied in the utility of human mesenchymal stem cells for wound healing. Recently, François et al. [30] studied the potential use of hMSC to limit radiation-induced skin lesions. Macroscopic and histologic analysis demonstrated a delay in development of lesions and a less severe degree of radiation dermatitis after hMSC transplant compared with irradiated non-transplanted controls, as well as a faster healing rate. However, the mechanisms involved in wound repair were not examined.
Interestingly, the human transplanted mesenchymal stem cells did not appear to incite inflammatory response in the rabbit model. These results suggest that allogenic human mesenchymal stem cells can be exploited for cutaneous wound repair. However, it remains to be explored how these approaches can be generalized in other wound healing models. For long-term purposes, such as chronic wounds another design of mesenchymal stem cell application may be more desirable. Other novel pathways may also need to be explored to enhance wound healing while preventing scar formation. For example, we demonstrated that adenovirus-mediated over expression of fibromodulin improves wound healing in vivo, suggesting that fibromodulin may be a key mediator in reduced scarring [31]. Perturbation of the mTOR signaling pathway by rapamycin was recently shown to down regulate expression of the extracellular matrix proteins fibronectin, collagen and alpha-smooth muscle actin, demonstrating its therapeutic potential in excessive scarring [32]. Follistatin may also have potential therapeutic value by reducing proliferation and suppressing activin-induced collagen expression [33]. Although our data are intriguing in their own right, we recognize the fact there is much more to understand about the biology of mesenchymal stem cells in tissue remodeling, which warrants further investigation.
Acknowledgments
This work was supported by Grant of the Deutsche Forschungsgemeinschaft Sto 647/1-1 (to M. A. Stoff-Khalili), by grants from the National Institutes of Health R01CA93796, R01CA98543 and AR46031 (to G. P. Siegal) and from the Louisiana Gene Therapy Research Consortium, Inc. (to J. M. Mathis).
References
- 1.Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–50. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci U S A. 2003;100 1:11917–23. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansilla E, Marin GH, Sturla F, Drago HE, Gil MA, Salas E, Gardiner MC, Piccinelli G, Bossi S, Petrelli L, Iorio G, Ramos CA, Soratti C. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplant Proc. 2005;37:292–4. doi: 10.1016/j.transproceed.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 6.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100:2397–402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99:3024–9. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–41. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 11.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 12.Hebda PA, Dohar JE. Transplanted fetal fibroblasts: survival and distribution over time in normal adult dermis compared with autogenic, allogenic, and xenogenic adult fibroblasts. Otolaryngol Head Neck Surg. 1999;121:245–51. doi: 10.1016/S0194-5998(99)70179-8. [DOI] [PubMed] [Google Scholar]
- 13.Liechty KW, Nesbit M, Herlyn M, Radu A, Adzick NS, Crombleholme TM. Adenoviral-mediated overexpression of platelet-derived growth factor-B corrects ischemic impaired wound healing. J Invest Dermatol. 1999;113:375–83. doi: 10.1046/j.1523-1747.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 14.Singer AJ, Thode HC, Jr, McClain SA. Development of a histomorphologic scale to quantify cutaneous scars after burns. Acad Emerg Med. 2000;7:1083–8. doi: 10.1111/j.1553-2712.2000.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 15.Hunt TK. Basic principles of wound healing. J Trauma. 1990;30:S122–8. doi: 10.1097/00005373-199012001-00025. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 17.Swanson NA, Tromovitch TA. Suture materials, 1980s: properties, uses, and abuses. Int J Dermatol. 1982;21:373–8. doi: 10.1111/j.1365-4362.1982.tb03154.x. [DOI] [PubMed] [Google Scholar]
- 18.Singer DD, Singer AJ, McClain SA, Tortora G. Histologic effects of laser-assisted topical anesthesia in a porcine model. Acad Emerg Med. 2005;12:1148–52. doi: 10.1197/j.aem.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 20.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM, Longoni PD. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 21.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implication in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 22.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naïve and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 23.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol. 2006;176:2864–71. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- 25.Daans J, Spaepen G, Chatterjee S, Vermeulen K, D'Haese P, Van Tendeloo VF, Van Marck E, Ysebaert D, Berneman ZN, Jorens PG, Ponsaerts P. Plasmid-based genetic modification of human bone marrow-derived stromal cells: analysis of cell survival and transgene expression after transplantation in rat spinal cord. Ronsyn MW, editor. BMC Biotechnol. 2007;7:90. doi: 10.1186/1472-6750-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Chen X, Armstrong MA, Li G. Survival of bone marrow-derived mesenchymal stem cells in a xenotransplantation model. J Orthop Res. 2007;25:926–32. doi: 10.1002/jor.20385. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence WT. Physiology of the acute wound. Clin Plast Surg. 1998;25:321–40. [PubMed] [Google Scholar]
- 28.Yamamoto M, Yanaga H, Nishina H, Watabe S, Mamba K. Fibrin stimulates the proliferation of human keratinocytes through the autocrine mechanism of transforming growth factor-alpha and epidermal growth factor receptor. Tohoku J Exp Med. 2005;207:33–40. doi: 10.1620/tjem.207.33. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005;153:29–36. doi: 10.1111/j.1365-2133.2005.06554.x. [DOI] [PubMed] [Google Scholar]
- 30.Francois S, Mouiseddine M, Mathieu N, Semont A, Monti P, Dudoignon N, Sache A, Boutarfa A, Thierry D, Gourmelon P, Chapel A. Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann Hematol. 2007;86:1–8. doi: 10.1007/s00277-006-0166-5. [DOI] [PubMed] [Google Scholar]
- 31.Stoff A, Rivera AA, Mathis JM, Moore ST, Banerjee NS, Everts M, Espinosa-de-los-Monteros A, Novak Z, Vasconez LO, Broker TR, Richter DF, Feldman D, Siegal GP, Stoff-Khalili MA, Curiel DT. Effect of adenoviral mediated overexpression of fibromodulin on human dermal fibroblasts and scar formation in full-thickness incisional wounds. J Mol Med. 2007;85:481–96. doi: 10.1007/s00109-006-0148-z. [DOI] [PubMed] [Google Scholar]
- 32.Ong CT, Khoo YT, Mukhopadhyay A, Do DV, Lim IJ, Aalami O, Phan TT. mTOR as a potential therapeutic target for treatment of keloids and excessive scars. Exp Dermatol. 2007;16:394–404. doi: 10.1111/j.1600-0625.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 33.Fumagalli M, Musso T, Vermi W, Scutera S, Daniele R, Alotto D, Cambieri I, Ostorero A, Gentili F, Caposio P, Zucca M, Sozzani S, Stella M, Castagnoli C. Imbalance between activin A and follistatin drives postburn hypertrophic scar formation in human skin. Exp Dermatol. 2007;16:600–10. doi: 10.1111/j.1600-0625.2007.00571.x. [DOI] [PubMed] [Google Scholar]