Abstract
In chronic lymphocytic leukemia (CLL), malignant B cells and nonmalignant T cells exhibit dysfunction. We previously demonstrated that infection of CLL cells with modified vaccinia Ankara (MVA) expressing the costimulatory molecules B7-1, ICAM-1, and LFA-3 (designated TRICOM) increased expression of these costimulatory molecules on the surface of CLL cells and thus augmented their antigen-presenting capability. Here, we evaluate the effect of MVA-TRICOM-modified CLL cells on T cells. Following incubation with irradiated MVA-TRICOM-modified CLL cells, allogeneic and autologous CD4+ and CD8+ T cells expressed significantly higher levels of B7-1, ICAM-1, and LFA-3. We show that this increase was the result of physical acquisition from the antigen-presenting cells (APCs), and that purified T cells that acquired costimulatory molecules from MVA-TRICOM-modified CLL cells were able to stimulate the proliferation of untreated T cells. These results demonstrate for the first time that T cells from CLL patients can acquire multiple costimulatory molecules from autologous CLL cells and can then act as APCs themselves. Given the immunodeficiencies characteristic of CLL, enhancing the antigen-presenting function of CLL cells and T cells simultaneously could be a distinct advantage in the effort to elicit antitumor immune responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-008-0611-5) contains supplementary material, which is available to authorized users.
Keywords: CLL, T cells, Acquisition, Costimulation, Immunotherapy
Introduction
Chronic lymphocytic leukemia (CLL) is a malignancy characterized by accumulation of clonal CD5+ B lymphocytes that are inefficient in antigen presentation [7, 25], largely due to an inadequate costimulatory capacity. The limited ability of CLL cells to present antigens to T cells is manifested as a failure to stimulate proliferation of both allogeneic and autologous T cells. CLL is also characterized by profound defects in the T-cell compartment, which are believed to increase the risk of infection and to hinder immune recognition and elimination of leukemic cells [2, 22]. It has been proposed that the distinctive phenotype and functional defects of T cells from CLL patients may result from T-cell interaction with the malignant B-cell clone [14]. Moreover, it has been demonstrated that contact with CLL cells induces several defects in healthy T cells in vitro [5].
Effective stimulation of T cells by antigen-presenting cells (APCs) involves two signaling events. Signal 1 is induced by T-cell receptor (TCR) recognition of peptide-MHC complexes, and signal 2 is triggered by interaction of costimulatory molecules on the surface of the APC with specific ligand(s) on the T-cell surface [13]. To form tight conjugates between T cells and APCs, various cell surface molecules (TCR/CD3, peptide/MHC, CD28/B7, CD2/LFA-3, LFA-1/ICAM-1, among others) segregate into distinct regions or clusters, designated supramolecular activation clusters (SMACs) [16] that create an organized interface referred to as an immunological synapse [6]. It has been previously demonstrated that as a result of this firm coupling of T cell and APC, molecules can be transferred to the T cell from the surface of various APCs, including insect cells, dendritic cells, and tumor cell lines [1, 8–10, 20, 23]. Both CD4+ and CD8+ T cells have been shown to acquire peptide/MHC complexes from the surface of APCs in an antigen-dependent manner [1, 8, 10, 20]. Following this molecular transfer, CD4+ T cells can induce proliferation of T cells with the same antigen specificity in a secondary culture [1], whereas CD8+ T cells that have acquired peptide/MHC complexes become susceptible to fratricide killing by neighboring T cells [8]. In addition to peptide/MHC molecules, it has also been demonstrated that T cells can extract from the surface of APCs costimulatory molecules such as B7-1 and B7-2, and that this transfer can be mediated by either TCR-peptide/MHC interaction or CD28-B7 interaction [10, 20, 23]. Whereas activated T cells efficiently acquire B7 molecules via CD28 [10], the level of B7-1 acquisition by naïve CD4+ T cells, with their lower expression of CD28, has been shown to be directly proportional to both the strength of signal 1 and the amount of B7-1 expressed on the surface of the APCs [20, 23]. Following acquisition of B7-1 and peptide/MHC complexes, the fates of naïve and effector/memory CD4+ T cells appear to be different. While naïve CD4+ T cells can act as APCs after acquisition, effector/memory CD4+ T cells can undergo apoptosis in the presence of increased levels of signal 1 [17, 20, 27].
We demonstrated in a previous report that in vitro infection of CLL cells with replication-defective modified vaccinia Ankara (MVA) engineered to express the human costimulatory molecules B7-1, ICAM-1, and LFA-3 (designated TRICOM) increased expression of these costimulatory molecules on the surface of CLL cells and thus augmented the ability of the CLL cells to stimulate proliferation of allogeneic and autologous T cells [18]. Importantly, all three costimulatory molecules were shown to contribute to the improvement of antigen-presenting capacity. Furthermore, cytotoxic T lymphocytes generated in vitro by stimulation of autologous T cells with MVA-TRICOM-modified CLL cells showed cytotoxicity against unmodified CLL cells [18]. To better understand the ability of MVA-TRICOM-modified CLL cells to elicit strong T-cell proliferation—taking into account that interaction with CLL cells can induce profound phenotypic alterations in T cells—we analyzed the phenotype of allogeneic and autologous T cells after contact with CLL cells that were unmodified or modified by infection with MVA-TRICOM. Our results demonstrate that after a brief contact with MVA-TRICOM-modified CLL cells, allogeneic and autologous CD4+ and CD8+ T cells express significantly higher levels of the costimulatory molecules B7-1 and ICAM-1 (and to a lesser extent LFA-3) on their cell surface, compared to T cells that have been in contact with unmodified CLL cells. This finding is demonstrably the result of physical acquisition of costimulatory molecules from the APCs and not of endogenous upregulation of each molecule. Moreover, we demonstrate that purified T cells that have acquired costimulatory molecules through brief contact with MVA-TRICOM-modified CLL cells have the ability to then stimulate the proliferation of untreated T cells, and therefore, have acquired the ability to act as APCs. These results show for the first time that T cells from CLL patients can acquire costimulatory molecules from autologous CLL cells and can then act as APCs themselves. While all previous studies demonstrated T cell acquisition of costimulatory molecules from a range of APCs including insect cells, dendritic cells, or tumor cell lines, to our knowledge, these studies provide the first direct demonstration that T cells can acquire costimulatory molecules from primary tumor cells. Finally, these results show the novel finding that APCs which exhibit high expression of B7-1, ICAM-1, and LFA-3 can transfer all three costimulatory molecules to T cells. We hypothesize that T cell acquisition of costimulatory function after a brief contact with TRICOM-modified CLL cells might provide an advantage in eliciting an effective immune response against the tumor cells.
Materials and methods
PBMCs from CLL patients and healthy donors
Peripheral blood was collected at the University of Pittsburgh Cancer Institute from patients diagnosed with CLL, after informed consent was obtained and following approval by the University of Pittsburgh Institutional Review Board. Peripheral blood was collected at NIH from healthy donors, after informed consent was obtained and following approval by the NIH Institutional Review Board. Peripheral blood mononuclear cells (PBMCs) were isolated as previously described [18]. Unless otherwise noted, cells were cultured in RPMI 1640 medium (Mediatech, Inc., Herndon, VA) supplemented with 2 mM glutamine, 1 × antibiotic/antimycotic solution (Mediatech, Inc.), and 10% human AB serum (Gemini Bio-Products, West Sacramento, CA).
MVA viruses
A replication-defective MVA vector expressing genes encoding for the human B7-1, ICAM-1, and LFA-3 costimulatory molecules (designated TRICOM) was used. Wild-type (WT) MVA virus was used as a control vector. Both viruses were kindly provided by Therion Biologics (Cambridge, MA).
Modification of CLL cells with MVA viruses
T cells were removed from PBMCs from patients with CLL by anti-CD4 and anti-CD8 microbeads (Miltenyi Biotec, Auburn, CA). The remaining CLL cells were resuspended at 4 × 106 cells/mL in Opti-MEM (Invitrogen, Carlsbad, CA), plated at 2 × 106 cells/well (in 0.5 mL) on a 24-well plate, and modified with five plaque-forming units/cell of MVA virus (multiplicity of infection = 5) for 1 h at 37°C. Following modification, 1.5 mL of prewarmed medium containing 10% human AB serum was added to the cells, and cells were cultured for an additional 24 h.
Flow cytometry
CD19-FITC, CD19-PE-Cy5, CD4-FITC, CD4-APC-Cy7, CD8-APC-Cy7, CD8-PE-Cy5, CD8-PE-Cy7, CD3-PE-Cy7, B7-1-PE, ICAM-1-PE, LFA-3-PE, and CD40-APC were purchased from BD Biosciences Pharmingen (San Diego, CA). HLA Class II (DQ, DP, DR)-PE was purchased from Serotec, Inc. (Raleigh, NC).
Mixed lymphocyte reaction (MLR)
Allogeneic MLR and autologous lymphocyte proliferation assays were conducted as previously described [18].
Acquisition of costimulatory molecules by T cells
T cells were negatively isolated from PBMCs of healthy donors by using the Pan T Cell Isolation Kit (Miltenyi Biotec). T cells were negatively isolated from PBMCs of CLL patients by depletion of CLL cells using anti-CD19 microbeads, followed by the Pan T Cell Isolation Kit (Miltenyi Biotec). Purified T cells from either healthy donors (allogeneic) or CLL patients (autologous) were cocultured with irradiated (75 Gy) CLL cells at a 5:1 ratio for 4 h at 37°C. CLL cells were either unmodified or modified with MVA vectors as described above.
In experiments with cycloheximide (CHX) treatment, T cells were pretreated with 20 μg/mL CHX (Sigma-Aldrich, St. Louis, MO) for 2 h at 37°C, then incubated with irradiated CLL cells for 4 h in the presence of the same concentration of CHX. As a control, T cells that were pretreated for 2 h with CHX were activated with 5 ng/mL phorbol myristate acetate (PMA) and 1 μM ionomycin for 4 h in the continued presence of CHX. For antibody (Ab) blocking experiments, CLL cells were pretreated with purified Ab to human B7-1, ICAM-1, or LFA-3 (or control mouse IgG1; Serotec, Inc.) at a concentration of 20 μg/mL for 1 h at 37°C, then incubated with T cells for 4 h in the presence of the same concentration of Ab. Alternatively, T cells were pretreated with purified Ab to human CD28, CD11a, or CD2 (or control mouse IgG1; Serotec, Inc.) at a concentration of 20 μg/mL for 1 h at 37°C, then incubated with CLL cells for 4 h in the presence of the same concentration of Ab.
RT-PCR
T cells were purified after 4-h acquisition by negative selection with two rounds of anti-CD19 microbeads (Miltenyi Biotec), and RNA was isolated using the RNeasy Kit (Qiagen, Valencia, CA). RT-PCR was performed on 0.04 μg RNA using the Titanium One-Step RT-PCR Kit (Clontech, Mountain View, CA) according to the manufacturer’s suggested protocol, using 30 cycles of denaturation (94°C, 30 s), annealing (65°C, 30 s), and polymerization (68°C, 1 min). Products (5 μL) were reamplified using the Platinum PCR SuperMix (Invitrogen) by a first denaturation at 94°C for 5 min, 30 cycles of denaturation (94°C, 30 s), annealing (55°C, 30 s), and polymerization (72°C, 1 min), followed by an extension step at 72°C for 7 min. The following primers were used: B7-1-F 5′ GAC CCT AAG CAT CTG AAG CCA TG 3′, B7-1-R 5′ TGA TCC CCA CGA TCC ATG TAT C 3′, CD3ζ-F 5′ GTG TCA TTC TCA CTG CCT TGT TCC 3′, CD3ζ-R 5′ TTC AGT GGC TGA GAA GAG TGA ACC 3′, CD19-F 5′ TCA CCG TGG CAA CCT GAC CAT G 3′, and CD19-R 5′ GAG ACA GCA CGT TCC CGT ACT G 3′. As a positive control, Epstein-Barr virus (EBV)-transformed B cells were diluted 1:100 in 1 × 107 T cells from a healthy donor. All PCR products were resolved on 1.2% agarose E-Gels (Invitrogen); expected product sizes for B7-1, CD3, and CD19 were 1 kb, 468 bp, and 278 bp, respectively.
Anti-CD3-mediated proliferation assay
For proliferation assays with T cells from healthy donors, T cells were purified after 2-h acquisition by negative selection with two rounds of anti-CD19 microbeads (Miltenyi Biotec). Untreated T cells from the same healthy donor (5 × 104 cells/well) were cocultured with irradiated (50 Gy) T cells from acquisition (5 × 104 cells/well) in triplicate wells of 96-well round-bottom culture plates pre-coated with 0.01 μg/mL anti-CD3 Ab (BD Biosciences Pharmingen). For proliferation assays with T cells from CLL patients, T cells from CLL patients were purified after 2-h acquisition by negative selection with three rounds of anti-CD19 microbeads (Miltenyi Biotec). Untreated T cells from a CLL patient (2.5 × 104 cells/well) were cocultured with irradiated (50 Gy) T cells from acquisition (2.5 × 104 cells/well) in triplicate wells of 96-well round-bottom culture plates pre-coated with 0.01 μg/mL anti-CD3 Ab (BD Biosciences Pharmingen). For all proliferation assays, cells were cultured for 6 days and pulsed with 3H-thymidine (1 μCi/well) for the final 16 h before harvesting. Stimulation index was calculated as average cpm (T cells + irradiated T cells)/average cpm (T cells alone).
Results
Phenotypic modification of allogeneic T cells from healthy donors following incubation with MVA-TRICOM-modified CLL cells
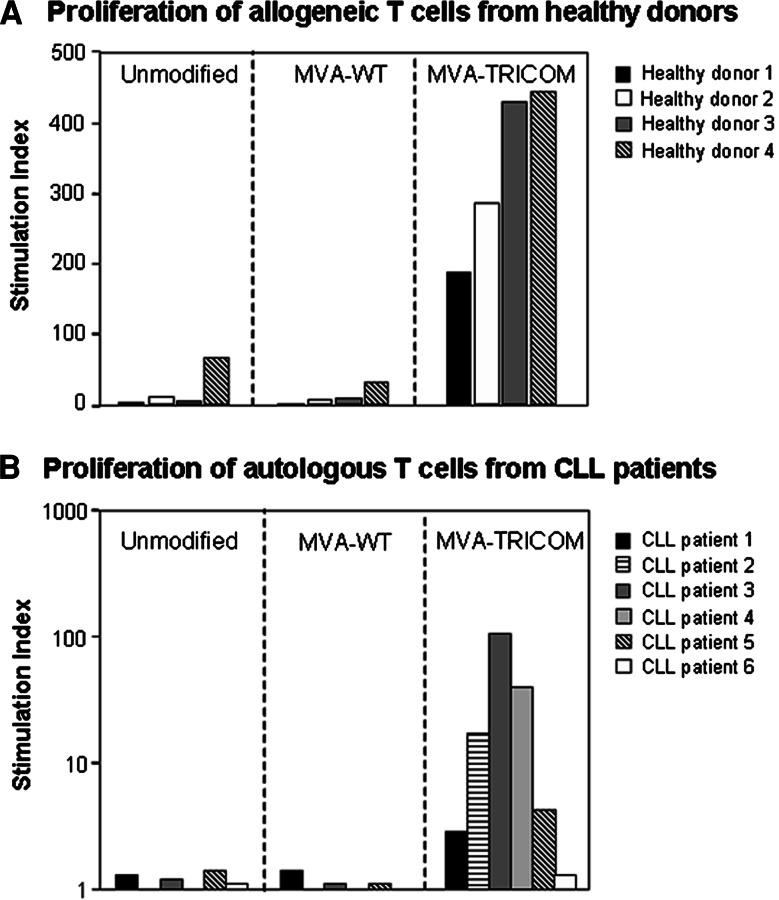
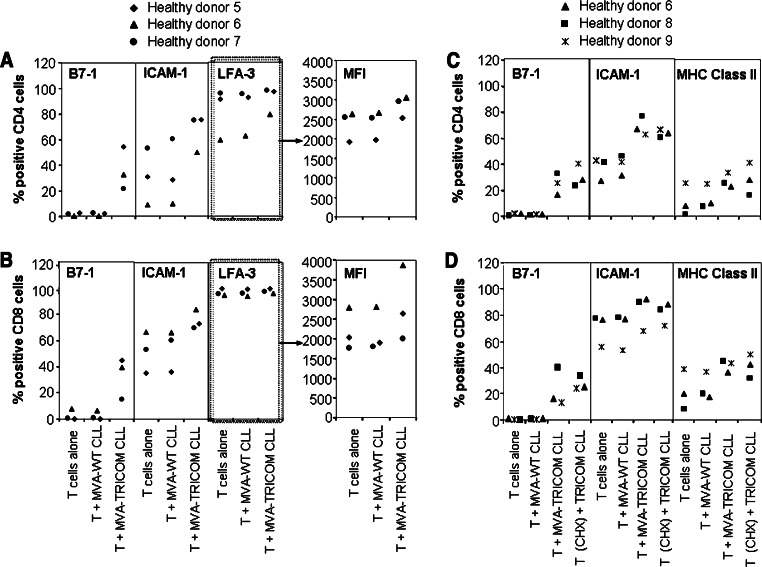
Infection of CLL cells with MVA-TRICOM was previously shown to increase expression of the costimulatory molecules B7-1, ICAM-1, and LFA-3 on their cell surface, resulting in an augmentation of their antigen-presenting ability [18]. In accordance with these previous observations, we show here that proliferation of T cells from four healthy donors was significantly enhanced after contact with allogeneic MVA-TRICOM-modified CLL cells, compared to contact with unmodified or MVA-WT-modified CLL cells (Fig. 1a). Similarly, proliferation of T cells from six CLL patients was increased following contact with autologous MVA-TRICOM-modified CLL cells (Fig. 1b). We then examined the phenotype of T cells that had been incubated with MVA-TRICOM-modified CLL cells. After allogeneic T cells from healthy donors were incubated with irradiated MVA-TRICOM-modified CLL cells for 4 h at 37°C, CD4+ and CD8+ T cells exhibited an increase in the percentage of cells with surface expression of B7-1 and ICAM-1; an increase in LFA-3 was evident from mean fluorescent intensity (MFI) of expression (Fig. 2a, b, showing results for three healthy donors assayed). As a control, T cells incubated with MVA-WT-modified CLL cells showed expression levels of B7-1, ICAM-1, and LFA-3 that were comparable to levels in untreated T cells (Fig. 2a, b). Comparable results were obtained from six healthy donors assayed (following incubation with MVA-TRICOM-modified CLL cells from six CLL patients). T cells from all of the healthy donors exhibited an increase in surface expression of B7-1 (by percentage of expression), ICAM-1 (by percentage and/or MFI of expression), and LFA-3 (by MFI of expression). However, the magnitude of the increase varied from donor to donor, as can be seen for three of the six healthy donors assayed in Fig. 2a, b. Increased expression of HLA class II molecules was also seen in allogeneic CD4+ and CD8+ T cells after contact with MVA-TRICOM-modified CLL cells (data not shown). HLA class I (HLA-A,B,C) expression on allogeneic T cells was not changed by incubation with MVA-TRICOM-modified CLL cells, nor was HLA-A2 surface expression evident on allogeneic HLA-A2− T cells after contact with HLA-A2+ MVA-TRICOM-modified CLL cells (data not shown). To rule out the possibility that a tight interaction between T cells and TRICOM-expressing CLL cells had skewed the flow cytometry results, the samples were simultaneously stained for CD19 expression. Results showed ≤0.3% expression of CD19, thus excluding the possibility of B-cell contamination within the T-cell fraction. These results demonstrate that a brief incubation with MVA-TRICOM-modified CLL cells results in phenotypic modification of allogeneic T cells from healthy donors.
Fig. 1.
T-cell proliferation in the presence of MVA-TRICOM-modified CLL cells. a CD3+ T cells from four healthy donors were cocultured with allogeneic irradiated CLL cells, either uninfected, MVA-WT-, or MVA-TRICOM-infected, at a ratio of effector to stimulator cells of 10:1. b CD3+ T cells from six CLL patients were cocultured with autologous irradiated CLL cells, either uninfected, MVA-WT-, or MVA-TRICOM-infected, at a ratio of effector to stimulator cells of 2.5:1. Proliferation was measured as 3H-thymidine incorporation on day 5 of culture. Stimulation index was calculated as average cpm (T cells + CLL cells)/average cpm (T cells alone)
Fig. 2.
Detection of costimulatory molecules on allogeneic T cells after incubation with MVA-TRICOM-modified CLL cells. CD3+ T cells from healthy donors were cultured alone or with MVA-WT-modified or MVA-TRICOM-modified irradiated CLL cells at a 5:1 ratio for 4 h at 37°C. CD4+ (a) and CD8+ (b) T cells from three healthy donors were analyzed for expression of the costimulatory molecules B7-1 (left), ICAM-1 (center), or LFA-3 (right) after the 4-h incubation. MFI of LFA-3 expression by the T cells is shown in the plots at the far right. As a control for the presence of CLL cells, T cells simultaneously stained for CD19 exhibited ≤0.3% expression of the B-cell marker. Comparable results were obtained for six healthy donors assayed. c, d One group of T cells was treated with CHX (20 μg/mL); CHX-treated T cells activated during the 4-h incubation with PMA and ionomycin did not exhibit upregulation of CD69 (74.3% of untreated T cells vs. 12.8% of CHX-treated T cells), demonstrating that CHX inhibited new protein synthesis. CD4+ (c) and CD8+ (d) T cells from three healthy donors (of three donors assayed in total) were analyzed for expression of B7-1 (left), ICAM-1 (center), and HLA class II (DQ, DP, DR) (right) at the end of the 4-h incubation
Effect of CHX treatment on expression of costimulatory molecules by allogeneic T cells from healthy donors
To determine whether the increase in costimulatory molecules on allogeneic T cells after incubation with MVA-TRICOM-modified CLL cells was due to upregulation of endogenous expression by the T cells or to acquisition from the CLL cells, allogeneic T cells were incubated with CHX before and during incubation with MVA-TRICOM-modified CLL cells and subsequently analyzed for expression of B7-1, ICAM-1, and HLA class II molecules. Following incubation of CD4+ and CD8+ T cells from healthy donors with MVA-TRICOM-modified CLL cells, levels of surface expression of B7-1, ICAM-1, and HLA class II on CHX-treated T cells (in which protein synthesis is abrogated) were equal to levels seen on untreated T cells (Fig. 2c, d, showing results for three healthy donors assayed). CHX-treated T cells activated during the 4-h incubation with PMA and ionomycin did not exhibit upregulation of CD69 (74.3% of untreated T cells vs. 12.8% of CHX-treated T cells), demonstrating that CHX inhibited new protein synthesis. These findings demonstrate that the increase in costimulatory and HLA class II molecules on allogeneic T cells is due to acquisition from the MVA-TRICOM-modified CLL cells.
Effect of Ab blocking on expression of costimulatory molecules by allogeneic T cells from healthy donors
We analyzed the respective contributions of B7-1, ICAM-1, and LFA-3 to the acquisition of costimulatory molecules by T cells by treating MVA-TRICOM-modified CLL cells with blocking Ab to each costimulatory molecule before and during incubation with allogeneic T cells from healthy donors. Blocking of LFA-3 on MVA-TRICOM-modified CLL cells produced the most striking effect on acquisition by allogeneic T cells, decreasing the acquisition of LFA-3, as evidenced by MFI (data not shown), as well as B7-1, ICAM-1, and HLA class II molecules (Table 1). In contrast, blocking of ICAM-1 did not significantly impair the acquisition of any of the molecules, while anti-B7-1 Ab significantly blocked the acquisition of B7-1 by CD4+ and CD8+ T cells (Table 1).
Table 1.
Decrease in acquisition of costimulatory molecules by allogeneic T cells with Ab blocking
| T alone | T + MVA-TRICOM CLL | ||||
|---|---|---|---|---|---|
| IgG1 | aB7-1 | aICAM-1 | aLFA-3 | ||
| CD4+ T cellsa | |||||
| B7-1 | |||||
| Healthy donor 6 | 1.9 | 35.0 | 14.0 | 44.4 | 2.0 |
| Healthy donor 8 | 0.3 | 42.0 | 12.8 | 34.4 | 7.0 |
| Healthy donor 9 | 1.9 | 35.4 | 17.4 | 34.5 | 12.5 |
| ICAM-1 | |||||
| Healthy donor 6 | 26.8 | 70.5 | 59.0 | 68.5 | 47.0 |
| Healthy donor 8 | 42.2 | 77.6 | 73.5 | 67.8 | 47.3 |
| Healthy donor 9 | 42.8 | 74.8 | 63.7 | 66.1 | 50.3 |
| HLA Class II | |||||
| Healthy donor 6 | 7.9 | 33.9 | 14.8 | 35.5 | 13.7 |
| Healthy donor 8 | 1.7 | 36.7 | 31.9 | 29.1 | 8.2 |
| Healthy donor 9 | 25.4 | 43.5 | 38.0 | 46.3 | 20.4 |
| CD8+ T cellsa | |||||
| B7-1 | |||||
| Healthy donor 6 | 1.2 | 33.7 | 16.5 | 36.4 | 2.0 |
| Healthy donor 8 | 0.2 | 50.6 | 21.9 | 41.1 | 8.3 |
| Healthy donor 9 | 0.7 | 25.6 | 12.0 | 20.3 | 3.6 |
| ICAM-1 | |||||
| Healthy donor 6 | 76.4 | 94.3 | 89.5 | 92.4 | 83.9 |
| Healthy donor 8 | 77.4 | 90.6 | 90.3 | 86.9 | 72.1 |
| Healthy donor 9 | 55.7 | 82.4 | 69.4 | 70.6 | 56.8 |
| HLA Class II | |||||
| Healthy donor 6 | 19.9 | 50.6 | 27.6 | 47.7 | 28.1 |
| Healthy donor 8 | 8.1 | 56.9 | 57.9 | 45.7 | 21.4 |
| Healthy donor 9 | 38.7 | 58.2 | 48.8 | 56.1 | 29.8 |
CD3+ T cells from 3 healthy donors were cultured alone or with MVA-TRICOM-modified irradiated CLL cells at a 5:1 ratio for 4 h at 37°C. MVA-TRICOM-modified CLL cells in indicated groups were treated with blocking Ab (anti-B7-1, anti-ICAM-1, anti-LFA-3, or isotype control mouse IgG1) before and during the 4-h acquisition. CD4+ and CD8+ T cells were analyzed for percentage of cells expressing B7-1, ICAM-1, and HLA class II (DQ, DP, DR) at the end of the 4-h incubation
aShown is percentage expression of each marker by CD4+ or CD8+ T cells
APC function of T cells from healthy donors that have acquired costimulatory molecules from MVA-TRICOM-modified CLL cells
We examined whether T cells from healthy donors that had acquired costimulatory molecules from MVA-TRICOM-modified CLL cells could function as APCs to untreated T cells. T cells from healthy donors were cultured with either unmodified, MVA-WT-modified, or MVA-TRICOM-modified irradiated CLL cells at a 5:1 ratio for 2 h at 37°C, after which the T cells were purified by depletion of B cells. Following incubation with MVA-TRICOM-modified CLL cells, purified CD3+ T cells exhibited an increase in the percentage of cells with surface expression of B7-1, ICAM-1, and HLA Class II (Figure S1A of Electronic Supplementary Material, showing results for one representative donor of two healthy donors assayed). As a control for possible CLL contamination, aliquots of the purified T cells were stained for CD19 and CD40; for two healthy donors assayed, all groups exhibited ≤0.3% expression of the B-cell markers (shown for T cells from acquisition with MVA-TRICOM-modified CLL cells from one representative healthy donor in Fig. S1B of Electronic Supplementary Material). The purified T cells from acquisition were irradiated and cocultured with untreated T cells from the same healthy donor at a 1:1 ratio in a proliferation assay, on plates pre-coated with anti-CD3 Ab. T cells that had acquired costimulatory molecules from MVA-TRICOM-modified CLL cells enhanced anti-CD3-mediated proliferation of the effector T cells. For a representative healthy donor, the stimulation index of effector T cells cultured with T cells that had acquired costimulatory molecules as APCs was 105.9 (Figure S1C of Electronic Supplementary Material). T cells that had been incubated with unmodified or MVA-WT-modified CLL cells (and had not acquired costimulatory molecules) exhibited poor APC function in the proliferation assay (Figure S1C of Electronic Supplementary Material). Thus, T cells from healthy donors that had acquired costimulatory molecules from TRICOM-expressing CLL cells were capable of functioning as APCs to untreated T cells.
Phenotypic modification of autologous T cells from CLL patients following incubation with MVA-TRICOM-modified CLL cells
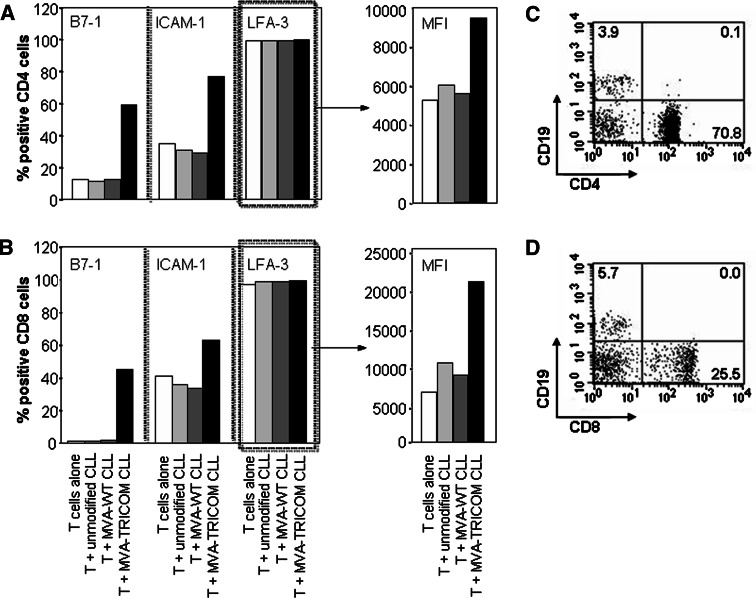
As observed with allogeneic T cells, after autologous T cells from CLL patients were incubated with irradiated MVA-TRICOM-modified CLL cells for 4 h at 37°C, CD4+ and CD8+ T cells exhibited increased surface expression of B7-1, ICAM-1, and LFA-3 (LFA-3 measured by MFI of expression; Fig. 3a, b, showing results for 1 representative donor of 12 CLL patients assayed). To rule out the possibility that doublet formation between T cells and TRICOM-expressing CLL cells had skewed the flow cytometry results, the samples were simultaneously stained for CD19 expression. Results showed ≤0.2% expression of CD19 by CD4+ and CD8+ T cells, thus excluding the possibility of B-cell contamination within the T-cell fractions (Fig. 3c, d). CHX-treated autologous T cells also showed enhanced surface expression of the three costimulatory molecules, indicating that the increase was due to acquisition from the MVA-TRICOM-modified CLL cells and not to endogenous expression by the T cells (Table 2, showing results for one representative donor of two CLL patients assayed). These results demonstrate that autologous T cells from CLL patients acquire costimulatory molecules following a brief incubation with MVA-TRICOM-modified CLL cells.
Fig. 3.
Acquisition of costimulatory molecules by autologous T cells from MVA-TRICOM-modified CLL cells. CD3+ T cells from a CLL patient were cultured alone or with autologous unmodified, MVA-WT-modified, or MVA-TRICOM-modified irradiated CLL cells at a 5:1 ratio for 4 h at 37°C. CD4+ (a) and CD8+ (b) T cells were analyzed for expression of B7-1 (left), ICAM-1 (center), and LFA-3 (right) at the end of the 4-h incubation. MFI of LFA-3 expression is shown in the plots at the far right. As a control for the presence of CLL cells, CD4+ (c) and CD8+ (d) T cells simultaneously stained for CD19 exhibited ≤0.2% expression of the B-cell marker. Results from a representative donor are shown; comparable results were obtained for 12 CLL patients assayed
Table 2.
Detection of costimulatory molecules on CHX-treated autologous T cells after incubation with MVA-TRICOM-modified CLL cells
| B7-1 % (MFI) | ICAM-1 % (MFI) | LFA-3 % (MFI) | |
|---|---|---|---|
| CD4+ | |||
| T (CHX) | 10.8 (471) | 70.6 (507) | 100.0 (3,550) |
| T (CHX) + MVA-WT CLL | 11.0 (420) | 72.2 (585) | 99.9 (3,175) |
| T (CHX) + MVA-TRICOM CLL | 75.9 (2,891) | 91.0 (7,368) | 100.0 (6,356) |
| CD8+ | |||
| T (CHX) | 5.2 (1,075) | 75.4 (942) | 99.9 (3,696) |
| T (CHX) + MVA-WT CLL | 2.4 (908) | 77.4 (1,083) | 99.7 (3,387) |
| T (CHX) + MVA-TRICOM CLL | 58.9 (4,394) | 81.5 (10,105) | 99.7 (6,375) |
CD3+ T cells from a CLL patient were treated with CHX (20 μg/mL) and were cultured alone or with autologous MVA-WT-modified or MVA-TRICOM-modified irradiated CLL cells at a 5:1 ratio for 4 h at 37°C. CD4+ and CD8+ T cells were analyzed for expression of B7-1, ICAM-1, and LFA-3 at the end of the 4-h incubation. Results from a representative donor are shown; comparable results were obtained for two CLL patients
As observed with allogeneic T cells, treatment of MVA-TRICOM-modified CLL cells with blocking Ab to B7-1 significantly decreased acquisition of B7-1 by autologous T cells from CLL patients; for a representative donor, acquisition of B7-1 by CD4+ T cells was reduced from 28.5% for the isotype control to 12.9% with anti-B7-1 Ab (Figure S2A of Electronic Supplementary Material). Further, treatment of MVA-TRICOM-modified CLL cells with blocking Ab to LFA-3 abrogated acquisition of B7-1, ICAM-1, and LFA-3 by autologous T cells. Acquisition of B7-1 by CD4+ T cells decreased from 28.5% for the isotype control to 9.3% with anti-LFA-3 Ab, while acquisition of ICAM-1 by CD4+ T cells was reduced from 56.5 to 37.1% with anti-LFA-3 treatment (Figure S2A of Electronic Supplementary Material). Ab blocking of T-cell ligands had a similar effect on acquisition of costimulatory molecules by autologous T cells. B7-1, ICAM-1, and LFA-3 on TRICOM-expressing CLL cells bind to CD28, CD11a, and CD2, respectively, on the surface of T cells. While T cells from CLL patients sometimes show marked reductions in the surface expression of key costimulatory and adhesion molecules, including CD28 and CD11a [21], CD3+ T cells from the patients assayed in this paper expressed CD28 (97.8% ± 3.5), CD11a (97.2% ± 2.5), and CD2 (98.7% ± 2.0). Ab blocking of CD28 decreased acquisition of B7-1 by CD4+ T cells from 26.7% (isotype control) to 12.8% (Figure S2B of Electronic Supplementary Material). Blocking of CD2 abrogated acquisition of B7-1, ICAM-1, and LFA-3 (Figure S2B of Electronic Supplementary Material; data not shown). These studies further demonstrate that transfer of costimulatory molecules from MVA-TRICOM-modified CLL cells to T cells is largely dependent upon ligation of LFA-3/CD2 and, to a lesser extent, B7-1/CD28.
Analysis of B7-1 mRNA expression in autologous T cells from CLL patients
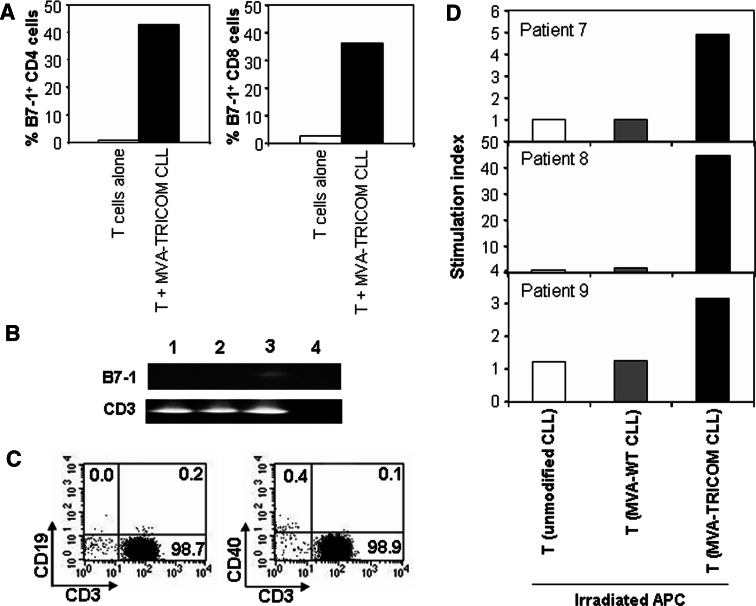
Of the three costimulatory molecules examined in this study, B7-1 alone is negligibly expressed on untreated T cells. To further demonstrate that T cells were acquiring B7-1 from MVA-TRICOM-modified CLL cells rather than upregulating endogenous expression, we performed RT-PCR analysis for B7-1, the T-cell marker CD3, and the B-cell marker CD19. Autologous T cells from CLL patients were incubated with irradiated MVA-TRICOM-modified CLL cells for 4 h at 37°C, purified by depletion of B cells, and subjected to RNA isolation. Autologous T cells exhibited increased surface expression of B7-1 by flow cytometry following incubation with TRICOM-expressing CLL cells (Fig. 4a). However, no amplification of B7-1 was observed by RT-PCR in unmodified T cells or T cells incubated with MVA-TRICOM-modified CLL cells, while CD3 was uniformly amplified in all samples (Fig. 4b). To establish the sensitivity of the RT-PCR for B7-1, EBV-transformed B cells were diluted into T cells from a healthy donor prior to RNA isolation. The B7-1 message could be detected from 10,000 EBV-transformed B cells diluted in 1 × 107 T cells (1:1,000) (data not shown). These results further demonstrate that the increase in surface expression of B7-1 on autologous T cells after incubation with MVA-TRICOM-modified CLL cells is not due to upregulation of endogenous B7-1, but rather to acquisition from the CLL cells. Because CD19 was not amplified from the purified T cells (data not shown), the results also indicate that after a 4-h incubation with MVA-TRICOM-modified CLL cells, purified T cells are free of contaminant CLL cells.
Fig. 4.
PCR analysis and APC function of T-cell fractions following acquisition. a CD3+ T cells from a CLL patient were cultured alone or with autologous MVA-TRICOM-modified irradiated CLL cells at a 5:1 ratio for 4 h at 37°C. B7-1 expression by CD4+ (left) and CD8+ (right) T cells was analyzed by flow cytometry at the end of the 4-h incubation. T cells simultaneously stained for CD19 exhibited ≤0.3% expression of the B-cell marker. b T cells from the same acquisition as shown in panel a were purified by negative selection. Isolated RNA (0.04 μg) was used in RT-PCR reactions for human B7-1 and CD3, and products were reamplified by PCR. Lane 1 T cells alone; lane 2 T cells from acquisition with MVA-TRICOM-modified CLL cells; lane 3 positive control for B7-1: EBV-transformed B cells diluted into T cells from a healthy donor; lane 4 negative control: no template. c, d T cells from CLL patients were cultured with either unmodified, MVA-WT-modified, or MVA-TRICOM-modified irradiated CLL cells at a 5:1 ratio for 2 h at 37°C, following which the T cells were purified by negative selection. c Purified CD3+ T cells from acquisition with MVA-TRICOM-modified CLL cells were analyzed for expression of CD19 (left) and CD40 (right). Results from one representative patient are shown. The level of contamination by CLL cells was ≤0.5%. d Purified T cells from acquisition were irradiated and cocultured with autologous (Patients 7 and 8) or allogeneic (Patient 9) untreated T cells from a CLL patient at a 1:1 ratio (2.5 × 104 cells/well) in triplicate wells of 96-well round-bottom culture plates that had been pre-coated with 0.01 μg/mL anti-CD3 Ab as stimulant. Proliferation was measured by 3H-thymidine incorporation on day 6 of culture. Stimulation index was calculated as average cpm (T cells + irradiated T cells)/average cpm (T cells alone)
APC function of T cells from CLL patients that have acquired costimulatory molecules from MVA-TRICOM-modified CLL cells
We examined whether T cells from CLL patients that had acquired costimulatory molecules from MVA-TRICOM-modified CLL cells could function as APCs to untreated T cells. T cells from CLL patients were cultured with either unmodified, MVA-WT-modified, or MVA-TRICOM-modified irradiated CLL cells at a 5:1 ratio for 2 h at 37°C, after which the T cells were purified by depletion of B cells. As a control for possible CLL contamination, aliquots of the purified T cells were stained for CD19 and CD40; all groups exhibited ≤0.5% expression of the B-cell markers (shown for T cells from acquisition with MVA-TRICOM-modified CLL cells from one representative patient in Fig. 4c). The purified T cells from acquisition were irradiated and cocultured with untreated T cells from a CLL patient at a 1:1 ratio in a proliferation assay, on plates pre-coated with 0.01 μg/mL anti-CD3 Ab as stimulant. T cells that had acquired costimulatory molecules from MVA-TRICOM-modified CLL cells induced anti-CD3-mediated proliferation of the effector T cells (stimulation indexes of 4.9, 44.6 and 3.2 for three patients assayed; Fig. 4d). T cells that had been incubated with unmodified or MVA-WT-modified CLL cells (and had not acquired costimulatory molecules) did not function as APCs in the proliferation assay (Fig. 4d). Thus, T cells from CLL patients that had acquired costimulatory molecules from TRICOM-expressing CLL cells were capable of functioning as APCs to untreated T cells.
Discussion
Chronic lymphocytic leukemia is a disease in which both malignant B cells and nonmalignant T cells exhibit dysfunction. CLL cells show decreased expression of costimulatory molecules such as B7-1 and B7-2, resulting in an inability to provide an adequate signal 2, which is required for T-cell activation [2, 7, 25]. Equally important, T cells from CLL patients show an inverted CD4/CD8 ratio, altered cytokine production, and decreased mitogenic and allogeneic responses, despite their increased absolute numbers and enhanced expression of activation markers [2, 22]. Current therapies for CLL, including the nucleoside analog fludarabine and the monoclonal Ab alemtuzumab, can further depress T cell-mediated immunity, thereby diminishing the potential for tumor control and increasing the risk of opportunistic infections [19]. Thus, there is substantial interest in developing CLL-specific immunotherapies that could limit tumor growth while boosting immune response.
We have previously demonstrated that modification of CLL cells with MVA-TRICOM, a recombinant virus encoding B7-1, ICAM-1, and LFA-3, results in increased surface expression of these three costimulatory molecules, with concomitant augmentation of the antigen-presenting ability of the CLL cells [18]. We show here that both allogeneic and autologous CD4+ and CD8+ T cells exhibit higher levels of surface expression of B7-1, ICAM-1, and LFA-3 after a 4-h exposure to MVA-TRICOM-modified CLL cells. We also demonstrate that allogeneic CD4+ and CD8+ T cells exhibit higher levels of HLA class II, but not HLA class I, following a 4-h incubation with MVA-TRICOM-modified CLL cells.
Protein synthesis inhibition experiments, and RT-PCR analysis in the case of B7-1, provided evidence that higher surface expression by T cells of the three costimulatory molecules and HLA class II was the result of physical acquisition from the CLL cells. These results are consistent with previous reports which show the transfer of B7 and ICAM-1 from APCs to T cells [10, 20, 23], as well as the transfer of MHC class II from allogeneic APCs to human T cells [4, 24]. For ICAM-1 and LFA-3, which are more widely expressed by T cells prior to incubation with MVA-TRICOM-modified CLL cells, the possibility remains that the increased surface expression results from redistribution of pre-formed molecules within the T cell. Further experiments could more directly assess whether acquisition is occurring by utilizing CLL cells which are infected with MVA virus expressing GFP-tagged TRICOM molecules.
We also show by Ab-mediated blocking experiments that the acquisition of the three costimulatory molecules and HLA class II was mainly dependent on the interaction between LFA-3/CD2, while the interaction between ICAM-1/LFA-1 had a negligible effect on acquisition. These results are consistent with previous reports in other cell types showing the importance of LFA-3 in the transfer of B7-1 and MHC class II from APCs to T cells [4, 24]. Acquisition of B7-1 was similarly abrogated by Ab blocking directed against B7-1 or the correspondent CD28 ligand, which is also in accordance with previous studies showing CD28-mediated absorption of B7 molecules by T cells [10]. The relative importance of these receptor/ligand pairs in the acquisition of costimulatory molecules may mirror their relative importance in forming and stabilizing the immunological synapse between T cell and APC. For instance, interaction between CD2 and LFA-3 is thought to form a complex with the same dimensions as the TCR-peptide/MHC complex, which favors close membrane proximity and stabilizes the synapse by excluding larger molecules, such as CD43 and CD45, from the contact interface [15]. In contrast, the larger ICAM-1 molecule is excluded from the central SMAC and, perhaps because of this exclusion, appears to play no role in the T-cell acquisition process, except in those cases where T cells are absorbing molecules from exosome-like membrane vesicles shed from APCs [11]. Consistent with this observation, our results also indicate that transfer of costimulatory molecules from CLL cells to T cells is independent of membrane vesicles.
Acquired molecules have previously been shown to alter the function of T cells in vitro and in vivo. Zhou et al. demonstrated that naïve T cells can acquire B7-1/MHC molecules from professional APCs, and that these acquired molecules can sustain T-cell activation/proliferation in the absence of APCs [27]. It has also been shown that CD4+ and CD8+ T cells from HIV-infected individuals express increased levels of B7-1 and B7-2, which allow the T cells to act as APCs [12, 26]. Further, T cells from myeloma patients have been demonstrated to acquire B7 antigens, and this phenotype seems to be associated with stable disease [3]. We have shown here for the first time that, after acquiring costimulatory molecules from MVA-TRICOM-modified CLL cells, T cells from CLL patients are capable of functioning in vitro as APCs to untreated T cells from CLL patients. T cells which acquired costimulatory molecules provided signal 2 to untreated T cells in an anti-CD3-mediated proliferation assay. To more closely mimic the situation in vivo, further experiments could assess the capacity of the modified T cells to stimulate an antigen-specific response against a recall antigen, for example by pulsing T cells which acquired costimulatory molecules with a peptide epitope from influenza virus. Previous studies from our laboratory have shown that CD4+ TCR-transgenic T cells which have acquired costimulatory molecules can effectively stimulate antigen-specific proliferation and cytokine production [27]. Undale et al. also demonstrated that T cells which have acquired MHC class II can present peptide to antigen-specific CD4+ T cells [24].
While it is difficult to deduce the implications of this acquisition in vivo, we speculate that T cells which acquire costimulatory molecules will amplify the immune stimulation induced by MVA-TRICOM-modified CLL cells used as a vaccine. Unmodified CLL cells express low levels of costimulatory molecules, and thus cannot provide a signal 2 adequate for T-cell activation. In vivo, circulating T cells that have contact with unmodified CLL cells will thus likely be activated only by signal 1, which could lead to anergy. However, T cells in vivo that acquire costimulatory capacity through contact with MVA-TRICOM-modified CLL cells administered via a vaccine may be able to deliver the missing signal 2 to those T cells activated by unmodified CLL cells, thereby enhancing immune response. We further showed that allogeneic T cells also acquire HLA class II from MVA-TRICOM-modified CLL cells, and other groups have previously shown that T cells can acquire entire peptide-MHC complexes from APCs [1, 8, 10, 20, 24], suggesting the possibility that T cells following acquisition could deliver both signals 1 and 2 to activate leukemia-specific T cells. This phenomenon of acquisition of costimulatory molecules by T cells has previously been shown in murine models to have potentially important biologic implications. In the literature, naïve CD4+ T cells which acquired B7 stimulated the proliferation of untreated T cells [20, 27], whereas memory CD4+ T cells which acquired B7 were more susceptible to apoptosis in vitro and in vivo, a susceptibility dependent on the strength of signal 1 [17, 20]. Also, in a previous report, CD8+ T cells which acquired peptide/MHC complexes became susceptible to fratricide killing by neighboring T cells [8]. Future experiments should be performed to clarify the distinction between effects on naïve versus memory T cells.
This study thus further supports the rationale for the use of CLL cells modified ex vivo with MVA-TRICOM as a whole tumor-cell vaccine for the immunotherapy of CLL. Given the profound immune deficiencies characteristic of CLL, the ability to enhance the antigen-presenting function of CLL cells and T cells simultaneously could be a distinct advantage in the effort to elicit an effective immune response.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We acknowledge the technical assistance of Margie Duberstein and the editorial assistance of Bonnie L. Casey and Debra Weingarten in the preparation of this manuscript. This research was supported by the NIH Intramural Research Program, Center for Cancer Research, National Cancer Institute.
References
- 1.Arnold PY, Davidian DK, Mannie MD. Antigen presentation by T cells: T cell receptor ligation promotes antigen acquisition from professional antigen-presenting cells. Eur J Immunol. 1997;27:3198–3205. doi: 10.1002/eji.1830271217. [DOI] [PubMed] [Google Scholar]
- 2.Bartik MM, Welker D, Kay NE. Impairments in immune cell function in B cell chronic lymphocytic leukemia. Semin Oncol. 1998;25:27–33. [PubMed] [Google Scholar]
- 3.Brown R, Murray A, Pope B, Sze D, Gibson J, Ho PJ, Joshua D. B7 + T cells in myeloma: an acquired marker of prior chronic antigen presentation. Leuk Lymphoma. 2004;45:363–371. doi: 10.1080/10428190310001607142. [DOI] [PubMed] [Google Scholar]
- 4.Game DS, Rogers NJ, Lechler RI. Acquisition of HLA-DR and costimulatory molecules by T cells from allogeneic antigen presenting cells. Am J Transplant. 2005;5:1614–1625. doi: 10.1111/j.1600-6143.2005.00916.x. [DOI] [PubMed] [Google Scholar]
- 5.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115:1797–1805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 7.Han T, Bloom ML, Dadey B, Bennett G, Minowada J, Sandberg AA, Ozer H. Lack of autologous mixed lymphocyte reaction in patients with chronic lymphocytic leukemia: evidence for autoreactive T-cell dysfunction not correlated with phenotype, karyotype, or clinical status. Blood. 1982;60:1075–1081. [PubMed] [Google Scholar]
- 8.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–954. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 9.Hudrisier D, Bongrand P. Intercellular transfer of antigen-presenting cell determinants onto T cells: molecular mechanisms and biological significance. FASEB J. 2002;16:477–486. doi: 10.1096/fj.01-0933rev. [DOI] [PubMed] [Google Scholar]
- 10.Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–1148. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci USA. 2003;100:6670–6675. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochli C, Wendland T, Frutig K, Grunow R, Merlin S, Pichler WJ. CD80 and CD86 costimulatory molecules on circulating T cells of HIV infected individuals. Immunol Lett. 1999;65:197–201. doi: 10.1016/S0165-2478(98)00107-2. [DOI] [PubMed] [Google Scholar]
- 13.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 14.Mellstedt H, Choudhury A. T and B cells in B-chronic lymphocytic leukaemia: faust, mephistopheles and the pact with the devil. Cancer Immunol Immunother. 2006;55:210–220. doi: 10.1007/s00262-005-0675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Merwe PA, Davis SJ, Shaw AS, Dustin ML. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin Immunol. 2000;12:5–21. doi: 10.1006/smim.2000.0203. [DOI] [PubMed] [Google Scholar]
- 16.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 17.Mostbock S, Catalfamo M, Tagaya Y, Schlom J, Sabzevari H. Acquisition of antigen presentasome (APS), an MHC/costimulatory complex, is a checkpoint of memory T-cell homeostasis. Blood. 2007;109:2488–2495. doi: 10.1182/blood-2006-09-047290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palena C, Foon KA, Panicali D, Yafal AG, Chinsangaram J, Hodge JW, Schlom J, Tsang KY. Potential approach to immunotherapy of chronic lymphocytic leukemia (CLL): enhanced immunogenicity of CLL cells via infection with vectors encoding for multiple costimulatory molecules. Blood. 2005;106:3515–3523. doi: 10.1182/blood-2005-03-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palma M, Kokhaei P, Lundin J, Choudhury A, Mellstedt H, Osterborg A. The biology and treatment of chronic lymphocytic leukemia. Ann Oncol. 2006;17(Suppl 10):x144–x154. doi: 10.1093/annonc/mdl252. [DOI] [PubMed] [Google Scholar]
- 20.Sabzevari H, Kantor J, Jaigirdar A, Tagaya Y, Naramura M, Hodge J, Bernon J, Schlom J. Acquisition of CD80 (B7-1) by T cells. J Immunol. 2001;166:2505–2513. doi: 10.4049/jimmunol.166.4.2505. [DOI] [PubMed] [Google Scholar]
- 21.Scrivener S, Kaminski ER, Demaine A, Prentice AG. Analysis of the expression of critical activation/interaction markers on peripheral blood T cells in B-cell chronic lymphocytic leukaemia: evidence of immune dysregulation. Br J Haematol. 2001;112:959–964. doi: 10.1046/j.1365-2141.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- 22.Scrivener S, Goddard RV, Kaminski ER, Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk Lymphoma. 2003;44:383–389. doi: 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- 23.Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol. 2002;169:6162–6169. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- 24.Undale AH, van den Elsen PJ, Celis E. Antigen-independent acquisition of MHC class II molecules by human T lymphocytes. Int Immunol. 2004;16:1523–1533. doi: 10.1093/intimm/dxh154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolos JA, Davey FR. B lymphocyte function in B cell chronic lymphocytic leukaemia. Br J Haematol. 1981;49:395–403. doi: 10.1111/j.1365-2141.1981.tb07242.x. [DOI] [PubMed] [Google Scholar]
- 26.Wolthers KC, Otto SA, Lens SM, Kolbach DN, van Lier RA, Miedema F, Meyaard L. Increased expression of CD80, CD86 and CD70 on T cells from HIV-infected individuals upon activation in vitro: regulation by CD4 + T cells. Eur J Immunol. 1996;26:1700–1706. doi: 10.1002/eji.1830260806. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Tagaya Y, Tolouei-Semnani R, Schlom J, Sabzevari H. Physiological relevance of antigen presentasome (APS), an acquired MHC/costimulatory complex, in the sustained activation of CD4 + T cells in the absence of APCs. Blood. 2005;105:3238–3246. doi: 10.1182/blood-2004-08-3236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.