Overview – spirochaetal lipoproteins in the postgenomic era
Anchoring of proteins to membranes by lipid modification is a universal strategy of both prokaryotic and eukaryotic cells. The spirochaetes represent a distinct bacterial phylum that have utilized the lipoprotein design extensively as evidenced by genome-sequencing studies revealing a large number of different paralogous families of lipoprotein genes, encoding 22 potential lipoproteins in Treponema pallidum (Fraser et al., 1998) and 105 potential lipoproteins in Borrelia burgdorferi (Fraser et al., 1997). Roughly 8% of Bor. burgdorferi open reading frames are predicted to encode lipoproteins (Fraser et al., 1997), which is a significantly higher frequency than that of any other bacterial genome sequenced to date (The Institute for Genomic Research, 2000). An understanding of spirochaetal lipoproteins requires an appreciation of the distinctive double-membrane architecture of spirochaetes, which shares characteristics of both Gram-positive and Gram-negative bacteria (see Fig. 1). As in Gram-positive bacteria, the cytoplasmic membrane of spirochaetes is closely associated with the peptidoglycan cell wall. Spirochaetes also have an outer membrane which provides a barrier shielding underlying antigens, such as the endoflagella, from the outside environment. However, the spirochaetal outer membrane appears to be fluid and labile, which contrasts it with the outer membrane of Gram-negative bacteria. This unique membrane architecture of spirochaetes and their ancient phylogeny suggest that the export, structure and function of spirochaetal lipoproteins have features which are unique to these organisms. As an indication of their importance in spirochaetes, lipoproteins are recognized to be the most abundant proteins in a wide array of organisms. Examples of lipoproteins that constitute a high percentage of the total protein composition of spirochaetes include OspA of Bor. burgdorferi, the Vmp proteins of the relapsing fever borreliae, SmpA of Brachyspira hyodysenteriae, LipL32 of the pathogenic Leptospira species and Tpp47 of T. pallidum.
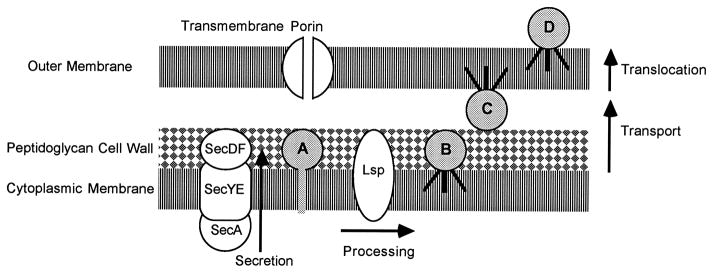
Fig. 1.
Model of spirochaetal lipoprotein export. The above model is based on what is known about bacterial lipoprotein export and the finding of orthologues of secretory proteins in spirochaetal genomes. The mechanism by which spirochaetal lipoproteins are exported has not been demonstrated experimentally. Abbreviations: A, prolipoprotein (with signal peptide) immediately after secretion; B, subsurface lipoprotein in the outer leaflet of the cytoplasmic membrane; C, subsurface lipoprotein in the inner leaflet of the outer membrane; D, surface-exposed lipoprotein in the outer leaflet of the outer membrane; Lsp, prolipoprotein signal peptidase.
This paper is intended as a broad review of key issues involving spirochaetal lipoproteins, relating fundamental biochemical and physiological issues to those of pathogenesis. Rather than an exhaustive survey of current knowledge, the goal of this review is to highlight issues of current interest and point out areas for future research. Readers desiring more detailed information about spirochaetal lipoproteins are referred to additional sources, including websites such as the TIGR Microbial Database (The Institute for Genomic Research, 2000) and other reviews (e.g. Norris, 1993). The starting point for this review is lipoprotein secretion, processing and sorting, which will allow formulation of questions regarding how cellular destination is determined. With the exponential expansion of sequence data, what should be the criteria for identification of spirochaetal lipoproteins? Characterization of lipoproteins should also include determination of whether it is exported to the outer membrane and/or surface exposed, since surface-exposed lipoproteins are likely to be involved in how spirochaetes interact with their environment. Surface-exposed lipoproteins that have been implicated in essential roles in the interaction with the mammalian host will be reviewed. What are the environmental signals that regulate differential expression of lipoproteins? Some of the lipoproteins upregulated in the mammalian host undergo antigenic variation as a strategy for evading the host immune response. Lipoproteins are also involved in disease pathogenesis through their ability to trigger the host inflammatory response. Ultimately, lipoproteins are of interest as vaccines for prevention of spirochaetal infections.
Secretion, processing and sorting –comparisons with Escherichia coli
Sequencing of the T. pallidum and Bor. burgdorferi genomes has revealed orthologues of genes encoding all the essential components of the bacterial secretory machinery, as shown in Table 1 (Fraser et al., 1997, 1998). Detailed reviews are available on secretion (e.g. Duong et al., 1997), which will be reviewed briefly here. As nascent lipoproteins emerge from the ribosome, an Ffh-containing signal-recognition particle binds to the signal peptide, piloting it to the preprotein translocase complex in the cytoplasmic membrane. As shown in Fig. 1, prolipoproteins are secreted across the cytoplasmic membrane through the preprotein translocase complex, which consists of a SecYE transmembrane channel with SecA on the cytoplasmic side and SecDF on the periplasmic side of the cytoplasmic membrane. Secretion through the translocase complex involves an energy-dependent cycle in which SecA binds the signal peptide, pushes it through the transmembrane channel, and then reaches back to bind the next part of the protein undergoing secretion. The cycle continues until the entire protein has been transported across the cytoplasmic membrane. It is likely that lipoproteins and all other proteins with signal peptides follow this pathway.
Table 1.
Putative lipoprotein secretion and processing proteins
| Description | E. coli | T. pallidum* | Bor. burgdorferi* |
|---|---|---|---|
| Signal-recognition particle | Ffh | TP0416 | BB0694 |
| Translocase, cytoplasmic domain | SecA | TP0379 | BB0154 |
| Translocase, periplasmic domain | SecD | TP0410 | BB0652 |
| Translocase, transmembrane domain | SecE | TP0235 | BB0395 |
| Translocase, periplasmic domain | SecF | TP0411 | BB0653 |
| Translocase, transmembrane domain | SecY | TP0208 | BB0498 |
| Diacylglyceryl transferase | Lgt | TP0852 | BB0362 |
| Prolipoprotein signal peptidase | Lsp | TP0978 | BB0469 |
| Apolipoprotein N-acyltransferase | Lnt | TP0252, TP0417 | BB0237 |
Numbers refer to the gene locus encoding the homologous protein.
After secretion across the cytoplasmic membrane, proteins are lipidated and cleaved at the cysteine residue in the lipoprotein signal-processing site. Lipoprotein processing is a three-step biosynthetic pathway that takes place on the periplasmic side of the cytoplasmic membrane and involves three separate enzymes located in the cytoplasmic membrane (Wu, 1996). Homologues for all three enzymes required for lipoprotein biosynthesis in E. coli have been found both in T. pallidum and in Bor. burgdorferi (Table 1). The details of lipoprotein processing were originally worked out in E. coli for the murein lipoprotein and are presumably similar in other Gram-negative bacteria and also in spirochaetes. The first of the three enzymes in the lipoprotein-processing pathway is phosphadidylglycerol:prolipoprotein diacylglyceryl transferase (Lgt). This enzyme transfers a diacylglyceryl group (containing two fatty acids) from phosphadidylglycerol to the sulfur atom of cysteine. The second enzyme in the pathway is prolipoprotein signal peptidase (Lsp), which proteolytically removes the signal peptide, making cysteine the new N-terminal amino acid. The third enzyme is phospholipid:apolipoprotein transacylase (Lnt) which transfers a third fatty acid from a membrane phospholipid to the nitrogen atom of cysteine. After processing, lipoproteins will have three fatty acids attached to the cysteine residue. It should be noted that modified proteins will be blocked to N-terminal amino acid sequencing using the Edman degradation technique.
Theoretically, a lipoprotein might become localized to one or more of four cellular compartments: the periplasmic leaflet of the cytoplasmic membrane, the periplasmic or outer leaflets of the outer membrane, or beyond the outer membrane in the surrounding milieu. The factors that determine an exported spirochaetal lipoprotein’s ultimate destination are not understood, but could include the sequence or physico-chemical properties of either the signal peptide or mature protein. A protein’s export ‘address’ could even be contained within its mRNA sequence, coupling translation and secretion, as has been demonstrated for type III secretion in Yersinia enterocolitica (Anderson & Schneewind, 1999).
In E. coli, two proteins have been identified that appear to be involved in localization of lipoproteins to the outer membrane (Matsuyama et al., 1997). A periplasmic chaperone, LolA, appears to transport lipoproteins to the outer membrane and a second protein, LolB, appears to be involved in the incorporation of murein lipoprotein into the outer membrane. No LolA or LolB sequence homologues were detected in the T. pallidum or Bor. burgdorferi genomes, suggesting that spirochaetal lipoproteins may be exported to the outer membrane by a unique pathway. In E. coli lipoproteins, the +2 residue (immediately after the N-terminal cysteine) functions as a sorting signal. Murein lipoprotein has a Ser at the +2 position and is sorted to the inner leaflet of the outer membrane. Proteins with Asp at the +2 position instead of Ser are retained in the cytoplasmic membrane (Yamaguchi et al., 1988). Acidic and/or basic residues following the N-terminal Cys may have relevance in sorting of spirochaetal lipoproteins. For example, the leptospiral lipoprotein LipL36 has an unusual series of six Asp residues located in positions +4 to +9 (Haake et al., 1998). We wondered whether an acidic region close to the N terminus explains why LipL36 is restricted to the inner leaflet of the outer membrane, while LipL41, which has only two acidic residues in this location, is exported to the outer leaflet of the outer membrane (Shang et al., 1996). Such a high concentration of negatively charged amino acids may function as a stop-transfer signal for translocation to the outer leaflet of the outer membrane. Alternatively, charged residues in this region could determine binding to a translocase protein involved in exporting lipoproteins to the outer leaflet of the outer membrane.
Criteria for identification of spirochaetal lipoproteins
Genome-sequencing studies have revealed a large number of genes encoding potential lipoproteins in spirochaetes (Fraser et al., 1997, 1998). The finding of a Cys residue after a signal peptide is suggestive evidence that a protein is lipidated. The E. coli lipobox has been defined by Wu (1996) as:
Leu occurs in the −3 position in about 75% of E. coli lipoproteins. The lipobox of Gram-positive bacteria is very similar to the E. coli lipobox, with Leu occurring at the −3 position in 87% of Gram-positive lipoproteins (Sutcliffe & Russell, 1995). In spirochaetes, the variability of amino acids in the lipoprotein signal peptidase cleavage site, including the −3 position relative to cysteine, makes recognition of lipoprotein signal peptidase cleavage sites less certain than in other bacteria. To define the spirochaetal lipobox, a literature search was performed to identify spirochaetal proteins for which experimental evidence of lipid modification was available. Twenty-six sequences were obtained from lipoproteins produced by Leptospira, Treponema, Borrelia and Brachyspira species (listed in Table 2). The resulting spirochaetal lipobox was found to be:
Table 2.
Spirochaetal proteins for which there is experimental evidence of lipidation
| Gene | Product | Cleavage site | Expression during mammalian infection | Surface-exposed | Immuno-protective | Description |
|---|---|---|---|---|---|---|
| Borrelia afzelii | ||||||
| nlpH | NlpH | MLSG↓CN | + | + | ? | Haemin-binding protein |
| Bor. burgdorferi | ||||||
| BB0328 | OppA-1 | SLIA↓CI | ? | ? | ? | Oligopeptide ABC transporter |
| BB0329 | OppA-2 | TFLC↓CN | ? | ? | ? | Oligopeptide ABC transporter |
| BB0330 | OppA-3 | SLIA↓CN | ? | ? | ? | Oligopeptide ABC transporter |
| BB0365 | LA7 | LVIA↓CT | − | − | ? | Membrane protein |
| BBA15 | OspA | ALIA↓CK | ↓ | + | + | Outer-surface protein |
| BBA16 | OspB | ALIG↓CA | − | + | + | Outer-surface protein |
| BBA24 | DbpA | LLIS↓CG | ↑ | + | + | Decorin-binding protein |
| BBB19 | OspC | LFIS↓CN | ↑ | + | + | Outer-surface protein |
| BBJ09 | OspD | LSIS↓CV | ? | + | − | Outer-surface protein |
| ospE (N40) | OspE | LIGA↓CK | + | + | − | Outer-surface protein |
| ospF (N40) | OspF | LIVS↓CK | + | + | + | Outer-surface protein |
| Bor. hermsii | ||||||
| vlp7 | Vlp7 | LMIG↓CG | ↑ | + | ? | Mammal-associated variable large protein |
| vsp33 | Vsp33 | LFIS↓CN | ↓ | + | ? | Tick-associated variable small protein |
| Bra. hyodysenteriae | ||||||
| smpA | SmpA | FAVS↓CN | − | ? | ? | Outer-membrane protein |
| Leptospira species | ||||||
| lipL32 | LipL32 | SITA↓CG | + | ? | ? | Outer-membrane protein |
| lipL36 | LipL36 | ALTA↓CK | − | − | − | Outer-membrane protein |
| lipL41 | LipL41 | FLGN↓CA | + | + | + | Outer-membrane protein |
| T. pallidum | ||||||
| TP0171 | TpN15 | LLGA↓CS | + | − | ? | Membrane protein |
| TP0257 | GlpQ/Gpd | LVAG↓CA | + | ? | +/− | Glycerophosphodiester phosphodiesterase |
| TP0319 | TmpC | LAVF↓CA | + | − | − | Cytoplasmic membrane protein |
| TP0435 | TpN17 | LCVS↓CT | + | − | ? | Outer-membrane protein |
| TP0574 | TpN47 | LVVG↓CG | + | − | ? | Zinc-dependent carboxypeptidase |
| TP0684 | MglB | LVVG↓CS | + | − | ? | Galactose-binding protein |
| TP0768 | TmpA | MLGS↓CA | + | − | − | Membrane protein |
| TP0971 | TpN29-35 | VFSA↓CG | + | − | ? | Pathogen-specific membrane protein |
The most notable departure from the E. coli lipobox is that the spirochaetal lipobox is much more loosely defined in the −3 position. As in E. coli, Leu occurred in the −3 position more frequently than any other amino acid, but was present in only 42% (11/26) of the spirochaetal sequences. In most of the other lipoproteins, Leu was substituted for by another hydrophobic amino acid such as Val, Phe or Ile. Occasionally, amino acids with lower hydrophobicity, including Ser, Thr and Ala, were found in the −3 position. However, in every case, the lack of a hydrophobic amino acid at the −3 position was compensated for by hydrophobic amino acids at the −4 (usually Leu) and −2 (usually Ile) positions. In fact, Leu occurs more frequently in the −4 position (54%) in the spirochaetal lipoproteins than in the −3 position (42%).
The variability of the spirochaetal lipobox in the −3 position may be a reflection of reduced substrate specificity of the spirochaetal prolipoprotein signal peptidases and/or the possibility that high rates of lipoprotein biosynthesis do not occur in spirochaetes. The latter idea is consistent with the slow physiology and remarkably long doubling times of spirochaetes. Studies performed on expression of the leptospiral lipoprotein LipL41 in E. coli are relevant to this question. It was observed that at a low level of expression, most of the recombinant LipL41 was lipidated while at a high level of expression, most of the recombinant LipL41 was not lipidated (Shang et al., 1996). This result suggested that E. coli prolipoprotein signal peptidase was capable of recognizing the LipL41 lipoprotein signal peptidase cleavage site, but that processing was rate limiting at high levels of induction. In spirochaetes, protein biosynthesis rates may be slow enough that spirochaetal lipoprotein signal peptidases do not need to process lipoproteins rapidly, allowing genetic drift to occur at the −3 position of the spirochaetal lipobox.
Sequence analysis alone is not sufficient to identify a spirochaetal protein as a lipoprotein. Experimental evidence is essential in confirming whether or not a protein is covalently modified by fatty acids at its N-terminal cysteine. Four types of experimental approaches have been used in studies of spirochaetal lipoproteins. 1. Incorporation of [3H]palmitate into the putative lipoprotein. 2. Demonstration of alkali- and/or acid-labile linkages of the [3H]palmitate to the lipoprotein. 3. Inhibition of processing by globomycin. 4. Behaviour of the protein in Triton X-114 fractionation.
Addition of [3H]palmitate to growth media containing metabolically active spirochaetes should result in the incorporation of tritium label into lipoproteins. An analysis of the fatty acids of T. pallidum and Bor. burgdorferi phospholipids and lipoproteins found that while fatty acids with different length side chains (C16 and C18)) were found in phospholipids, palmitate (C16) predominated in the lipoproteins, indicating a specificity for palmitate by both Lgt and Lnt acyl transferases (Belisle et al., 1994). A caveat here is that bacteria, including spirochaetes, that beta-oxidize fatty acids could potentially incorporate the tritium label into amino acids. However, labelling of proteins by incorporation of tritiated amino acids would be relatively inefficient in comparison with modification of lipoproteins by one or more molecules of [3H]palmitate. This concern can be dealt with experimentally by acid and/or alkaline hydrolysis of tritium-labelled proteins with recovery of [3H]palmitate (Brandt et al., 1990; Swancutt et al., 1990) or by demonstration of label removal from an otherwise intact protein (Haake et al., 2000).
Globomycin is a cyclic peptide antibiotic which selectively inhibits prolipoprotein signal peptidase. Examination of lipoprotein expression in the presence and absence of globomycin is a very useful tool for determining whether a protein is a substrate for prolipoprotein signal peptidase. Globomycin has been found to be active in inhibiting lipidation of native spirochaetal lipoproteins in T. pallidum (Swancutt et al., 1990) and Borrelia hermsii (Carter et al., 1994), and of recombinant spirochaetal lipoproteins expressed in E. coli (Erdile et al., 1993; Haake et al., 1998; Purcell et al., 1990; Shang et al., 1996; Thomas & Sellwood, 1993).
Lipid modification not only provides a membrane anchor for lipoproteins but also imparts a strongly hydrophobic quality, which can be assessed using the detergent Triton X-114 (Brandt et al., 1990; Chamberlain et al., 1989a). Similar in properties and structure to Triton X-100, Triton X-114 has a slightly shorter polyoxyethylene side chain that gives it the property of having an unusually low cloud point. In a typical experiment, spirochaetes are extracted in a buffer containing a low concentration of Triton X-114 (usually 0.1–1.0%), followed by centrifugation to remove insoluble material (the protoplasmic cylinder, including cytoplasmic membrane and cell wall). These initial steps must be carried out at 4 °C to prevent premature phase partitioning. The supernatant containing the solubilized spirochaetal membrane is then warmed to 37 °C, at which point Triton X-114 partitions into two phases, a detergent-rich ‘hydrophobic’ phase and a detergent-poor ‘hydrophilic’ phase. Membrane proteins, including lipoproteins, typically partition selectively into the Triton X-114 hydrophobic phase. In contrast, non-membrane proteins would be expected to partition into the Triton X-114 hydrophilic phase. The importance of lipidation on the phase-partitioning characteristics of a protein can be confirmed by performing the Triton X-114 phase-partitioning experiment on recombinant protein expressed without its lipoprotein signal peptidase cleavage site, with the expectation that it would now partition into the hydrophilic aqueous phase (Chamberlain et al., 1989b).
Controversies in outer membrane and surface localization
Lipoproteins exported to the outer leaflet of the outer membrane are of special importance in terms of understanding the interactions of spirochaetes with their environment. Surface-exposed proteins are a double-edged sword for pathogens: proteins may be utilized by bacteria to facilitate adhesion or invasion of the host, but also may serve as a target for the host immune system. Despite this apparent dilemma, all pathogenic spirochaetes share the property of being able to cause persistent infections in mammalian hosts. Spirochaetes evade the host immune response by utilizing highly evolved strategies for reducing surface exposure of protein antigens. One strategy is to restrict trans-membrane outer-membrane-protein expression to low levels (Radolf et al., 1989; Walker et al., 1989, 1991). There is also evidence that some surface lipoproteins may hinder the access of antibodies to transmembrane outer-membrane proteins (Bunikis & Barbour, 1999). Still other lipoproteins may be restricted to subsurface locations to avoid antibody exposure (Cox et al., 1992). Another mechanism for evasion of the host immune response is antigenic variation of surface lipoproteins (see Antigenic variation and evasion of the mammalian immume response section below).
Since we do not understand the signals involved in determining whether a lipoprotein will be exported to the outer membrane and/or the cell surface, experimental determination of surface exposure is critical in identifying lipoproteins that are involved in the pathogenesis of spirochaetal disease. Determining which lipoproteins are outer-membrane components and to what extent they are surface-exposed is highly controversial and continues to be an area of intense investigation (Brusca et al., 1991; Cox et al., 1995, 1996; Shevchenko et al., 1999). The degree of difficulty in resolving these issues is probably a reflection of the remarkable ability of spirochaetes to evade the host immune response. Before assigning a cellular location to a lipoprotein, multiple approaches should be utilized and great caution should be exercised in interpretation of the results. Regardless of which techniques are used to support the designation of a lipoprotein as a surface-exposed outer-membrane protein, use of appropriate markers to control for subsurface antigens and the presence of cytoplasmic-membrane components are essential to verify the accuracy of the procedure(s).
Triton X-114 extraction and phase partitioning (see previous section) has been applied to isolation of the outer membrane of a variety of different spirochaetes. The advantage of Triton X-114 partitioning is the ease with which it can be performed. However, this approach suffers from a serious limitation in terms of specificity. Cytoplasmic membrane lipoproteins may be accessible to Triton X-114 solubilization even though the peptidoglycan cell wall and cytoplasmic membrane appear to be intact following extraction. For example, much of the prominent T. pallidum lipoprotein TpN47 is solubilized by Triton X-114 (Radolf et al., 1988). However, TpN47 was not among the protein components of the T. pallidum outer membrane obtained using more accurate and laborious methods of isolating T. pallidum outer-membrane vesicles by isopycnic ultracentrifugation (Blanco et al., 1994; Radolf et al., 1995a). T. pallidum outer-membrane vesicles isolated in this way were found to contain a number of minor proteins, including potential transmembrane proteins and the lipoproteins TpN17 and glycerophosphodiester phosphodiesterase (GlpQ) (Shevchenko et al., 1997).
Identification of transmembrane proteins in isolated outer-membrane vesicles is significant because such proteins are exposed on both faces of the outer membrane. Unfortunately, this is not true for lipoproteins which are potentially restricted to either the inner or outer leaflet of the outer membrane. For this reason, experimental approaches for assessment of surface exposure are essential in analysis of spirochaetal outer-membrane lipoproteins. Due to the lability of spirochaetal outer membranes, the best techniques for identifying surface-exposed antigens take measures to avoid disrupting the bacteria, utilize multiple approaches and include relevant controls. T. pallidum lipoproteins Tpp17 and GlpQ appear to be restricted to the periplasmic leaflet of the outer membrane (Cox et al., 1995; Shevchenko et al., 1999). Surface exposure was determined on the basis of accessibility to surface proteolysis and immunofluorescence of T. pallidum encapsulated in agarose beads using antiserum raised against recombinant proteins.
The initial description of Bor. burgdorferi outer-surface proteins utilized four independent approaches involving exposure of intact cells to antibodies and proteases (Barbour et al., 1984). A monoclonal antibody specific for OspB was found to agglutinate Bor. burgdorferi at a low concentration and was shown to bind to the Bor. burgdorferi surface in immuno-electron microscopy and radiolabelling experiments. Although most Bor. burgdorferi proteins were intact following treatment of intact cells with proteinase K, OspA and OspB were completely digested. The localization of these proteins to the cell surface is consistent with the sensitivity of Bor. burgdorferi to OspA- and OspB-specific antibodies. Even in the absence of complement, the growth of Bor. burgdorferi strain B31 is inhibited by certain OspA- and OspB-specific monoclonal antibodies at concentrations less than 1 μg ml−1 (Sadziene et al., 1993). This concentration is actually lower on a gram-for-gram basis than the minimal concentration of penicillin G (6.4 μg ml−1) required to kill Bor. burgdorferi (Johnson et al., 1987).
Persuasive as these studies are, research involving alternative approaches for examining the cellular location of antigens questioned the extent to which these outer-surface proteins were surface exposed. Immuno-electron microscopy using ultrathin-sectioned organisms found binding of OspA- and OspB-specific monoclonal antibodies not only to the outer leaflet of the outer membrane but also to the inner leaflet of the outer membrane, as well as to the outer leaflet of the cytoplasmic membrane (Brusca et al., 1991). An independent approach involving binding of OspA- and OspB-specific monoclonal antibodies to Bor. burgdorferi encapsulated in agarose beads found that immunofluorescence increased dramatically after the outer membrane was removed or disrupted by treatment with Triton X-100 (Cox et al., 1996). These studies suggest that the OspA and OspB are not exclusively on the cell surface, but rather that there is a distribution of the population of OspA and OspB molecules between surface and subsurface locations. Additional Bor. burgdorferi surface-exposed lipoproteins have been identified on the basis of accessibility to proteases and/or antibodies in intact cells, including OspC (Wilske et al., 1993), OspD (Norris et al., 1992), OspE and OspF (Lam et al., 1994), and decorin-binding protein (Guo et al., 1998).
A surface immunoprecipitation technique has been used to assess the exposure of antigens on the surface of the pathogen Leptospira kirschneri (Haake et al., 1991). Intact leptospires were incubated in the presence of antiserum to whole cells, followed by gentle washes to remove unbound antibodies. The surface antigen– antibody complexes were solubilized and isolated using protein A–Sepharose beads. Electrophoresis and immunoblotting of the surface-exposed antigens revealed that leptospiral LPS and proteins of 33 and 41 kDa were among the antigens isolated by this procedure. The 33 kDa protein was subsequently identified as a porin, OmpL1 (Haake et al., 1993; Shang et al., 1995), while the 41 kDa protein was found to be a lipoprotein, designated LipL41 (Shang et al., 1996). The surface-immunoprecipitation characteristics of OmpL1 and LipL41 were then compared to a second lipoprotein, LipL36. LipL36 was immunoprecipitable in a Triton X-100 extract of the outer membrane, but was inaccessible to antibody in the surface immunoprecipitation procedure, indicating that it is restricted to the periplasmic leaflet of the outer membrane (Shang et al., 1996). This technique is widely applicable to analysis of surface exposure but requires a protein to be immunogenic for it to be detected.
Evidence for involvement of lipoproteins in spirochaetal pathogenesis
While a number of subsurface lipoproteins are thought to have roles in cellular physiology (Table 2) (Fraser et al., 1997, 1998), the functions of the vast majority of spirochaetal lipoproteins remain unclear, and only a few have been implicated to participate directly in pathogenesis. Even though the crystal structure of OspA has been known for some time (Li et al., 1997), an understanding of its role in the biology of Bor. burgdorferi is only now beginning to emerge. Mutants deficient in OspA are more sensitive to complement and digestion of transmembrane OmpP66 by proteases (Bunikis & Barbour, 1999; Sadziene et al., 1995). These data suggest that OspA expression by Bor. burgdorferi during tick infection protects the organism both from tick midgut proteases and from mammalian complement when the tick takes a blood meal.
The ability of spirochaetes to attach to eukaryotic cell surfaces and extracellular matrix proteins is essential in pathogenesis. Decorin is a collagen-associated proteoglycan found in various tissues, including skin and joints, sites typically associated with Lyme disease. Bor. burgdorferi was shown to attach selectively to decorin, which led to the subsequent isolation of the decorin-binding protein (Dbp) genes by screening an expression library with digoxigenin-labelled decorin (Guo et al., 1995, 1998). The two-gene operon encoding the lipoprotein DbpA and the related lipoprotein DbpB were independently identified by screening a Bor. burgdorferi expression library with infection-derived serum (Feng et al., 1998; Skare et al., 1999) and by sequencing of DbpA found in Bor. burgdorferi outer membranes (Hagman et al., 1998).
A strong case has been made for the importance of DbpA in Bor. burgdorferi adhesion. DbpA was subject to surface proteolysis when intact cells were incubated in the presence of proteinase K (Guo et al., 1998; Hagman et al., 1998). Recombinant DbpA binds decorin and inhibits the adherence of Bor. burgdorferi to decorin (Guo et al., 1998). There is increased expression of DbpA at 37 °C relative to ambient temperature, consistent with the importance of decorin binding after Bor. burgdorferi is transmitted from the tick to the mammalian host (Skare et al., 1999). Because DbpA demonstrates considerable heterogeneity among Bor. burgdorferi strains, it was important to define the portion of the molecule involved in decorin binding. Chemical modification of lysine residues was found to abrogate DbpA binding to decorin. A comparison of 30 DbpA sequences was performed that identified 5–6 conserved lysine residues, three of which were shown to be essential for decorin binding by site-directed mutagenesis (Brown et al., 1999).
There is evidence implicating a 47 kDa Bor. burgdorferi lipoprotein (BBK32), designated P47, in fibronectin binding (Probert & Johnson, 1998). P47 was originally identified by probing blots of Bor. burgdorferi whole-cell lysates with alkaline phosphatase-labelled fibronectin. This general approach to identifying adhesins is potentially hazardous given the ‘stickiness’ of fibronectin. However, the possibility that P47 is involved in Bor. burgdorferi adhesion is supported by the demonstration that Bor. burgdorferi adhesion to fibronectin-coated cover slips is strongly inhibited by addition of a purified P47 fusion protein. Based upon sequence homology, the TIGR website designates BBK32 as a cell-envelope protein involved in ‘biosynthesis of surface polysaccharides and lipopolysaccharides’; an assignment which is difficult to reconcile with the lack of such molecules in Bor. burgdorferi.
Environmental adaptation by differential expression
Like DbpA (see previous section), many spirochaetal lipoproteins are differentially expressed, presumably for the purpose of adapting to different environmental conditions. Differential expression has been demonstrated for lipoproteins of Bor. burgdorferi, the relapsing fever borreliae and the pathogenic Leptospira species. These are organisms that exist in different environmental conditions during their life cycle. The life cycle of the borreliae consists of two phases, one phase inside a tick or louse and the other phase inside the mammalian host. Although leptospiral infection may occur through direct transmission from one animal to another, infection commonly occurs through exposure to water or soil contaminated with urine from a carrier host, requiring adaptation of Leptospira species to the environment outside the mammalian host. Although Treponema species do not exist outside the mammalian host, there may be differential expression of proteins depending on the location within the host, since treponemes do have genes involved in chemotaxis and sensory transduction (Fraser et al., 1998; Greene et al., 1997; Hagman et al., 1997). However, it is not known whether environmental responses include differential expression of treponemal lipoproteins.
Bor. burgdorferi undergoes a dramatic metamorphosis, including changes in the expression of many different lipoproteins, when it goes from the tick to the mammalian environment. Inside the midgut of unfed ticks, Bor. burgdorferi expresses the OspA lipoproteins in large amounts (Burkot et al., 1994). Elegant immunofluorescence studies show that after the tick attaches to a mammalian host and begins feeding, Bor. burgdorferi downregulates OspA expression and begins rapid synthesis of OspC (Schwan et al., 1995). At the same time, Bor. burgdorferi migrates from the tick midgut to the salivary glands in preparation for mammalian-host infection. Once inside the mammalian host, the transition from OspA to OspC expression is complete: Bor. burgdorferi no longer expresses OspA, while OspC is readily detectable in mammalian-host-adapted organisms (Montgomery et al., 1996). Temperature is at least one cue that Bor. burgdorferi uses to undergo this transition, since it was found that changes in lipoprotein expression could be examined by comparing Bor. burgdorferi grown in culture at 23–24 °C with those grown at 35 °C (Schwan et al., 1995). Effects of temperature on differential expression of OspA and OspC are enhanced by cocultivation of Bor. burgdorferi with tick cells (Obonyo et al., 1999).
In addition to OspC, a large number of other lipoproteins are upregulated during infection of the mammalian host. Examination of Bor. burgdorferi grown at low and high temperatures by immunoblotting using sera from infected mice, it was found that the expression of two other outer-surface proteins, OspE and OspF, also appeared to be expressed only during mammalian infection (Stevenson et al., 1995). It has become evident that many proteins with upregulated expression during mammalian infection are members of a large family of OspEF-related proteins, designated Erps (Stevenson et al., 1996). Further studies have shown that expression of the Erp proteins could be induced in cultivated Bor. burgdorferi strain B31 by a shift in temperature from 23 to 35 °C (Stevenson et al., 1998a).
Incubation of spirochaetes at different temperatures is one method of replicating environmental signals that regulate lipoprotein expression. Other environmental stimuli that appear to affect spirochaetal lipoprotein expression include cell density (Haake et al., 1998; Indest et al., 1997) and pH (Carroll et al., 1999). The existence of other, as yet unidentified, environmental signals is suggested by effects of growth in peritoneal dialysis bags on expression of various lipoproteins, including Erps (Akins et al., 1998).
Molecular approaches for identifying environmentally regulated genes, such as in vivo expression technology, are not available in spirochaetes. However, a number of studies have utilized the immune response as a tool to study differential regulation by screening expression libraries with sera from infected animals (Fikrig et al., 1997; Skare et al., 1999; Suk et al., 1995; Wallich et al., 1995). For obvious reasons, these experiments rely heavily on the quality of the infection-derived sera used to identify recombinant antigens. An effective approach has been to use sera from rabbits that had recovered from infection with low-passage Bor. burgdorferi strain B31 and had become immune to reinfection (Foley et al., 1997). The expression library was then screened with this immune rabbit serum after absorption with culture-attenuated, noninfectious Bor. burgdorferi strain B31. A number of proteins expressed during mammalian infection were identified in this way including a number of novel and previously characterized proteins, including Rev, DbpA and VraA (Skare et al., 1999).
Changes in lipoprotein expression have also been found to occur in Leptospira species. Immunohistochemistry was used to evaluate whether lipoproteins expressed by cultivated Leptospira species were also expressed during infection. These experiments took advantage of the fact that in kidneys of infected animals, pathogenic Leptospira species are found in high numbers within proximal renal tubules. Experiments revealed expression of a number of membrane antigens, including leptospiral LPS, the OmpL1 porin and the lipoproteins LipL32 and LipL41 (Barnett et al., 1999; Haake et al., 2000). However, a third lipoprotein, LipL36, which is expressed at high levels in cultivated organisms, was undetectable by immunohistochemistry (Barnett et al., 1999). These observations were validated by examination of the antibody response in animals experimentally infected with host-derived organisms (Barnett et al., 1999). Research is ongoing in our laboratory to determine the signals that regulate differential expression of LipL36.
Antigenic variation and evasion of the mammalian immune response
The Borrelia species that cause relapsing fever produce a major surface lipoprotein, which in Bor. hermsii has been shown to undergo antigenic variation with each successive febrile episode. These serotype-determining lipoproteins are referred to collectively as variable major proteins (Vmps), but are classified by size and sequence homology differences into two groups: variable large proteins (Vlps) and variable small proteins (Vsps) (Hinnebusch et al., 1998). Interestingly, the Vsps share a high degree of amino acid and DNA sequence identity with OspC of Bor. burgdorferi (Carter et al., 1994). A single Bor. hermsii isolate may have more than 40 different complete or partial vsp and vlp genes (Hinnebusch et al., 1998). Antigenic variation occurs when the gene in the 28 kb linear-plasmid expression locus is replaced by a copy of one of the silent vsp or vlp genes. Recently it was discovered that as Bor. hermsii cycles between ticks and mice, the Vmp expressed on its surface alternates between tick-associated (Vsp33) and mammal-associated (e.g. Vlp7 or Vsp8) Vmps (Schwan & Hinnebusch, 1998). Temperature appears to be a primary signal for which type of Vmp is expressed; Bor. hermsii grown at 23 °C produce the tick-associated Vmp (Vsp33) while those grown at 37 °C produce a mammal-associated Vmp (e.g. Vsp8). This is consistent with the idea that the mammal-associated vmp genes must be inserted into the 28 kb linear plasmid expression locus for expression to occur, while the tick-associated vsp33 gene is expressed by its own promoter on a 53 kb linear plasmid (Carter et al., 1994). These findings indicate that Vsp33 serves a special, though yet not understood, role for Bor. hermsii during tick infection.
The diversity of vmp variants allows Bor. hermsii to evade the host immune response. Expression of a new Vmp allows re-emergence in the bloodstream, which facilitates transmission to the insect vector. Studies involving Borrelia turicatae suggest that Vmp expression may also determine the sites of infection and manifestations of disease. Bor. turicatae causes a form of relapsing fever in man and experimental animals that is similar to Lyme disease, including arthritis and neurological disease. Bor. turicatae serotype A causes central nervous system infection, while infection with serotype B is associated with arthritis and higher levels of organisms in blood and joint tissue (Cadavid et al., 1994). The only apparent difference between serotypes A and B is the Vmp that they express on their surface. Both VspA (expressed by serotype A) and VspB (expressed by serotype B) belong to the Vsp–OspC family of lipoproteins. VspA is a relatively hydrophobic molecule, while VspB has an unusually basic pI (Pennington et al., 1999). It is not known whether the link between Vmp type and the pattern of disease is causal or an association with more direct determinants of pathogenesis.
Antigenic variation has also been found in Bor. burgdorferi in a manner similar to that in the relapsing fever borreliae. The vmp-like sequence (vls) locus was found using a subtractive hybridization technique designed to identify genetic elements lost during culture attenuation of Bor. burgdorferi virulence (Zhang et al., 1997). The vls expression site (vlsE) is a lipoprotein gene with a cassette region bounded by 17 bp direct repeats. Antigenic recombination occurs by promiscuous recombination between the cassette region of vlsE and segments of the silent vls cassettes located on the same 28 kb linear plasmid (Zhang & Norris, 1998a). Antigenic recombination was found to occur at a faster rate in immunologically intact mice than in mice with severe combined immunodeficiency, indicating the importance of the mammalian immune response in production of antigenic variants (Zhang & Norris, 1998b).
During chronic mammalian infections, intergenic recombination among other Bor. burgdorferi lipoproteins, such as the Erp lipoproteins (El Hage et al., 1999), OspA, OspC or OspD (Persing et al., 1994; Stevenson et al., 1994), is not detectable. The multiplicity of highly related Erp proteins, and the sequence variations in OspA, OspC and OspD homologues among different Bor. burgdorferi isolates are presumably due to other mechanisms of genetic mutability.
Stimulation of inflammation and the innate immune response
Many of the complications of spirochaetal diseases are due to the host inflammatory response. In Lyme disease, the mammalian host responds to the presence of Bor. burgdorferi in skin and articular tissues by an influx of inflammatory cells. Tissue inflammation is also a hallmark of other spirochaetal diseases, including dermatitis and vasculitis in syphilis, interstitial nephritis in leptospirosis and periodontititis in oral treponeme infections. Although LPS is likely to play a role in inflammation in disease caused by oral treponemes, Brachyspira and Leptospira species, this is not the case for T. pallidum and Bor. burgdorferi which lack LPS. Spirochaetal lipoproteins have been found to be potent stimulators of inflammation. Treponemal and borrelial lipoproteins are capable of activating a variety of cell types in vitro, including monocytes, macrophages, lymphocytes and endothelial cells (Radolf et al., 1995b; Sellati et al., 1996; Vincent et al., 1998). The stimulatory effects are dependent on lipidation and can be reproduced using synthetic acylated hexapeptides (Radolf et al., 1995b; Sellati et al., 1996; Vincent et al., 1998). The inflammatory effects of lipoproteins can also be reproduced in animal models of disease. For example, dermal inflammation including monocytic cellular infiltrates can be elicited by intradermal injection of acylated hexapeptides that are synthetic analogues of borrelial and treponemal lipoproteins (Norgard et al., 1995).
It has recently come to light that the innate immune response is responsible for cellular activation by borrelial and treponemal lipoproteins. The innate immune response provides a rapid mechanism for recognizing and responding to microbial pathogens (Ulevitch, 1999). In the case of Gram-negative pathogens, a serum LPS-binding protein (LBP) presents LPS to CD14. Activation of myeloid (monocyte/macrophage/polymorphonuclear leukocyte) cells occurs through presentation of LPS to CD14 on the cell membrane. A soluble form of CD14 is involved in activating non-myeloid (endothelial/epithelial) cells. After binding LPS, CD14 interacts with transmembrane Toll-like receptors (primarily TLR4 in the case of Gram-negative LPS) that transmit the signal across the cell membrane, resulting in induction of NF-κB nuclear translocation.
Induction of the NF-κB pathway by spirochaetal lipoproteins primarily involves CD14, and lipoproteins bind to CD14 at the LPS-binding site (Giambartolomei et al., 1999; Sellati et al., 1998; Wooten et al., 1998). However, there are important differences between Gram-negative LPS- and spirochaetal lipoprotein-mediated cellular activation. First, spirochaetal lipoproteins are able to interact with CD14 without LBP (Sellati et al., 1998). Another important difference is that signalling of cellular activation by spirochaetal lipoproteins occurs via TLR2 instead of TLR4 (Aliprantis et al., 1999; Hirschfeld et al., 1999). The sensitivity of the TLR2 pathway to spirochaetal lipoproteins is demonstrated by the finding that TLR2-transfected cells had ten-fold greater sensitivity to spirochaetal lipoproteins than Gram-negative LPS (Hirschfeld et al., 1999). Cellular activation by the TLR2 pathway may explain some of the clinical differences between Gram-negative sepsis and the spirochaetemia that occurs in Lyme disease and syphilis. Another relevant question is why some animals, such as the white-footed mouse, Peromyscus leucopus, are able to tolerate persistent infection by Bor. burgdorferi, allowing them to serve as a reservoir for tick transmission (Barthold et al., 1990). A potential reason for these phenotypic differences in infection and disease susceptibility among mammalian hosts could involve polymorphisms in genes that regulate the intensity of the host inflammatory response, including TLR2-mediated innate immunity (Weis et al., 1999).
Spirochaetal lipoproteins as protective immunogens
Ultimately, the relevance of spirochaetal lipoprotein research will be measured by whether it enables us to change the paradigms of how diseases caused by these pathogens are managed or prevented. One of the most direct ways in which this can happen is through vaccine development. Subunit vaccines consisting of recombinant spirochaetal lipoproteins is an area of intense investigation. Rather than to provide a comprehensive review on spirochaetal vaccines, it is the intent of this section to review the role of lipoproteins in immunity.
Much of the information available on the subject of lipoproteins and immunity involves Bor. burgdorferi. The potential of surface-exposed proteins as protective immunogens led to work on Lyme disease vaccine studies shortly after the genes encoding Osps were cloned into E. coli expression plasmids. Early studies showed that a number of recombinant lipoproteins were protective immunogens in animal models of Lyme disease, including OspA, OspB, OspC and OspF (Fikrig et al., 1990; Nguyen et al., 1994; Probert & LeFebvre, 1994). However, the heterogeneity of these proteins among Bor. burgdorferi isolates led to problems with cross-protection, which limits their usefulness as vaccines. OspA has received the most interest in vaccine studies because it showed sufficient antigenic conservation among North American isolates to be effective in commercial vaccines.
While there is clearly a need for development of new vaccine strategies for prevention of Lyme disease, the experience gained from work on OspA is relevant to an understanding of lipoproteins as immunoprotective molecules. An important feature of immunity based on OspA is lipidation of the recombinant antigen (Erdile et al., 1993). Nonlipidated OspA requires addition of adjuvant to elicit full protection, indicating that lipidation functions as a kind of internal adjuvant (Erdile et al., 1993). Although lipidation is important in generating a protective immune response, the lipidated N-terminal cysteine is not the target of protective antibodies. An important protective epitope has been mapped to the C terminus of OspA (Sears et al., 1991). This region is structurally distinct from the rest of OspA. Crystallographic studies have shown that most of OspA is a repetitive antiparallel beta sheet with an exposed C-terminal alpha helix (Li et al., 1997). Monoclonal antibody LA-2, which is directed at this region of OspA, has been found to be useful in quantitating bactericidal antibody titres (Johnson et al., 1995).
The mechanism of action of OspA-induced immunity involves blocking transmission of Bor. burgdorferi from the tick to the mammalian host. Bor. burgdorferi in the tick midgut is susceptible to the effects of OspA antibodies in the bloodstream of the mammalian host. When an infected tick takes a blood meal from an immunized animal, OspA antibodies appear to inhibit Bor. burgdorferi growth in the midgut, prevent dissemination to the salivary gland and prevent transition to the OspC expression phenotype (de Silva et al., 1999). Each of these effects serve to reduce the likelihood of transmission. If the antibody titre is high enough, OspA antibodies in combination with complement are also able to kill Bor. burgdorferi in the tick midgut (Nowling & Philipp, 1999).
Decorin-binding protein A (DbpA) is another potential Bor. burgdorferi lipoprotein vaccine candidate. Immunization of mice with recombinant DbpA protects against homologous, and in some cases, heterologous challenge (Hanson et al., 1998). Because DbpA is expressed during mammalian infection and is involved in the interaction of Bor. burgdorferi with host tissues, the mechanism of action of DbpA immunization is different to OspA immunization. OspA immunization protects by prevention of transmission, while DbpA immunization acts by aborting infection after transmission. Passive immunization was able to prevent infection as long as 4 d after challenge (Hanson et al., 1998). DbpA antibodies appear to act by inhibiting Bor. burgdorferi growth and blocking binding to decorin, a collagen-associated proteoglycan. The biological significance of this effect is demonstrated by the finding that when DbpA immunization resulted in partial protection, cultures were usually positive only in the bladder, and not in the ear or joints; tissues rich in collagen (Hanson et al., 1998). The usefulness of DbpA as a vaccinogen will depend on finding conserved protective epitopes since there is a fairly high degree of sequence heterogeneity among Bor. burgdorferi strains (Roberts et al., 1998).
Given their prevalence in spirochaetal membranes, lipoproteins are also being considered as immunoprotective antigens in spirochaetes other than Bor. burgdorferi. The rabbit is the best characterized animal model of syphilis. Intradermal inoculation of T. pallidum into the backs of naïve rabbits results in ulcerative lesions that mimic human syphilitic chancres. Recently, there has been interest in whether T. pallidum glycerophosphodiester phosphodiesterase is an immunoprotective antigen. In one study, rabbits immunized with recombinant, enzymically active glycerophosphodiester phosphodiesterase had accelerated formation of atypical, nonulcerative, darkfield-negative lesions after intradermal challenge with T. pallidum (Cameron et al., 1998). However, all immunized rabbits were infected and a second study found no alterations in lesion formation after challenge, suggesting that differences in recombinant protein, adjuvant, and/or immunization protocol accounted for the observed differences (Shevchenko et al., 1999). It is unclear whether the atypical lesions observed in the first study represented partial immunity or an accelerated immune response consistent with what has been observed in earlier studies involving subsurface antigens (Champion et al., 1990).
LipL41 is a surface-exposed lipoprotein of pathogenic Leptospira species, which has been shown to play a synergistic role in immunity (Haake et al., 1999). Although hamsters immunized with lipidated LipL41 alone did not have improved survival over control immunized hamsters challenged with virulent leptospires (23% and 20% survival, respectively), hamsters immunized with both the porin OmpL1 and lipidated LipL41 had significantly better survival than hamsters immunized with OmpL1 alone (71% and 42% survival, respectively; P < 0.05). The finding that immunization with a combination of transmembrane porin (OmpL1) and a lipoprotein provides synergistic protection suggests that the lipoprotein is not merely an additional surface target. One possible explanation for why immunoprotection is amplified by combining two different types of antigens is that surface lipoproteins ‘shield’ porins. If this were the case, antibody binding to LipL41 could inactivate its shielding effect, facilitating antibody binding to OmpL1. The shielding concept is supported by recent studies with Bor. burgdorferi indicating that Osp lipoproteins interfere with the access of antibody and trypsin to the integral outer-membrane protein P66 (Bunikis & Barbour, 1999). These studies indicate the importance of considering immunoprotection strategies which combine different classes of membrane proteins.
Conclusion
The T. pallidum and Bor. burgdorferi genome sequences have given us a greater appreciation for the importance of spirochaetal lipoproteins (Fraser et al., 1997, 1998). As ongoing genome-sequencing projects involving Treponema denticola and Leptospira interrogans (The Institute for Genomic Research, 2000) continue to expand the number of genes known to encode potential lipoproteins, there will be an increased need to develop effective strategies for genetic manipulation of spirochaetes. Promising techniques are already available for T. denticola (Li et al., 1996), Bor. burgdorferi (Stevenson et al., 1998b) and Bra. hyodysenteriae (Rosey et al., 1995). Genes of spirochaetes which can not be cultivated can potentially be expressed and studied in a related spirochaetal host (Chi et al., 1999). Introduction of heterologous DNA, selective gene inactivation and site-specific mutagenesis will be extremely powerful tools for elucidating lipoprotein export and function. It will also be necessary to be able to complement inactivated genes as a control for the inactivation process. These new approaches promise to enhance existing methods for studying lipoproteins and in so doing will greatly expand our understanding of the roles lipoproteins play in the biology of spirochaetes.
Acknowledgments
This work was supported in part by funding from a Public Health Service Grant AI-34431 (to D.A.H.). I thank Susan K. Haake, James N. Miller, Isabelle Saint Girons and Jon T. Skare for their helpful suggestions and critical review of the manuscript.
References
- Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Schneewind O. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol Microbiol. 1999;31:1139–1148. doi: 10.1046/j.1365-2958.1999.01254.x. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Tessier SL, Hayes SF. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984;45:94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JK, Barnett D, Bolin CA, Summers TA, Wagar EA, Cheville NF, Hartskeerl RA, Haake DA. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect Immun. 1999;67:853–861. doi: 10.1128/iai.67.2.853-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- Belisle JT, Brandt ME, Radolf JD, Norgard MV. Fatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteins. J Bacteriol. 1994;176:2151–2157. doi: 10.1128/jb.176.8.2151-2157.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco DR, Reimann K, Skare J, Champion CI, Foley D, Exner MM, Hancock RE, Miller JN, Lovett MA. Isolation of the outer membranes from Treponema pallidum and Treponema vincentii. J Bacteriol. 1994;176:6088–6099. doi: 10.1128/jb.176.19.6088-6099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt ME, Riley BS, Radolf JD, Norgard MV. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EL, Guo BP, O’Neal P, Hook M. Adherence of Borrelia burgdorferi: identification of critical lysine residues in DbpA required for decorin binding. J Biol Chem. 1999;274:26272–26278. doi: 10.1074/jbc.274.37.26272. [DOI] [PubMed] [Google Scholar]
- Brusca JS, McDowall AW, Norgard MV, Radolf JD. Localization of outer surface proteins A and B in both the outer membrane and intracellular compartments of Borrelia burgdorferi. J Bacteriol. 1991;173:8004–8008. doi: 10.1128/jb.173.24.8004-8008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J, Barbour AG. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkot TR, Piesman J, Wirtz RA. Quantitation of the Borrelia burgdorferi outer surface protein A in Ixodes scapularis: fluctuations during the tick life cycle, doubling times, and loss while feeding. J Infect Dis. 1994;170:883–889. doi: 10.1093/infdis/170.4.883. [DOI] [PubMed] [Google Scholar]
- Cadavid D, Thomas DD, Crawley R, Barbour AG. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med. 1994;179:631–642. doi: 10.1084/jem.179.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CE, Castro C, Lukehart SA, Van Voorhis WC. Function and protective capacity of Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase. Infect Immun. 1998;66:5763–5770. doi: 10.1128/iai.66.12.5763-5770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JA, Garon CF, Schwan TG. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CJ, Bergstrom S, Norris SJ, Barbour AG. A family of surface-exposed proteins of 20 kilodaltons in the genus Borrelia. Infect Immun. 1994;62:2792–2799. doi: 10.1128/iai.62.7.2792-2799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain NR, Brandt ME, Erwin AL, Radolf JD, Norgard MV. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989a;57:2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain NR, DeOgny L, Slaughter C, Radolf JD, Norgard MV. Acylation of the 47-kilodalton major membrane immunogen of Treponema pallidum determines its hydrophobicity. Infect Immun. 1989b;57:2878–2885. doi: 10.1128/iai.57.9.2878-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion CI, Miller JN, Borenstein LA, Lovett MA, Blanco DR. Immunization with Treponema pallidum endoflagella alters the course of experimental rabbit syphilis. Infect Immun. 1990;58:3158–3161. doi: 10.1128/iai.58.9.3158-3161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi B, Chauhan S, Kuramitsu H. Development of a system for expressing heterologous genes in the oral spirochete Treponema denticola and its use in expression of the Treponema pallidum flaA gene. Infect Immun. 1999;67:3653–3656. doi: 10.1128/iai.67.7.3653-3656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DL, Chang P, McDowall AW, Radolf JD. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect Immun. 1992;60:1076–1083. doi: 10.1128/iai.60.3.1076-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DL, Akins DR, Porcella SF, Norgard MV, Radolf JD. Treponema pallidum in gel microdroplets: a novel strategy for investigation of treponemal molecular architecture. Mol Microbiol. 1995;15:1151–1164. doi: 10.1111/j.1365-2958.1995.tb02288.x. [DOI] [PubMed] [Google Scholar]
- Cox DL, Akins DR, Bourell KW, Lahdenne P, Norgard MV, Radolf JD. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA. 1996;93:7973–7978. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong F, Eichler J, Price A, Leonard MR, Wickner W. Biogenesis of the gram-negative bacterial envelope. Cell. 1997;91:567–573. doi: 10.1016/s0092-8674(00)80444-4. [DOI] [PubMed] [Google Scholar]
- El Hage N, Lieto LD, Stevenson B. Stability of erp loci during Borrelia burgdorferi infection: recombination is not required for chronic infection of immunocompetent mice. Infect Immun. 1999;67:3146–3150. doi: 10.1128/iai.67.6.3146-3150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdile LF, Brandt MA, Warakomski DJ, Westrack GJ, Sadziene A, Barbour AG, Mays JP. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993;61:81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Hodzic E, Stevenson B, Barthold SW. Humoral immunity to Borrelia burgdorferi N40 decorin-binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Sun W, Feng W, Telford SR, III, Flavell RA. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- Foley DM, Wang YP, Wu XY, Blanco DR, Lovett MA, Miller JN. Acquired resistance to Borrelia burgdorferi infection in the rabbit: comparison between outer surface protein A vaccine- and infection-derived immunity. J Clin Invest. 1997;99:2030–2035. doi: 10.1172/JCI119371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Fraser CM, Norris SJ, Weinstock GM, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- Giambartolomei GH, Dennis VA, Lasater BL, Philipp MT. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SR, Stamm LV, Hardham JM, Young NR, Frye JG. Identification, sequences, and expression of Treponema pallidum chemotaxis genes. DNA Seq. 1997;7:267–284. doi: 10.3109/10425179709034046. [DOI] [PubMed] [Google Scholar]
- Guo BP, Norris SJ, Rosenberg LC, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BP, Brown EL, Dorward DW, Rosenberg LC, Hook M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- Haake DA, Walker EM, Blanco DR, Bolin CA, Miller MN, Lovett MA. Changes in the surface of Leptospira interrogans serovar grippotyphosa during in vitro cultivation. Infect Immun. 1991;59:1131–1140. doi: 10.1128/iai.59.3.1131-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake DA, Champion CI, Martinich C, Shang ES, Blanco DR, Miller JN, Lovett MA. Molecular cloning and sequence analysis of the gene encoding OmpL1, a transmembrane outer membrane protein of pathogenic Leptospira spp. J Bacteriol. 1993;175:4225–4234. doi: 10.1128/jb.175.13.4225-4234.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake DA, Martinich C, Summers TA, Shang ES, Pruetz JD, McCoy AM, Mazel MK, Bolin CA. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late log-phase growth and mammalian infection. Infect Immun. 1998;66:1579–1587. doi: 10.1128/iai.66.4.1579-1587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake DA, Mazel MK, McCoy AM, Milward F, Chao G, Matsunaga J, Wagar EA. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect Immun. 1999;67:6572–6582. doi: 10.1128/iai.67.12.6572-6582.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M, Matsunaga J, Levett PN, Bolin CA. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect Immun. 2000;68:2276–2285. doi: 10.1128/iai.68.4.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman KE, Porcella SF, Popova TG, Norgard MV. Evidence for a methyl-accepting chemotaxis protein gene (mcp1) that encodes a putative sensory transducer in virulent Treponema pallidum. Infect Immun. 1997;65:1701–1709. doi: 10.1128/iai.65.5.1701-1709.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman KE, Lahdenne P, Popova TG, Porcella SF, Akins DR, Radolf JD, Norgard MV. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect Immun. 1998;66:2674–2683. doi: 10.1128/iai.66.6.2674-2683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MS, Cassatt DR, Guo BP, Patel NK, McCarthy MP, Dorward DW, Hook M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch BJ, Barbour AG, Restrepo BI, Schwan TG. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect Immun. 1998;66:432–440. doi: 10.1128/iai.66.2.432-440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- Indest KJ, Ramamoorthy R, Sole M, Gilmore RD, Johnson BJ, Philipp MT. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RC, Kodner C, Russell M. In vitro and in vivo susceptibility of the Lyme disease spirochete, Borrelia burgdorferi, to four antimicrobial agents. Antimicrob Agents Chemother. 1987;31:164–167. doi: 10.1128/aac.31.2.164. (erratum 34, 1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJ, Sviat SL, Happ CM, Dunn JJ, Frantz JC, Mayer LW, Piesman J. Incomplete protection of hamsters vaccinated with unlipidated OspA from Borrelia burgdorferi infection is associated with low levels of antibody to an epitope defined by mAb LA-2. Vaccine. 1995;13:1086–1094. doi: 10.1016/0264-410x(95)00035-y. [DOI] [PubMed] [Google Scholar]
- Lam TT, Nguyen TP, Montgomery RR, Kantor FS, Fikrig E, Flavell RA. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ruby J, Charon N, Kuramitsu H. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol. 1996;178:3664–3667. doi: 10.1128/jb.178.12.3664-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Dunn JJ, Luft BJ, Lawson CL. Crystal structure of Lyme disease antigen outer surface protein A complexed with an FAb. Proc Natl Acad Sci USA. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Yokota N, Tokuda H. A novel outer membrane lipoprotein, LolB (He mM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J. 1997;16:6947–6955. doi: 10.1093/emboj/16.23.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RR, Malawista SE, Feen KJ, Bockenstedt LK. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TP, Lam TT, Barthold SW, Telford SR, III, Flavell RA, Fikrig E. Partial destruction of Borrelia burgdorferi within ticks that engorged on OspE- or OspF-immunized mice. Infect Immun. 1994;62:2079–2084. doi: 10.1128/iai.62.5.2079-2084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard MV, Riley BS, Richardson JA, Radolf JD. Dermal inflammation elicited by synthetic analogs of Treponema pallidum and Borrelia burgdorferi lipoproteins. Infect Immun. 1995;63:1507–1515. doi: 10.1128/iai.63.4.1507-1515.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SJ. Polypeptides of Treponema pallidum: progress toward understanding their structural, functional, and immunologic roles. Microbiol Rev. 1993;57:750–779. doi: 10.1128/mr.57.3.750-779.1993. (erratum 58, 291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SJ, Carter CJ, Howell JK, Barbour AG. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowling JM, Philipp MT. Killing of Borrelia burgdorferi by antibody elicited by OspA vaccine is inefficient in the absence of complement. Infect Immun. 1999;67:443–445. doi: 10.1128/iai.67.1.443-445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonyo M, Munderloh UG, Fingerle V, Wilske B, Kurtti TJ. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J Clin Microbiol. 1999;37:2137–2141. doi: 10.1128/jcm.37.7.2137-2141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington PM, Cadavid D, Barbour AG. Characterization of VspB of Borrelia turicatae, a major outer membrane protein expressed in blood and tissues of mice. Infect Immun. 1999;67:4637–4645. doi: 10.1128/iai.67.9.4637-4645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing DH, Mathiesen D, Podzorski D, Barthold SW. Genetic stability of Borrelia burgdorferi recovered from chronically infected immunocompetent mice. Infect Immun. 1994;62:3521–3527. doi: 10.1128/iai.62.8.3521-3527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- Probert WS, LeFebvre RB. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell BK, Swancutt MA, Radolf JD. Lipid modification of the 15 kilodalton major membrane immunogen of Treponema pallidum. Mol Microbiol. 1990;4:1371–1379. doi: 10.1111/j.1365-2958.1990.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Chamberlain NR, Clausell A, Norgard MV. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988;56:490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Norgard MV, Schulz WW. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci USA. 1989;86:2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Robinson EJ, Bourell KW, Akins DR, Porcella SF, Weigel LM, Jones JD, Norgard MV. Characterization of outer membranes isolated from Treponema pallidum, the syphilis spirochete. Infect Immun. 1995a;63:4244–4252. doi: 10.1128/iai.63.11.4244-4252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Arndt LL, Akins DR, Curetty LL, Levi ME, Shen Y, Davis LS, Norgard MV. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995b;154:2866–2877. [PubMed] [Google Scholar]
- Roberts WC, Mullikin BA, Lathigra R, Hanson MS. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect Immun. 1998;66:5275–5285. doi: 10.1128/iai.66.11.5275-5285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosey EL, Kennedy MJ, Petrella DK, Ulrich RG, Yancey RJ., Jr Inactivation of Serpulina hyodysenteriae flaA1 and flaB1 periplasmic flagellar genes by electroporation-mediated allelic exchange. J Bacteriol. 1995;177:5959–5970. doi: 10.1128/jb.177.20.5959-5970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Thompson PA, Barbour AG. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993;167:165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- Sadziene A, Thomas DD, Barbour AG. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect Immun. 1995;63:1573–1580. doi: 10.1128/iai.63.4.1573-1580.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Hinnebusch BJ. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears JE, Fikrig E, Nakagawa TY, Deponte K, Marcantonio N, Kantor FS, Flavell RA. Molecular mapping of Osp-A mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 1991;147:1995–2000. [PubMed] [Google Scholar]
- Sellati TJ, Abrescia LD, Radolf JD, Furie MB. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect Immun. 1996;64:3180–3187. doi: 10.1128/iai.64.8.3180-3187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellati TJ, Bouis DA, Kitchens RL, et al. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–5464. [PubMed] [Google Scholar]
- Shang ES, Exner MM, Summers TA, Martinich C, Champion CI, Hancock REW, Haake DA. The rare outer membrane protein, OmpL1, of pathogenic Leptospira species is a heat-modifiable porin. Infect Immun. 1995;63:3174–3181. doi: 10.1128/iai.63.8.3174-3181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang ES, Summers TA, Haake DA. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun. 1996;64:2322–2330. doi: 10.1128/iai.64.6.2322-2330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko DV, Akins DR, Robinson EJ, Li M, Shevchenko OV, Radolf JD. Identification of homologs for thioredoxin, peptidyl prolyl cis-trans isomerase, and glycerophosphodiester phosphodiesterase in outer membrane fractions from Treponema pallidum, the syphilis spirochete. Infect Immun. 1997;65:4179–4189. doi: 10.1128/iai.65.10.4179-4189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko DV, Sellati TJ, Cox DL, Shevchenko OV, Robinson EJ, Radolf JD. Membrane topology and cellular location of the Treponema pallidum glycerophosphodiester phosphodiesterase (GlpQ) ortholog. Infect Immun. 1999;67:2266–2276. doi: 10.1128/iai.67.5.2266-2276.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Zeidner NS, Zhang Y, Dolan MC, Piesman J, Fikrig E. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect Immun. 1999;67:30–35. doi: 10.1128/iai.67.1.30-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare JT, Foley DM, Hernandez SR, Moore DC, Blanco DR, Miller JN, Lovett MA. Cloning and molecular characterization of plasmid-encoded antigens of Borrelia burgdorferi. Infect Immun. 1999;67:4407–4417. doi: 10.1128/iai.67.9.4407-4417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Bockenstedt LK, Barthold SW. Expression and gene sequence of outer surface protein C of Borrelia burgdorferi reisolated from chronically infected mice. Infect Immun. 1994;62:3568–3571. doi: 10.1128/iai.62.8.3568-3571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Schwan TG, Rosa PA. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Tilly K, Rosa PA. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol. 1996;178:3508–3516. doi: 10.1128/jb.178.12.3508-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Bono JL, Schwan TG, Rosa P. Borrelia burgdorferi erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998a;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Bono JL, Elias A, Tilly K, Rosa P. Transformation of the Lyme disease spirochete Borrelia burgdorferi with heterologous DNA. J Bacteriol. 1998b;180:4850–4855. doi: 10.1128/jb.180.18.4850-4855.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk K, Das S, Sun W, Jwang B, Barthold SW, Flavell RA, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe IC, Russell RR. Lipoproteins of gram-positive bacteria. J Bacteriol. 1995;177:1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt MA, Radolf JD, Norgard MV. The 34-kilodalton membrane immunogen of Treponema pallidum is a lipoprotein. Infect Immun. 1990;58:384–392. doi: 10.1128/iai.58.2.384-392.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W, Sellwood R. Molecular cloning, expression, and DNA sequence analysis of the gene that encodes the 16-kilodalton outer membrane lipoprotein of Serpulina hyodysenteriae. Infect Immun. 1993;61:1136–1140. doi: 10.1128/iai.61.3.1136-1140.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Institute for Genomic Research. Trends Genet. Microbial Database: A listing of microbial genomes and chromosomes completed and in progress. 2000. http://www.tigr.org/tdb/mdb/mdb.html.
- Ulevitch RJ. Endotoxin opens the Tollgates to innate immunity. Nature Med. 1999;5:144–145. doi: 10.1038/5504. [DOI] [PubMed] [Google Scholar]
- Vincent MS, Roessner K, Sellati T, Huston CD, Sigal LH, Behar SM, Radolf JD, Budd RC. Lyme arthritis synovial gamma delta T cells respond to Borrelia burgdorferi lipoproteins and lipidated hexapeptides. J Immunol. 1998;161:5762–5771. [PubMed] [Google Scholar]
- Walker EM, Zampighi GA, Blanco DR, Miller JN, Lovett MA. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J Bacteriol. 1989;171:5005–5011. doi: 10.1128/jb.171.9.5005-5011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EM, Borenstein LA, Blanco DR, Miller JN, Lovett MA. Analysis of outer membrane ultrastructure of pathogenic Treponema and Borrelia species by freeze-fracture electron microscopy. J Bacteriol. 1991;173:5585–5588. doi: 10.1128/jb.173.17.5585-5588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R, Brenner C, Kramer MD, Simon MM. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis JJ, McCracken BA, Ma Y, et al. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J Immunol. 1999;162:948–956. [PubMed] [Google Scholar]
- Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten RM, Morrison TB, Weis JH, Wright SD, Thieringer R, Weis JJ. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J Immunol. 1998;160:5485–5492. [PubMed] [Google Scholar]
- Wu HC. Biosynthesis of lipoproteins. In: Neidhardt FC, et al., editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. Vol. 2. Washington, DC: American Society for Microbiology; 1996. pp. 1005–1014. [Google Scholar]
- Yamaguchi K, Yu F, Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988;53:423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Zhang JR, Norris SJ. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect Immun. 1998a;66:3698–3704. doi: 10.1128/iai.66.8.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JR, Norris SJ. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun. 1998b;66:3689–3697. doi: 10.1128/iai.66.8.3689-3697.1998. (erratum 67, 468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]