Abstract
The contracted tissues from clubfeet resemble tissues from other fibroproliferative disorders such as palmar fibromatosis. Beta-catenin-mediated signaling is a crucial pathway controlling the fibroproliferative response in many fibroproliferative disorders. To determine if beta-catenin signaling plays a role in clubfoot, contracted and less contracted tissues from clubfeet were studied using Western analysis to determine the protein level of beta-catenin. Primary cell cultures were established from these tissues, and they were treated with either lithium to increase beta-catenin or Dickkopf-1 to inhibit beta-catenin. RNA was extracted from the cells and analyzed to determine how beta-catenin regulates expression of Type III collagen, an extracellular matrix protein upregulated in contracted clubfoot tissue. There was a more than twofold increase in beta-catenin protein in the contracted tissues. Treatment with either lithium or Dickkopf-1 showed Type III collagen RNA expression positively correlated with the protein level of beta-catenin. These data support the concept that beta-catenin-mediated signaling plays an important role regulating contracture in clubfeet. Because pharmacologic agents are under development to block this signaling pathway, such drugs could be used in cases of severe stiffness to improve range of motion or to decrease the need for radical surgical approaches.
Introduction
Clubfoot is one of the most common musculoskeletal anomalies in newborns [3]. Although a genetic cause is suggested, because it tends to run in families [27], data from early amniocentesis suggest oligohydramnios as a cause [35]. In either case, osseous deformities [39], muscle abnormalities [19, 22, 45], and arrested fetal development [18] are all hypothesized to play a role in its pathogenesis. Manipulation and casting has become the most frequently used treatment for clubfoot with generally good results reported [32, 34]. Long-term study of clubfoot shows feet that maintain a good range of motion and are not contracted have better function [10, 11, 15, 23].
Ultrastructural study of the contracted tissue in clubfeet shows cells from the medial side of clubfeet contain microfilaments, identical in nature to those described in palmar fibromatosis [36]. Cells from the contracted tissue express Type III collagen at high levels [18, 25]. Type III collagen is expressed in healing wounds, hypertrophic scar tissues, and in contracted tissues in conditions such as palmar fibromatosis [12, 33, 37]. Cells from palmar fibromatosis and the contracted tissues in clubfoot express a number of growth factors such as platelet-derived growth factor and transforming growth factor beta [25]. However, expression of these factors may not be the primary cause of the contracture, but instead may be secondary to activation of another signaling pathway.
Work over the past decade demonstrates that beta-catenin-mediated signaling plays a central role regulating fibroblast activity [6, 7, 9, 21, 38]. This was initially suggested by the finding that mutations activating beta-catenin cause the fibroproliferative tumor aggressive fibromatosis [1, 42], but more recent data show hyperplastic wounds and palmar fibromatosis are also associated with activation of this pathway. Indeed, pharmacologic agents that activate beta-catenin signaling will cause hyperplastic wounds in mice [9].
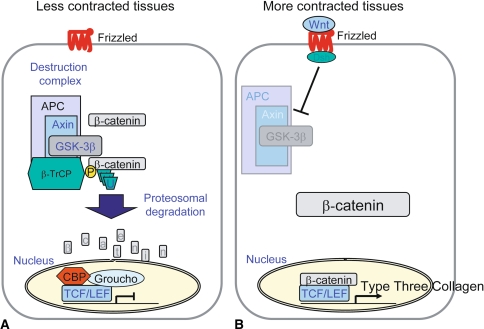
Beta-catenin is a key member of the canonical Wnt signaling pathway. This pathway is activated by specific circulating Wnt ligands. In the absence of a Wnt ligand, beta-catenin is processed for degradation by phosphorylation by a multiprotein complex consisting of APC, GSK3-beta, and Axin. Phosphorylation of beta-catenin targets it for ubiquitin-mediated degradation at the proteasome. In the presence of Wnt signaling proteins, stabilized beta-catenin can translocate to the nucleus, bind to the members of the Tcf-Lef family of transcription factors to form a transcriptional activation complex, and induce expression of target genes [2, 10, 28, 31, 41] (Fig. 1). In the case of aggressive fibromatosis, specific mutations cause activation of beta-catenin independent of Wnt ligands [1, 42]. In the case of palmar fibromatosis, beta-catenin is activated in the absence of an identified mutation [30, 44].
Fig. 1A–B.
β-catenin regulation and function in less contracted and more contracted clubfoot tissues. (A) When Wnt signaling is quiescent, in less contracted tissues, a multiprotein complex phosphorylates amino terminal serine and threonine residues, resulting in β-catenin degradation by a ubiquitin-mediated pathway. (B) Wnt activation, as is the case in contracted clubfoot tissues, inhibits the ubiquitin-mediated degradation of β-catenin, elevating β-catenin protein level. Stabilized β-catenin translocates to the nucleus and binds members of the tcf-lef family, resulting in transcriptional activation. One of the targets in clubfoot is Type III collagen.
An adjuvant treatment to reduce stiffness would result in a better outcome for patients with clubfeet. This might be useful in those rare feet that are resistant to manipulation and casting. Such adjuvant therapy may improve motion and function after treatment. Although agents to block beta-catenin signaling are not yet available, there are several approaches to reduce its activity that are under investigation [14, 29]. As such, if beta-catenin is indeed found to play a major role in clubfoot, once such agents become available, they can be applied to this condition.
We therefore asked whether beta-catenin signaling is activated in the contracted tissues in clubfeet and if this signaling pathway regulates the expression of genes important in contracture formation.
Materials and Methods
We studied tissues from patients undergoing surgery for clubfeet. These tissues were the same ones that were used in a previous investigation of growth factors in patients with clubfeet [25]. Contracted tissues were obtained from the medial aspect of the talonavicular joint, and less contracted tissues were obtained from the plantar surface of the calcaneocuboid joint. Sufficient materials from 10 feet were available for study. We obtained institutional approval for the study, and all patients completed informed consent to participate.
We processed tissue as soon as possible after surgical excision as previously reported [6, 8, 24, 25]. A portion of each sample was cryopreserved in liquid nitrogen vapor, and this was used for protein analysis. We used another portion of the tissue, from the more contracted side of the clubfoot, to establish primary cell cultures. Cultures were established by mechanical and enzymatic dissociation (trypsin and collagenase) in culture medium (Dulbecco’s modified Eagle’s medium) with 10% fetal calf serum.
We extracted protein from samples by homogenization in lysis buffer (1% sodium dodecyl sulfate [SDS], 10 mM Tris-HCl, pH 7.4) followed by centrifugation at 12,000 g for 5 minutes. Equal amounts of total protein were electrophoresed on an SDS polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, and stained to verify equal amounts of transferred protein from each sample. Western blot was performed using an antibody to activated beta-catenin (R & D Systems, Minneapolis, MN), Phospho, and total GSK-3-beta (Cell Signaling Technology, Danvers, MA). Hybridization was carried out overnight at 4°C and detected using an antimouse IgG-horseradish peroxidase secondary antibody and chemiluminescence. The membranes were stripped and reprobed with an antibody to GAPDH (R & D Systems) as an additional protein loading control.
To determine the role of beta-catenin in these cells, they were treated with lithium, which activates beta-catenin-mediated signaling [4, 9], or with Dickkopf-1 (DKK-1), which inhibits canonical Wnt signaling [4, 5, 20, 43]. We performed in vitro experiments with either 50 mM LiCl (Sigma, St Louis, MO) or an equal concentration of NaCl as a control to activate signaling. Cells were treated with 200 multiplicity of infection Ad DKK1-HA in serum-free media using identical conditions as previously reported [4, 5, 20, 43]. As a control, we used the same adenovirus but expressing an empty vector. After 1.5 hours, cells were returned to regular 10% fetal bovine serum media and left to grow overnight. RNA and protein were extracted and used for expression studies and Western analysis to determine that beta-catenin levels did indeed change. In additional cell cultures, we added bromodexyuridine (BrDU) to the cells overnight and the relative proportion of cells incorporating BrDU was measured using a commercially available colorimetric assay (Roche, Indianapolis, IN).
Previous work suggests Type III collagen is upregulated in contracted tissues in clubfeet and as such is a marker of tissue contracture [25, 36]. We used real-time polymerase chain reaction to determine differences in Type III collagen expression between cultures, using GAPDH as a housekeeping control, using previously reported primers and conditions [16].
Means, standard deviations, and 95% confidence intervals were calculated for the data. We used two-way Student’s t-test to compare comparisons between data sets (Microsoft Excel; Microsoft, Redmond, WA).
Results
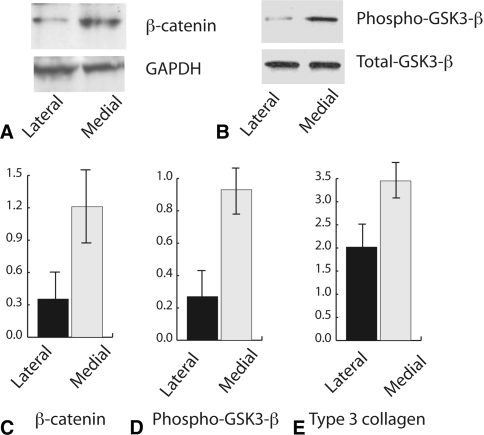
We found Type III collagen, a gene expressed at high levels in contracted tissues, was expressed at a higher (p = 0.006) level in the more contracted (medial) tissues compared with the less contracted lateral tissues (Fig. 2). This confirms the medial tissues examined are indeed representative of contracted clubfoot tissues. Using Western analysis, we found a more than twofold increase (p = 0.003) in activated beta-catenin in the medial (contracted) tissues compared with the lateral (less contracted) tissues. This increase was found in tissues from all 10 feet we studied (Fig. 2). We then examined GSK-3-beta, a protein that is phosphorylated with canonical Wnt signaling activation, and found a higher (p = 0.01) level of phosphorylation in the medial capsular tissues (Fig. 2).
Fig. 2A–E.
Contracted clubfoot tissues have higher levels of beta-catenin protein. (A) Western analysis from a protein extract from a representative clubfoot sample shows a higher level of protein expression in the medial, more contracted tissues compared with GAPDH as a loading control. (B) Western analysis from a representative clubfoot sample shows a higher level of GSK-3-beta phosphorylation in the medial contracted tissues compared with the lateral, less contracted tissues compared with total GSK-3-beta. (C) Densitometry results from Western analysis for beta-catenin level from all 10 clubfeet are shown. Band density is given relative to GAPDH as a loading control. Results are shown as means and 95% confidence intervals. (D) Densitometry results from Western analysis for phosphorylated GSK-3-beta level from all 10 clubfeet are shown. Band density is given relative to total GSK-3-beta as a loading control. Results are shown as means and 95% confidence intervals. (E) Results of real-time polymerase chain reaction for RNA expression of Type III collagen compared with GAPDH showing a higher level of expression in the medial, more contracted tissues. Results are shown as means and 95% confidence intervals.
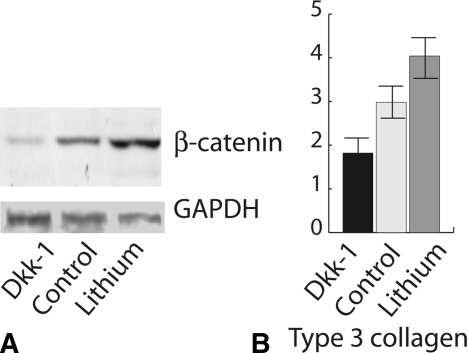
Using Western analysis, we found DKK-1 decreased (p = 0.002) and lithium increased (p = 0.005) beta-catenin in the cell cultures derived from the contracted clubfeet compared with controls (Fig. 3). Quantitative real-time polymerase chain reaction showed an increase (p = 0.02) in expression of Type III collagen with lithium treatment and a decrease (p = 0.01) in the expression level of Type III collagen with DKK-1 treatment (Fig. 3).
Fig. 3A–B.
Beta-catenin regulates expression of Type III collagen in the contracted tissues from clubfeet. (A) Western analysis shows treatment with DKK-1 decreases and treatment with lithium increases beta-catenin protein level compared with GAPDH as a loading control. (B) The level of RNA expression for Type III collagen as detected using real-time polymerase chain reaction shows a positive relationship between beta-catenin levels and Type III collagen expression. Results are shown as means and 95% confidence intervals.
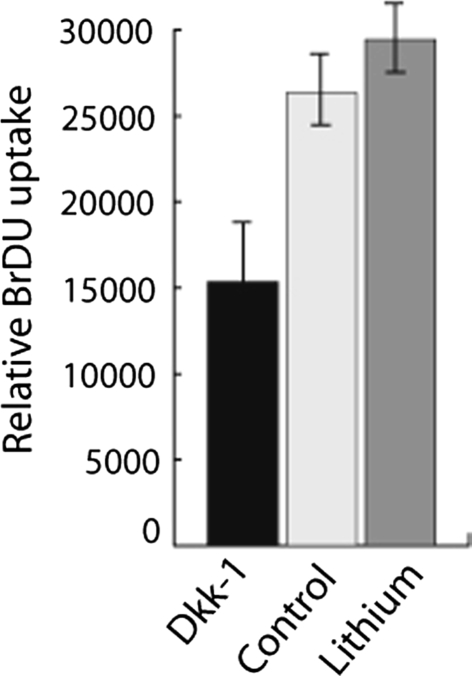
BrDU incorporation, as a measure of proliferation, was assessed in the cell cultures subjected to the same treatments. There was an increase (p = 0.01) in the relative uptake of BrDU with lithium treatment and a decrease (p = 0.03) in uptake with DKK-1 treatment compared with controls (Fig. 4).
Fig. 4.
Beta-catenin regulates proliferation in the contracted tissues from clubfeet. Proliferation is measured using BrDU incorporation in cell cultures from the contracted tissues from clubfeet. Results are shown as means and 95% confidence intervals for relative BrDU incorporation with a higher level indicating a higher proliferation rate. There is a positive relationship between beta-catenin level and proliferation rate.
Discussion
The contracted tissues from clubfeet share histologic and ultrastructural similarities with contracted tissues from fibroproliferative conditions such as palmar fibromatosis [14, 29]. Beta-catenin-mediated signaling is a cell regulatory pathway that is activated in fibroproliferative conditions causing cell proliferation and the deposition of matrix components. Pharmacologic agents to modulate beta-catenin signaling activation are in development. As such, it is possible beta-catenin levels are elevated in the contracted tissues from clubfeet and therapeutically targeting this signaling pathway could be used to decrease contracture and improve outcome for children being treated for clubfeet. In this study, we tested the hypothesis that beta-catenin protein level was elevated in contracted tissues from clubfeet and that modulation of beta-catenin could decrease cell proliferation and the expression of extracellular matrix components in a way that might decrease the severity of the contracture.
One limitation of this study is clubfoot samples were obtained from feet that underwent operative treatment almost always after a period of corrective casting. It is therefore possible the change in beta-catenin protein level is the result of the period of immobilization rather than the foot deformity itself. Although this possibility cannot be completely excluded, it is not likely to be the case, because all of the feet had at least a 6-week period without immobilization before operative treatment, the entire foot was immobilized in the cast, and the changes resulting from immobilization would likely be present throughout the foot. Furthermore, the medial tissues from fetal clubfeet have the same cytologic features characteristic of contracture and express matrix components associated with contracture at a similar level as medial tissues from surgically treated clubfeet [14, 29] suggesting changes associated with tissue contracture are not related to postnatal therapy. Another potential limitation is that using the current treatment approach, most clubfeet are treated with manipulation and casting; one could argue these are abnormally severe feet that were available for study. However, these samples were obtained several years ago, at a time when more than half of all clubfeet that presented to our institution were treated with open circumferential releases. In an ideal situation, fetal tissues would be used for such a study. Because of difficulties in obtaining appropriately preserved tissue from prenatal clubfeet for these analyses, it is impractical to test for differences in beta-catenin using fetal clubfoot tissues. The control tissues used in this study were from the less contracted tissues from clubfeet rather than from normal feet or other capsular tissues that were not related to a pathologic process. Obtaining such normal tissues is rather difficult and, as such, they are not included in this study. However, the level of beta-catenin protein is approximately the same as that found in normal control tissues from other sites in the body as previously reported in a number of studies [1, 6, 31, 38] suggesting the level of expression of the less contracted tissues is at approximately a baseline level for normal connective tissues.
We found the contracted capsular tissues in clubfoot were associated with increased protein levels of beta-catenin. Furthermore, regulating beta-catenin in cell cultures from the contracted tissues shows a positive correlation with expression of Type III collagen and proliferation rate with the protein level of beta-catenin. Because Type III collagen is an important extracellular component of the contracted clubfoot tissues, this suggests the beta-catenin signaling pathway plays an important role regulating contracture in clubfoot. Beta-catenin is also elevated in contracted tissues in a variety of other soft tissue fibroproliferative processes (eg, palmar fibromatosis) [2, 31]. Many of these processes are associated with myofibroblast cells and soft tissue contracture. The level of beta-catenin protein we found in the contracted clubfoot tissues is approximately the same as that found in palmar fibromatosis [38]. As such, it seems the regulation of canonical Wnt signaling through beta-catenin is a common factor in fibrosis. Although growth factors and extracellular matrix components also play a role in these processes, and because beta-catenin directly regulates transcriptional control of many of the genes important in fibrosis [9], it is likely a central regulator of this process. Because treatment with DKK-1 inhibited beta-catenin, it is likely that in clubfoot, a Wnt-ligand-dependent process activates beta-catenin.
Intriguingly, a genetic linkage study searching for the gene(s) predisposing to idiopathic clubfoot found a relationship between Wnt 7A and clubfoot [13]. Because Wnt ligands activate beta-catenin, it is possible this could play a role as a causative factor in clubfeet. However, a second study of genetic linkage showed Wnt 7A is not likely to be a causative gene for clubfoot [26]. Taken together, this suggests the beta-catenin-mediated fibrosis is more likely an end result rather than a causative factor in clubfoot. Despite this, it is possible genetic changes that alter the level of expression or activity of a Wnt ligand will alter the level of beta-catenin and thus the severity of clubfoot. As such, genes in the beta-catenin signaling pathway might play important roles regulating severity of contracture and response to treatment.
The data from this study raise several intriguing possibilities for the management of clubfoot, which could be explored as part of future work. For instance, levels of beta-catenin could be a prognostic factor, and lower levels may be related to less contracted tissues. Beta-catenin levels also may differ between idiopathic and syndromic or atypical clubfeet. They might also predict recurrence after successful manipulative treatment, the response to manipulative treatment, or a better response to anterior tibialis transfers for recurrence. Data supporting these possibilities could be obtained from samples from patients undergoing surgery for relapsed clubfeet.
Although drugs that regulate beta-catenin are not yet available for clinical use, there is a great deal of research into developing such agents [14, 17, 40, 46]. In addition, screening of known compounds may identify drugs that modulate beta-catenin activity [40]. Such agents may be used as an adjuvant in the treatment of stiff or syndromic clubfeet or for feet in which there is a recurrence requiring additional surgeries. They may decrease contracture and increase the ease of manipulative correction and increase the posttreatment range of motion. As such agents become available, they would be good candidates for clinical trials in children with clubfeet.
Footnotes
One of the authors (BAA) has received funding from the Canadian Institutes of Health Research.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors) Am J Pathol. 1997;151:329–334. [PMC free article] [PubMed] [Google Scholar]
- 2.Bowley E, O’Gorman DB, Gan BS. Beta-catenin signaling in fibroproliferative disease. J Surg Res. 2007;138:141–150. doi: 10.1016/j.jss.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Cardy AH, Barker S, Chesney D, Sharp L, Maffulli N, Miedzybrodzka Z. Pedigree analysis and epidemiological features of idiopathic congenital talipes equinovarus in the United Kingdom: a case-control study. BMC Musculoskelet Disord. 2007;8:62. doi: 10.1186/1471-2474-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Medicine. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Whetstone HC, Youn A, Nadesan P, Chow EC, Lin AC, Alman BA. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem. 2007;282:526–533. doi: 10.1074/jbc.M602700200. [DOI] [PubMed] [Google Scholar]
- 6.Cheon S, Poon R, Yu C, Khoury M, Shenker R, Fish J, Alman BA. Prolonged beta-catenin stabilization and tcf-dependent transcriptional activation in hyperplastic cutaneous wounds. Lab Invest. 2005;85:416–425. doi: 10.1038/labinvest.3700237. [DOI] [PubMed] [Google Scholar]
- 7.Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, Alman BA. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A. 2002;99:6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheon SS, Nadesan P, Poon R, Alman BA. Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp Cell Res. 2004;293:267–274. doi: 10.1016/j.yexcr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. Faseb J. 2006;20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 10.Clevers H, Wetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485–489. doi: 10.1016/S0168-9525(97)01305-X. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DM, Dietz FR. Treatment of idiopathic clubfoot. A thirty-year follow-up note. J Bone Joint Surg Am. 1995;77:1477–1489. doi: 10.2106/00004623-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Dasu MR, Hawkins HK, Barrow RE, Xue H, Herndon DN. Gene expression profiles from hypertrophic scar fibroblasts before and after IL-6 stimulation. J Pathol. 2004;202:476–485. doi: 10.1002/path.1539. [DOI] [PubMed] [Google Scholar]
- 13.Dietz FR, Cole WG, Tosi LL, Carroll NC, Werner RD, Comstock D, Murray JC. A search for the gene(s) predisposing to idiopathic clubfoot. Clin Genet. 2005;67:361–362. doi: 10.1111/j.1399-0004.2005.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dihlmann S, l Doeberitz M. Wnt/beta-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int J Cancer. 2005;113:515–524. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- 15.Dobbs MB, Nunley R, Schoenecker PL. Long-term follow-up of patients with clubfeet treated with extensive soft-tissue release. J Bone Joint Surg Am. 2006;88:986–996. doi: 10.2106/JBJS.E.00114. [DOI] [PubMed] [Google Scholar]
- 16.Eikmans M, Baelde HJ, Heer E, Bruijn JA. Effect of age and biopsy site on extracellular matrix mRNA and protein levels in human kidney biopsies. Kidney Int. 2001;60:974–981. doi: 10.1046/j.1523-1755.2001.060003974.x. [DOI] [PubMed] [Google Scholar]
- 17.Eisinger AL, Nadauld LD, Shelton DN, Prescott SM, Stafforini DM, Jones DA. Retinoic acid inhibits beta-catenin through suppression of Cox-2: a role for truncated adenomatous polyposis coli. J Biol Chem. 2007;282:29394–29400. doi: 10.1074/jbc.M609768200. [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara K, Schollmeier G, Uhthoff HK. The pathogenesis of club foot. A histomorphometric and immunohistochemical study of fetuses. J Bone Joint Surg Br. 1994;76:450–457. [PubMed] [Google Scholar]
- 19.Herceg MB, Weiner DS, Agamanolis DP, Hawk D. Histologic and histochemical analysis of muscle specimens in idiopathic talipes equinovarus. J Pediatr Orthop. 2006;26:91–93. doi: 10.1097/01.bpo.0000188994.90931.e8. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman J, Kuhnert F, Davis CR, Kuo CJ. Wnts as essential growth factors for the adult small intestine and colon. Cell Cycle. 2004;3:554–557. [PubMed] [Google Scholar]
- 21.Howard JC, Varallo VM, Ross DC, Roth JH, Faber KJ, Alman B, Gan BS. Elevated levels of beta-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16. doi: 10.1186/1471-2474-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kranicz J, Trombitas K, Szabo G. Results of ultrastructural analysis of the calf muscles in clubfoot. Orthopedics. 1991;14:73–75. doi: 10.3928/0147-7447-19910101-13. [DOI] [PubMed] [Google Scholar]
- 23.Laaveg SJ, Ponseti IV. Long-term results of treatment of congenital club foot. J Bone Joint Surg Am. 1980;62:23–31. [PubMed] [Google Scholar]
- 24.Li C, Bapat B, Alman BA. Adenomatous polyposis coli gene mutation alters proliferation through its beta-catenin-regulatory function in aggressive fibromatosis (desmoid tumor) Am J Pathol. 1998;153:709–714. doi: 10.1016/s0002-9440(10)65614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Nguyen Q, Cole WG, Alman BA. Potential treatment for clubfeet based on growth factor blockade. J Pediatr Orthop. 2001;21:372–377. doi: 10.1097/00004694-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Inglis J, Cardy A, Shaw D, Sahota S, Hennekam R, Sharp L, Miedzybrodzka Z. Variation in WNT7A is unlikely to be a cause of familial congenital talipes equinovarus. BMC Med Genet. 2008;9:50. doi: 10.1186/1471-2350-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lochmiller C, Johnston D, Scott A, Risman M, Hecht JT. Genetic epidemiology study of idiopathic talipes equinovarus. Am J Med Genet. 1998;79:90–96. doi: 10.1002/(SICI)1096-8628(19980901)79:2<90::AID-AJMG3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 28.Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- 29.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W, He TC. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery E, Lee JH, Abraham SC, Wu TT. Superficial fibromatoses are genetically distinct from deep fibromatoses. Mod Pathol. 2001;14:695–701. doi: 10.1038/modpathol.3880374. [DOI] [PubMed] [Google Scholar]
- 31.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 32.Morcuende JA, Abbasi D, Dolan LA, Ponseti IV. Results of an accelerated Ponseti protocol for clubfoot. J Pediatr Orthop. 2005;25:623–626. doi: 10.1097/01.bpo.0000162015.44865.5e. [DOI] [PubMed] [Google Scholar]
- 33.Murrell GA, Francis MJ, Bromley L. The collagen changes of Dupuytren’s contracture. J Hand Surg. [Br] 1991;16:263–266. doi: 10.1016/0266-7681(91)90050-x. [DOI] [PubMed] [Google Scholar]
- 34.Ponseti IV. Treatment of congenital club foot. J Bone Joint Surg Am. 1992;74:448–454. [PubMed] [Google Scholar]
- 35.Randomised trial to assess safety, fetal outcome of early, midtrimester amniocentesis The Canadian Early and Mid-trimester Amniocentesis Trial (CEMAT) Group. Lancet. 1998;351:242–247. doi: 10.1016/S0140-6736(97)12346-7. [DOI] [PubMed] [Google Scholar]
- 36.Sano H, Uhthoff HK, Jarvis JG, Mansingh A, Wenckebach GF. Pathogenesis of soft-tissue contracture in club foot. J Bone Joint Surg Br. 1998;80:641–644. doi: 10.1302/0301-620X.80B4.8526. [DOI] [PubMed] [Google Scholar]
- 37.Satish L, Laframboise WA, O’Gorman DB, Johnson S, Janto B, Gan BS, Baratz ME, Hu FZ, Post JC, Ehrlich GD, Kathju S. Identification of differentially expressed genes in fibroblasts derived from patients with Dupuytren’s contracture. BMC Med Genomics. 2008;1:10. doi: 10.1186/1755-8794-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 38.Sato M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–307. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro F, Glimcher MJ. Gross and histological abnormalities of the talus in congenital club foot. J Bone Joint Surg Am. 1979;61:522–530. [PubMed] [Google Scholar]
- 40.Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, Gupta S. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–6935. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 41.Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18:215–230. doi: 10.1023/A:1006369223282. [DOI] [PubMed] [Google Scholar]
- 42.Tejpar S, Nollet F, Li C, Wunder JS, Michils G, dal Cin P, Cutsem E, Bapat B, Roy F, Cassiman JJ, Alman BA. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor) Oncogene. 1999;18:6615–6620. doi: 10.1038/sj.onc.1203041. [DOI] [PubMed] [Google Scholar]
- 43.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 44.Varallo VM, Gan BS, Seney S, Ross DC, Roth JH, Richards RS, McFarlane RM, Alman B, Howard JC. Beta-catenin expression in Dupuytren’s disease: potential role for cell-matrix interactions in modulating beta-catenin levels in vivo and in vitro. Oncogene. 2003;22:3680–3684. doi: 10.1038/sj.onc.1206415. [DOI] [PubMed] [Google Scholar]
- 45.Windisch G, Anderhuber F, Haldi-Brandle V, Exner GU. Additional muscle in idiopathic club foot. Eur J Pediatr Surg. 2006;16:294–296. doi: 10.1055/s-2006-924372. [DOI] [PubMed] [Google Scholar]
- 46.Wunder JS, Nielsen TO, Maki RG, O’Sullivan B, Alman BA. Opportunities for improving the therapeutic ratio for patients with sarcoma. Lancet Oncol. 2007;8:513–524. doi: 10.1016/S1470-2045(07)70169-9. [DOI] [PubMed] [Google Scholar]