Abstract
Walking flexibility depends on use of feedback or reactive control to respond to unexpected changes in the environment, and the ability to adapt feedforward or predictive control for sustained alterations. Recent work has demonstrated that cerebellar damage impairs feedforward adaptation, but not feedback control, during human split-belt treadmill walking. In contrast, focal cerebral damage from stroke did not impair either process. This led to the suggestion that cerebellar interactions with the brainstem are more important than those with cerebral structures for feedforward adaptation. Does complete removal of a cerebral hemisphere affect either of these processes? We studied split-belt walking in 10 children and adolescents (age 6–18 years) with hemispherectomy (i.e. surgical removal of one entire cerebral hemisphere) and 10 age- and sex-matched control subjects. Hemispherectomy did not impair reactive feedback control, though feedforward adaptation was impaired in some subjects. Specifically, some showed reduced or absent adaptation of inter-leg timing, whereas adaptation of spatial control was intact. These results suggest that the cerebrum is involved in adaptation of the timing, but not spatial, elements of limb movements.
Keywords: locomotion, children, motor learning
Introduction
We must constantly adjust our locomotor pattern for changes in the environment (e.g. a slippery patch of ice) and body mechanics (e.g. growth, injury). Feedback or reactive locomotor adjustments are used to immediately change an ongoing motor pattern in response to sensory information about an unexpected perturbation (Reisman et al., 2005; Lam et al., 2006). Feedforward or predictive locomotor control is used to account for systematic perturbations by incorporating the expected effects into the planning of upcoming steps. Feedforward control therefore anticipates and counteracts expected perturbations so that they do not disrupt the movement. Adapting feedforward control mechanisms requires practice with the constant perturbation, and results in the storage of new motor plans. When the constant perturbation is removed, the updated motor plan remains and is expressed in the form of an after-effect that must actively be ‘unlearned’ (Reisman et al., 2005; Lam et al., 2006).
Many levels of the nervous system, including the spinal cord, brainstem, cerebellum and motor cortex differentially contribute to these control processes during locomotion (Pearson, 2000; Rossignol, 2006). A recent split-belt walking study demonstrated that feedback control is intact in individuals with cerebellar damage (Morton and Bastian, 2006). Subjects could immediately react to the split treadmill by changing the stride length and amount of time spent in stance on the faster and slower belts in order to maintain one-to-one stepping. In contrast, cerebellar damage disrupted feedforward adaptation: subjects were unable to optimize spatial (e.g. step lengths) and temporal (e.g. phasing between the legs) elements of the walking pattern in the face of sustained split treadmill (Morton and Bastian, 2006). In contrast, we found that adults with cerebral damage were able to make both feedback adjustments and adapt feedforward control (Reisman et al., 2007). Since cerebral damage did not impair the tested locomotor capabilities (Reisman et al., 2007), we speculated that cerebellar projections to the brainstem motor areas are more important for feedforward adaptation than those to cerebral areas. However, since the lesions in the cerebrum were often focal, overall involvement of the cerebrum in feedback control or feedforward adaptive process cannot be definitively ruled-out. Indeed, studies have shown that neural activity in cerebral motor areas relates to modifications in the locomotor pattern (Armstrong, 1986; Drew et al., 2002), and that lesions of the corticospinal tract cause deficits in voluntary modifications (Drew, 1993; Drew et al., 2002).
To further investigate cerebral contributions to locomotor adaptation, we studied split-belt walking adaptation in children and adolescents who had undergone surgical removal of one entire cerebral hemisphere to treat intractable epileptic seizures. We tested whether cerebral structures are required for feedback mediated locomotor adjustments that allow individuals to react to split belts, and predictive feedforward locomotor adaptation as measured by the presence of after-effects. Our results suggest that hemispherectomized subjects have normal feedback control because they were able to rapidly adjust walking to account for split belts. Feedforward adaptation was partially impaired however; hemispherectomized subjects had some difficulty adapting and storing a new timing pattern, even though they could adapt and store the spatial pattern.
Methods
Subjects
We studied 10 children and adolescents who had undergone hemispherectomy for intractable epileptic seizures (Table 1). All of the patients had hemidecortication, a procedure in which potentially epileptogenic, unihemispheric, cortical grey matter is removed while the underlying white matter and ventricles are left intact (Kossoff et al., 2003). Post-operative brain scans show that the basal ganglia and thalamus are partially preserved to different extents across patients. Hemispherectomized subjects had hemiparesis of varying degree and were excluded if they could not walk on the treadmill at a maximum speed of 0.8 m/s. One of the patients tested (H5) had post-operative seizures but did not experience any during testing. All patients, except for H8, started walking before the surgery. We included 10 age- and sex-matched healthy control subjects (age range 7–18 years; median age 12.5 years; seven females, three males). All subjects and parents gave informed consent prior to participating. The protocol was approved by the Johns Hopkins Institutional Review Board.
Table 1.
Subject characteristics
| Subject | age | sex | Diagnosisa | Age at seizure onset (months) | Age at surgery (months) | Time since surgery (months) | Fast over ground walking speed (m/s) | LE Fugl-Meyer score (/34) | GT touch thresholdb (g) | BITc (/54) |
|---|---|---|---|---|---|---|---|---|---|---|
| H1 | 7 | M | L Sturge-Weber | 4 | 11 | 77 | 1.74 | 25 | 6.65 | 53 |
| H2d | 6 | F | R Rasmussen | 3 | 78 | 6 | NT | 22 | 6.65 | 27 |
| H3 | 16 | F | R Rasmussen | 101 | 119 | 81 | 1.45 | 24 | 3.61 | 54 |
| H4 | 10 | M | R Stroke | 5 | 27 | 105 | 1.28 | 23 | 4.56 | 53 |
| H5 | 13 | F | R Rasmussen | 103 | 163 | 5 | 1.18 | 21 | 6.65 | 53 |
| H6 | 16 | M | L Stroke | 89 | 138 | 58 | NT | NT | 4.31 | 54 |
| H7 | 13 | F | R Rasmussen | 38 | 46 | 120 | 1.45 | 24 | 4.31 | 54 |
| H8d | 18 | F | R Dysplasia | 13 | 13 | 211 | NT | NT | 4.56 | 52 |
| H9 | 9 | F | R Dysplasia | 62 | 84 | 29 | 1.58 | 28 | 3.61 | 53 |
| H10 | 12 | F | R Stroke | 52 | 69 | 81 | 1.24 | 25 | 3.61 | 54 |
aSturge-Weber syndrome is a congenital neurocutaneous condition. The majority of seizures begin before age of 1. Rasmussen's syndrome is a chronic, presumably autoimmune, encephalitis, that leads to a progressive unilateral seizure disorder, functional decline and hemiplegia. It occurs primarily in children. Cortical dysplasia refers to a heterogeneous group with congenital malformations of cortical development.
bNormal ≤ 3.61g; cNormal = 52–54; dslow = 0.4 m/s; fast = 0.8 m/s; NT = not tested.
LE, lower extremity; GT, great toe; BIT, behavioural inattention test.
Clinical assessment
Motor function on the paretic leg was assessed using the Fugl-Meyer scale, which measures reflexes, ability to move in and out of synergy and movement coordination (Fugl-Meyer, 1975). Walking speed was measured across a 25-feet walkway at their fastest speed. Touch sensation was tested using Semmes Winstein graded monofilaments on the great toe; the threshold was determined as the lowest gram filament that could be correctly detected on four out of five trials. The behavioural inattention test (BIT) Star Cancellation was used to measure visuospatial neglect (Wilson et al., 1987).
Experimental setup and design
We studied walking adaptation using a split-belt treadmill (Woodway, Waukesha, WI, USA) that had two separate belts driven by independent motors. Subjects were positioned in the middle of the treadmill, with one foot on each belt. They held onto a front rail adjusted to elbow height, and wore a safety harness, that did not support body weight. At the beginning of each trial, the treadmill belts were stationary. Subjects were informed when the belts were going to start moving but not the speeds. Rest breaks were given when needed. All subjects were able to perform at least 2 min non-stop bouts of walking on the treadmill.
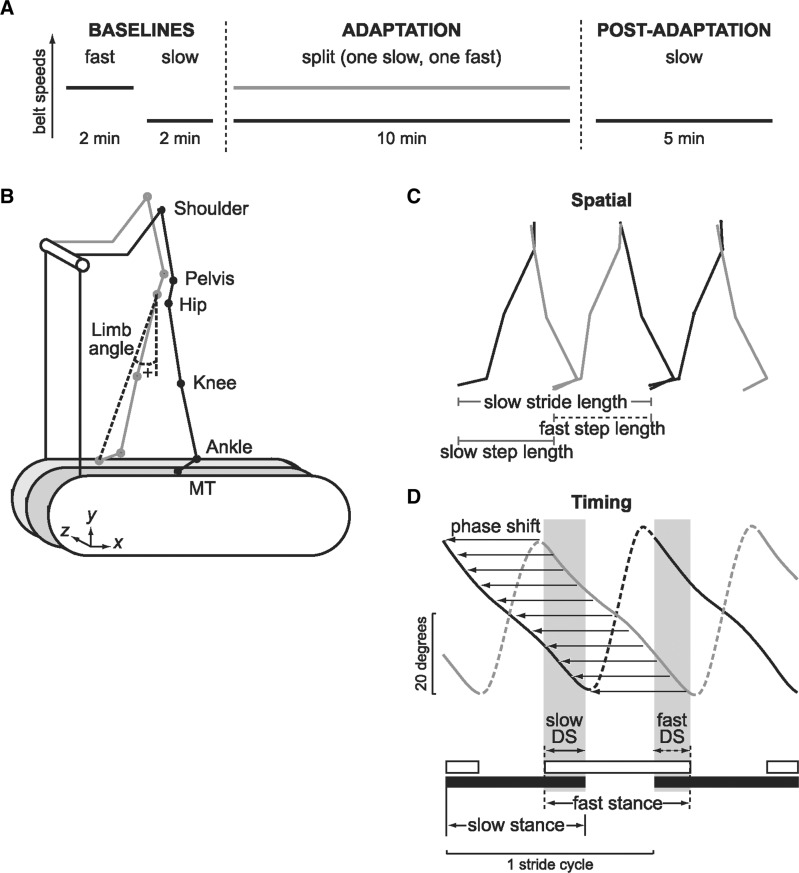
The paradigm (Fig. 1A) consisted of a ‘Baseline period’, where gait was measured for fast walking (1.0 m/s) and slow walking (0.5 m/s) on tied-belts (i.e. both belts set at the same speed) over 2 min bouts. During ‘Adaptation period’, subjects walked on split-belts with one belt moving slow (0.5 m/s) and the other moving fast (1.0 m/s) for 10 min. During ‘post-adaptation period’, gait was tested again on slow (0.5 m/s) tied-belts for 5 min. Two patients (H2 and H8) walked 0.4 m/s as the slow speed and 0.8 m/s as the fast speed.
Figure 1.
Experimental paradigm and walking measurements. (A) Experimental paradigm consisting of Baselines, Adaptation and Post-adaptation periods. (B) Stick figure illustrates marker location (dots) and limb angle. (C) Spatial aspects of walking are measured by calculating stride length and step lengths. Stride length in treadmill walking is the distance travelled by ankle marker from foot contact to lift off, and step length is the distance between left and right ankle markers at time of foot contact. (D) Temporal aspects of walking are measured by calculating stance time and double support time. Horizontal bars represent stance (time from foot contact to lift off) for the slow leg (black bar) and fast leg (white bar). Double support is the period when both legs are in stance. Another temporal measure is interlimb phasing, determined by calculating the cross-correlation function between limb angle trajectories for the slow leg (black line) and fast leg (grey line). Interlimb phase was defined as the lag time at peak correlation. While double support captures timing of foot contact, interlimb phase depends on the time shift (thin arrows) and trajectory of limb kinematics across the whole stride cycle.
Hemispherectomy patients were tested in two sessions: one where we trained their paretic leg on the fast belt and a second with the paretic leg on the slow belt. Seven patients participated in both sessions; three patients participated in only the first session. We trained patients in both directions to test whether they could change interlimb coordination during walking in both conditions (i.e. impaired leg going faster or slower). Matched controls were tested only once, half with left versus right leg on the fast belt.
Data collection
Kinematic data were collected at 100 Hz using Optotrak (Northern Digital, Inc.). Infrared-emitting markers were placed bilaterally over the following joints (Fig. 1B): foot (fifth metatarsal head), ankle (lateral malleolus), knee (lateral femoral epicondyle), hip (greater trochanter), pelvis (iliac crest) and shoulder (acromion process). Foot-switches placed on the bottom of shoes were used to record the times of foot contact and lift off.
Data analysis
A stride cycle is defined as the onset of foot contact to the onset of the next foot contact on the same leg. We measured spatial characteristics of walking (Fig. 1C) by calculating stride length (distance travelled by ankle marker from foot contact to lift off) and step lengths (distance between two ankle markers at time of foot contact). While stride length is measured from a single leg, step length depends on the spatial relationship between both legs. Our previous study (Reisman et al., 2005) have shown that stride length is adjusted immediately (within first few strides) in response to split-belt perturbation, which reflects reactive feedback control. In contrast, step length is gradually adapted over time and show after-effects when the perturbation is removed, which suggests involvement of predictive feedforward adaptation.
Temporal characteristics of walking (Fig. 1D) are measured by calculating stance time (period from foot contact to lift off) as a percent of stride cycle, and double support time (period toward end of stance when both legs are in ground contact). Stance phase is determined from foot contact of one leg, while double support phase describes the temporal relationship of foot contact on both legs. Our previous study (Reisman et al., 2005) have shown that stance time is adjusted immediately (within first few strides) in response to split-belt perturbation, which reflects reactive feedback control. In contrast, double support time is gradually adapted over time and show after-effects when the perturbation is removed, which suggests involvement of predictive feedforward adaptation.
For all measures, a symmetry index was used to quantify the difference between two legs for each gait parameter: symmetry = (fast leg – slow leg)/(fast leg + slow leg), where ‘slow leg’ and ‘fast leg’ refers to the leg on the slower and faster belt during split-belt condition, respectively. An index value of 0 would indicate that a gait parameter is symmetric and equal for both legs. Positive values indicate that the fast leg is taking a longer step, and negative values indicate that the slow leg is taking a longer step.
We also measured interlimb coordination by calculating the cross-correlation function (Signal Processing Toolbox, MATLAB) between limb angle trajectories (Fig. 1D). Limb angle was defined as the angle between the vertical and vector from hip to foot on the x–y plane; positive values indicate that the foot is in front of the hip (Fig. 1B). Each correlation function was calculated using limb angle data spanning three consecutive foot contacts on the slow leg (i.e. two stride cycles). The maximum time lag is limited to one stride cycle. Interlimb phase was defined as the lag time at peak correlation, and expressed as a fraction of the stride cycle. A phase of 0.5 cycle means that the legs are moving out-of-phase, and a phase of 0 means in-phase. By convention, positive lag times indicate a lead of the fast leg relative to the slow leg. This method of calculating interlimb phase was also used and described in our previous papers (Morton and Bastian, 2006; Choi and Bastian, 2007; Reisman et al., 2007).
Double support time and interlimb phase both capture temporal aspects of walking. While double support focuses on the discrete timing of foot contact and lift off, interlimb phase considers limb kinematics across the whole stride cycle. The value for interlimb phase depends on both the timing and the shape of limb trajectories. The rate at which the hip rolls over the supporting foot shapes the limb trajectory during stance. Hence, the two temporal parameters measure different aspects of walking (foot contact versus limb kinematics), and could vary separately to some extent.
Statistical analysis
We compared gait parameters across experiment periods using the last five values in each baseline period, the first and last five values in each adaptation period (early and late adaptation, respectively) and the first and last five values in each post-adaptation period (early and late post-adaptation, respectively). Repeated-measures ANOVA was used with experimental groups (control, paretic leg fast, paretic leg slow) as the between-subjects variable and experimental period as the within-subjects variable; post hoc analyses were performed using the Tukey's significant different test. Watson's U2 (circular statistic) was used to compare phase across groups (Batschelet, 1981). We also conducted specific comparisons between controls with the average of hemispherectomy groups, and between paretic leg fast with paretic leg slow. Planned comparisons were conducted to test components of interaction for the adaptation period (A1 and A2) and post-adaptation period (P1 and P2). We used α = 0.05 as the alpha level for each planned comparison. Pearson product-moment correlations were used to test for relationships between clinical and adaptation measures.
Results
Table 1 shows demographic and clinical information for the subjects with hemispherectomy. All subjects walked independently without an assistive device, had voluntary active movement of the paretic leg (i.e. Fugl-Meyer scores were in the twenties), and had some tactile sensation of the paretic leg. One child (H2) who had a right hemispherectomy 6 months prior showed some evidence of neglect. Two subjects were tested <6 months after their surgery; all others were >2 years post surgery. Fugl-Meyer assessments and over ground walking measurements were conducted on the second visit, and were not tested in three subjects who only participated in the first session (H2, H6 and H8).
Feedback control
We first asked whether the patients with hemispherectomy could maintain alternating leg movements during split-belt walking, which requires an immediate reaction to the treadmill. All children and adolescents in this study produced alternating leg movements similar to healthy adults (Reisman et al., 2005). None of the subjects from either group produced walking patterns where the fast leg took more steps then the slow leg on split-belts, which is more common during split-belt stepping in younger infants (Yang et al., 2005).
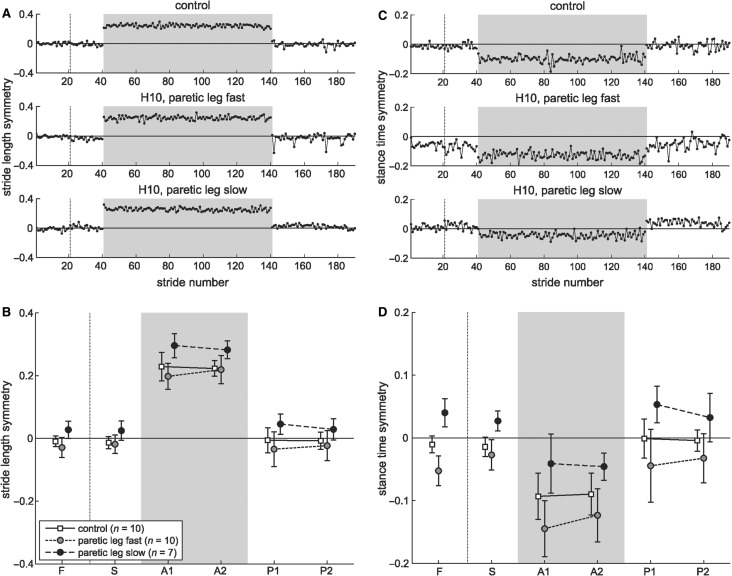
Alternating stepping was maintained by immediately adjusting the stride length and stance time symmetry to match feedback about the belt speeds. Figure 2A illustrates stride length asymmetry from a typical control subject and hemispherectomy patient. During baseline, the stride lengths are close to symmetric; during split-belt walking, the fast leg immediately takes a longer stride and the slow leg immediately takes a shorter stride. The change is sustained throughout adaptation and subjects quickly switch back to their baseline stride lengths in post-adaptation.
Figure 2.
Feedback control: ability to make immediate reactive responses. Stride-by-stride values for stride length symmetry (A) and stance time symmetry (C) from a typical control (top row) and hemispherectomy subject (bottom two rows) for the first 20 strides of fast and slow baselines, first 100 strides of split-belt adaptation, and first 60 strides of post-adaptation. Zero indicates symmetric stride length or stance time, negative value indicates that stride length or stance time on the fast leg is shorter relative to slow leg. Dotted vertical line indicates stop between fast and slow baseline. Shaded area indicates split-belts condition. Group averages for stride length (B) and stance time symmetry (D) during baseline conditions (slow, S and fast, F), split-belt adaptation (early, A1 and late, A2) and post-adaptation (early, P1 and late P2) for control (white squares) and hemispherectomy group with paretic leg fast (grey circles) or paretic leg slow (black circles). Each data point represents mean ± 1 SD over five strides. Note that symmetry index was always calculated with the slow leg as reference. Since we tested hemispherectomy subjects with their paretic leg fast in one experiment and slow in another, the symmetry index reversed sign at baseline due to this convention (grey versus black circles).
The hemispherectomy group showed normal feedback adjustments during split-belt walking (Fig. 2B). Stride length symmetry in slow and fast baseline was not different between control and hemispherectomy with paretic leg fast (P ≥ 0.5) or paretic leg slow (P = 0.3). Note that baseline values for two hemispherectomy groups differ because symmetry calculations were always referenced to the slow leg (i.e. non-paretic leg in session one and paretic leg in session two).
Feedback adjustments of stride length symmetry during adaptation and post-adaptation, relative to baseline, was not significantly different between controls and hemispherectomy subjects (group effect, P = 0.3; group × period interaction effect, P = 0.2), demonstrating that all groups made similar changes across testing periods. Intact feedback control in spatial parameters is reflected in the main effect of testing period (P < 0.001), where stride length symmetry immediately changed from baseline to early adaptation (P < 0.001). The adjustment was maintained from early to late adaptation (P ≥ 0.5). On return to tied-belts, stride length symmetry switched back and was not different between baseline and post-adaptation (P ≥ 0.5).
Figure 2C illustrates stance time symmetry changes for example subjects. During split-belt walking, control subjects shortened stance time on the fast leg within the first few steps, and switched back to baseline pattern on return to tied-belts post-adaptation. This hemispherectomy subject showed similar behaviour despite some stance asymmetries at baseline.
As a group, hemispherectomy subjects started out with baseline stance time asymmetry, however their feedback control was comparable to control subjects (Fig. 2D). Stance time asymmetry in the paretic leg fast group during baseline fast (mean ± 1 SD: –0.05 ± 0.02) and slow (–0.03 ± 0.02) walking was driven by an increase in stance time on the non-paretic leg. Control subjects had symmetric stance times during baseline fast (–0.01 ± 0.01) and slow (–0.01 ± 0.02) walking. Feedback adjustments of stance time symmetry during adaptation and post-adaptation, relative to baseline, was not significantly different across groups (group effect, P = 0.3; group × period interaction effect, P = 0.6). Stance time symmetry changed across testing period (P < 0.001); there was a change from baseline to early adaptation (P < 0.001), no change from early to late adaptation (P ≥ 0.5) and a switch back during post-adaptation. There was no after-effect in stance time, as baseline and post-adaptation were not different (P ≥ 0.5). Hence, all subjects showed rapid feedback changes regardless of whether the paretic or non-paretic leg was on the fast belt during adaptation and regardless of baseline asymmetry in some patients.
Feedforward adaptation
During the adaptation period of split-belt condition, control subjects show a walking pattern that is initially asymmetric, with unequal step lengths (spatial) and double support times (temporal), as well as time shifted phasing between legs (temporal). Normally these parameters would adapt back to symmetry over the course of split-belt adaptation and show post-adaptation after-effects (Reisman et al., 2005); the presence of after-effects indicates that a predictive feedforward mechanism was involved. Here, we saw that hemispherectomy subjects adapted feedforward spatial parameters, but not necessarily feedforward temporal parameters during split-belt walking.
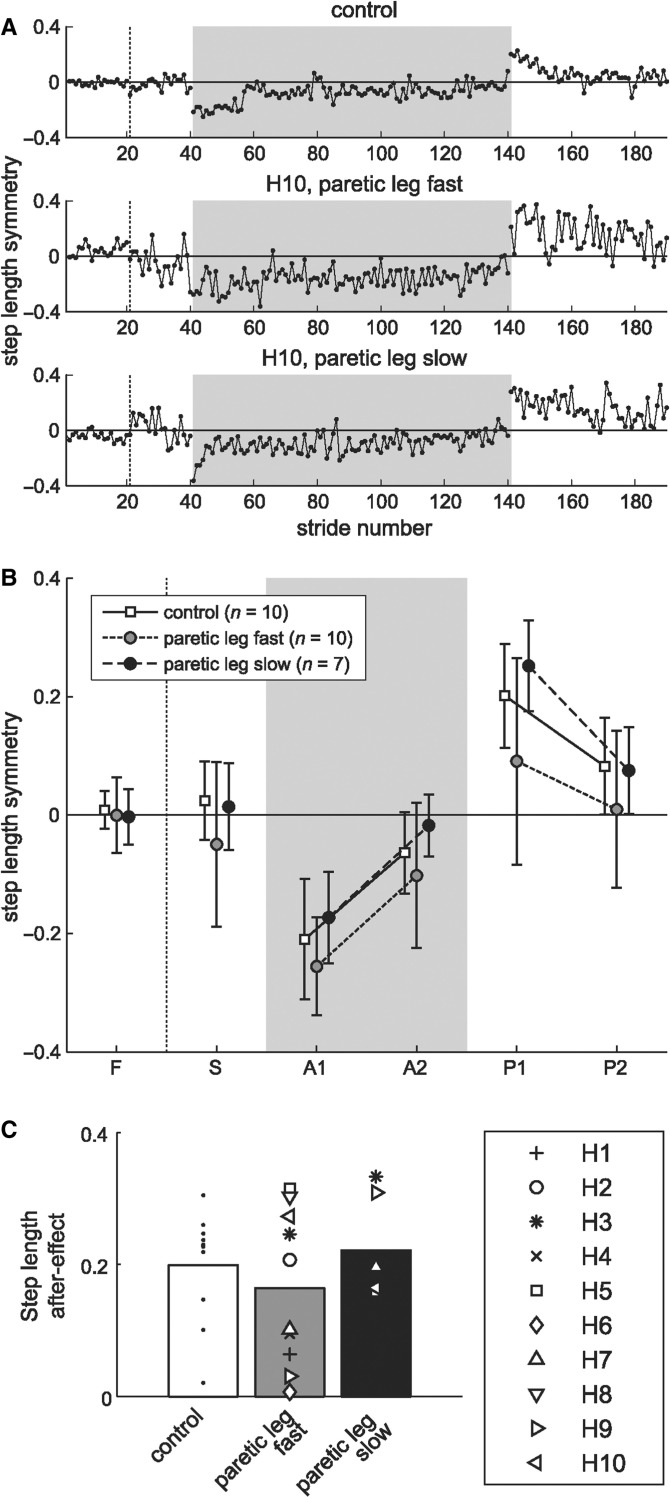
Figure 3A shows step length symmetry in a typical control and hemispherectomy patient from both sessions. Subjects in both groups took approximately equal step lengths in ‘baseline’. In split-belt ‘adaptation’, the slow leg initially took a longer step than the fast leg, but gradually became symmetric over 10 min of adaptation. During ‘post-adaptation’, subjects showed after-effects, taking a shorter step on the slow leg relative to the fast; this after-effect was gradually washed out.
Figure 3.
Feedforward adaptation: measured by the presence of after-effects. (A) Stride-by-stride values for step length from a typical control (top row) and hemispherectomy subject (bottom two rows) for the first 20 strides of fast and slow baseline, first 100 strides of split-belt adaptation, and first 60 strides of post-adaptation. Zero indicates symmetric step length; negative value indicates that step length on fast leg is shorter relative to slow leg. Shaded area indicates split-belts condition. (B) Group averages for step length during baseline conditions (slow, S and fast, F), split-belt adaptation (early, A1 and late, A2) and post-adaptation (early, P1 and late P2) for control (white squares) and hemispherectomy group with paretic leg fast (grey circles) or paretic leg slow (black circles). Each data point represents mean ± 1 SD over five strides. (C) Post-adaptation after-effects relative to baseline (P1 minus S). Bars represent mean after-effect in control (white) and hemispherectomy subjects with paretic leg fast (grey) or paretic leg slow (black), with individual data overlaid. The presence of an after-effects indicated involvement of a feedforward adaptation.
As a group, hemispherectomy subjects showed normal control of step length symmetry (Fig. 3B). There was no significant difference in step length symmetry across groups during baseline slow and fast walking (P = 0.6). Feedforward adjustments of step length symmetry during adaptation and post-adaptation, relative to baseline, were not different between groups (group effect, P = 0.1; group × period, P = 0.2), indicating that all subjects could recalibrate step length over the split-belt period and subsequently showed after-effects in tied-belts condition. Step length symmetry was perturbed from baseline to early adaptation (P < 0.001), re-established from early to late adaptation (P < 0.001), and showed significant after-effects in post-adaptation (P < 0.001). Figure 3C illustrates the mean after-effect magnitude relative to baseline and individual subject data. Means were not statistically different between control and hemispherectomy groups (P ≥ 0.5), and not different between paretic leg fast and paretic slow experiments (P = 0.2). There was substantial variability in the after-effect size of the control and hemispherectomy group with paretic leg fast, with some subjects storing larger after-effects.
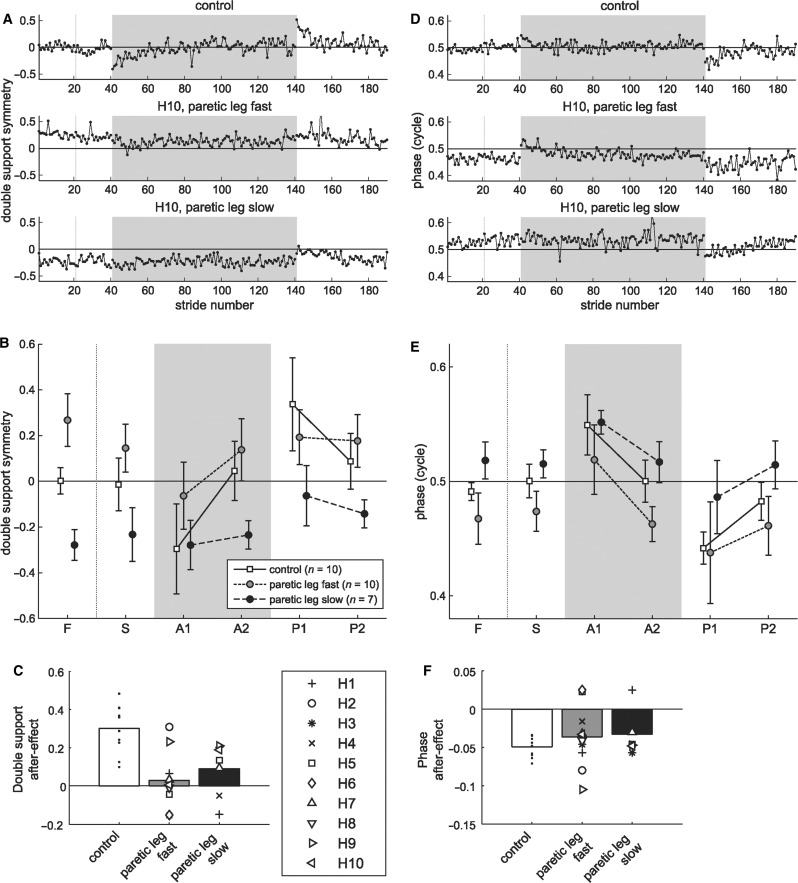
Hemispherectomy subjects had greater deficits in adapting temporal aspects of walking. Figure 4A shows symmetry in double support duration (i.e. when both legs are in stance) for a typical control and a hemispherectomy subject tested in both sessions. Note that symmetry index is calculated from two double support periods: one at the end of stance phase on each leg (Fig. 1D). The control subject showed a normal pattern of adaptation: symmetry at baseline, asymmetries during adaptation that were gradually reduced with practice and an after-effect of the opposite asymmetry during ‘post-adaptation’. The hemispherectomy subject has a baseline asymmetry due to a shorter double support at the end of non-paretic stance, and a longer one at the end of paretic leg stance. This subject also had difficulty adapting and showed limited after-effects.
Figure 4.
Feedforward adaptation of temporal parameters. Stride-by-stride double support symmetry (A) and interlimb phase (D) from a typical control (top row) and hemispherectomy subject (bottom two rows) for the first 20 strides of fast and slow baselines, first 100 strides of split-belt adaptation (grey shaded area), and first 60 strides of post-adaptation. For double support, zero indicates symmetry and negative values indicates shorter double support on fast leg relative to slow leg. For interlimb phase, 0.5 indicates out of phase coordination and a positive value indicates phase lead by the fast leg. (B and E) Group averages during baseline tied-belts conditions (slow, S and fast, F), split-belt adaptation (early, A1 and late, A2) and post-adaptation (early, P1 and late P2) for the control (white squares) and hemispherectomy group with paretic leg fast (grey circles) or paretic leg slow (black circles). Each data point represents mean ± 1 SD over five strides. (C and F) Post-adaptation after-effects relative to baseline (P1 minus S). Bars represent mean after-effects in control (white) and hemispherectomy subjects with paretic leg fast (grey) or paretic leg slow (black), with individual data is overlaid. The presence of after-effects indicated involvement of feedforward adaptation. Note that by convention, the slow leg was always used as reference. Values at baseline differ (grey versus black circles) since hemispherectomy subjects were trained with paretic leg fast in one experiment and paretic leg slow in another.
As a group, hemispherectomy subjects had asymmetric double support during baseline tied-belts walking, and their ability to adapt double support timing was also impaired (Fig. 4B). Double support asymmetry in the paretic leg fast group during baseline fast (mean ± 1 SD: 0.27 ± 0.12) and slow (0.15 ± 0.11) walking was due to a longer time in double support before transitioning to single limb support on the non-paretic leg. Control subjects had symmetric double support times during baseline fast (–0.00 ± 0.06) and slow (–0.01 ± 0.12) walking. Note that hemispherectomy subjects showed greater asymmetry in double support compared with adult stroke subjects in baseline (0.09 ± 0.15) walking from our previous paper (Reisman et al., 2007).
Feedforward adaptation of double support across adaptation and post-adaptation periods was significantly different across groups (group × period effect, P < 0.001), reflecting impaired double support adaptation in hemispherectomy patients. Hemispherectomy showed reduced changes during adaptation period compared with controls (P = 0.003), and they also had smaller after-effects in post-adaptation (P = 0.002). Comparisons between two hemispherectomy groups suggest reduced adaptation when the paretic leg was slow (P = 0.06), and no difference in post-adaptation (P = 0.4). In other words, hemispherectomy subjects showed similar after-effects during post-adaptation in both experiments, even though there was a smaller change during adaptation in the paretic leg slow experiment (see ‘Discussion’ section). Mean after-effect in double support (Fig. 4C) was reduced in hemispherectomy subjects compared with controls (P < 0.01), and not different between paretic leg fast and paretic leg slow experiments (P = 0.4).
A second temporal measure was interlimb phase, which captures timing of limb kinematics across the whole cycle (Fig. 1D). At ‘baseline’, interlimb phase was close to 0.5 cycles reflecting anti-phase coordination in both example subjects (Fig. 4D). During split-belt ‘adaptation’, the fast leg was phase advanced relative to the slow leg and gradually shifted back. In ‘post-adaptation’, both subjects had a negative after-effect in interlimb phase, though this effect was stronger in the control subject. This hemispherectomy subject had an offset in baseline, due to a delay of the paretic leg cycle relative to the non-paretic leg.
As a group, hemispherectomy subjects were offset from anti-phase during baseline fast (mean ± 1 SD: 0.47 ± 0.02) and slow (0.47 ± 0.02) walking, while controls showed normal anti-phasing for fast (0.49 ± 0.01) and slow (0.50 ± 0.01) walking (Fig. 4E). Feedforward adaptation of phase was less impaired than double support in hemispherectomy subjects. There was no group effect (P ≥ 0.5) but a near significant group by period interaction (P = 0.06). We saw no difference between controls and hemispherectomy subjects in adaptation (P = 0.6) and post-adaptation (P = 0.2). Planned comparisons suggest that an interaction component may lie in the difference between paretic leg fast versus paretic leg slow during adaptation (P = 0.06). There was no difference between the two sessions during post-adaptation (P = 0.7). Mean after-effect in phase (Fig. 4F) was not statistically different between control and hemispherectomy groups (P = 0.2), and not different between paretic leg fast and paretic leg slow experiment (P ≥ 0.5).
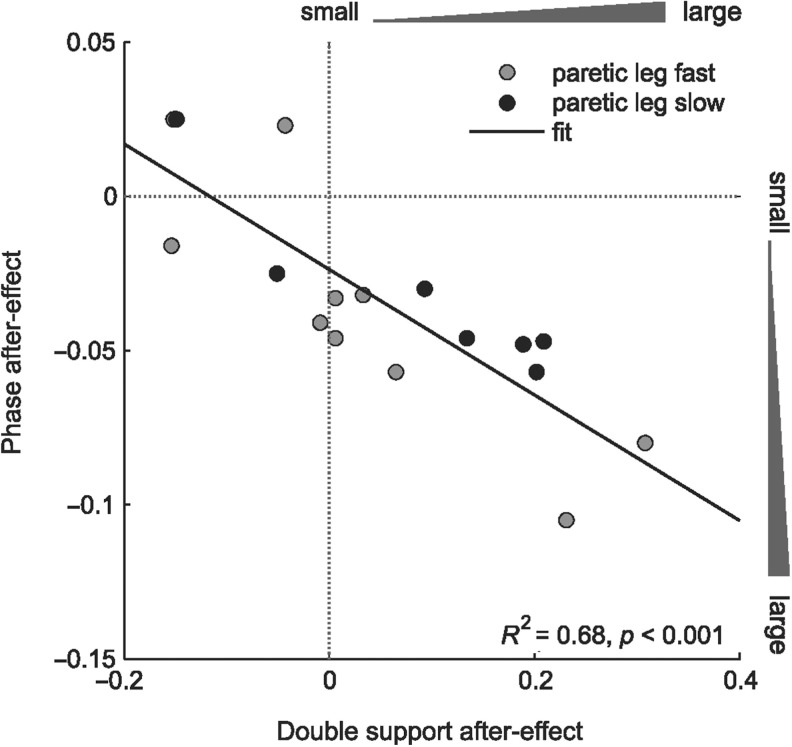
Since double support and interlimb phase both reflect timing aspects of walking, we expected to see similar changes in both parameters. Indeed, there was a relationship between after-effects in double support and phase (Fig. 5, R2 = 0.68, P < 0.001); smaller after-effect in double support corresponded with smaller after-effect in phase. However, our results also showed that some hemispherectomy subjects had no after-effect in double support, but yet showed after-effect in phase. Figure 5 illustrates this offset (i.e. line does not pass through origin) where double support is more impaired overall. Taken together, our results suggest that the precise timing of foot contact and lift off may be more difficult to control than moving the limbs forward and backward.
Figure 5.
Relationship between adaptation of double support and interlimb phase. Each data point represents the after-effect in double support (x-axis) and phase (y-axis) for individual hemispherectomy subjects trained with paretic leg fast (grey circles) or paretic leg slow (black circles). There is a clear correlation (linear fit) between the two temporal parameters, where smaller after-effect in double support corresponded with smaller after-effect in phase. Notice offset in this relationship (i.e. line shifted left of origin). Some hemispherectomy subjects had small after-effect in double support, yet showed after-effect in phase.
What predicts locomotor adaptive ability?
We hypothesized that some clinical measurements such as age at surgery, time since surgery, Fugl-Meyer score or sensory touch threshold might correlate with adaptive ability in hemispherectomy patients. We were surprised to find no significant relationships between these measures and after-effects in step length, double support or interlimb phase (all Ps > 0.1). This suggests that there is no linear relationship between, for example, time since surgery and adaptive ability. Since we had patients with different etiologies, we considered whether diagnoses influenced outcome. We saw no difference between Rasmussen's and stroke in feedforward adaptation of step length (P ≥ 0.5), double support (P = 0.2) and phase (P = 0.1).
The only relationship that we found was a negative correlation (r = –0.59, P = 0.01) between baseline asymmetry and the after-effect size for double support in patients. After-effects are normally due to the fast double support being lengthened relative to the slow double support. At baseline hemispherectomy subjects’ paretic double support was longer than their non-paretic double support. When patient's paretic leg was the fast leg during adaptation, they did not show substantial after-effects; presumably because they could not further lengthen the paretic leg double support (i.e. asymmetry did not get worse). Conversely, hemispherectomy subjects showed more substantial after-effects when the non-paretic leg was the fast leg. Hence, the magnitude of after-effect in double support seems to depend on initial asymmetry and whether the paretic leg was adapted fast or slow. This suggests a ceiling effect that limits the range of possible double support asymmetry under experimental treadmill conditions.
Discussion
We have demonstrated that a complete lesion of one cerebral hemisphere does not impair many aspects of split-belt walking. All hemispherectomized subjects were able to use feedback to immediately change their locomotor pattern and maintain alternating stepping during split-belt walking. Hemispherectomy patients could also adapt feedforward control of spatial interlimb coordination, but some subjects had difficulty adapting temporal coordination of the legs.
Feedback control does not require cerebral mechanisms
Our results indicate that the ability to make quick, feedback-driven adjustments to inter-limb coordination in walking do not depend on cerebral mechanisms. This finding is consistent with other studies showing that spinalized cats can also quickly adjust the stance and swing time on each limb during split-belt walking (Forssberg et al., 1980). Moreover, human infants whose descending pathways are not fully myelinated (Yang et al., 2004) show reactive feedback adjustments during split-belt walking (Yang et al., 2005). Subjects with cerebellar damage can also make reactive feedback adjustments, even though they are impaired in feedforward control (Morton and Bastian, 2006). Hence, spinal circuits seem to be sufficient for making quick reactive feedback adjustments on the split-belt treadmill.
Cerebral versus cerebellar contributions to feedforward adaptation
Our previous studies have demonstrated that cerebellar lesions in adults impair the ability to adapt feedforward locomotor coordination (Morton and Bastian, 2006). In contrast, cerebral strokes in adults do not impair feedforward or feedback adaptive abilities (Reisman et al., 2007). In the current study, subjects with hemispherectomy showed partially disrupted feedforward adaptive ability (i.e. temporal but not spatial parameters) and intact feedback control.
There are at least two possible explanations for impaired adaptation of timing parameters in these subjects. First, cerebral structures may play a role in adapting the timing of locomotor activities. Studies of motor cortex in cats have suggested it has exclusive capability (over brainstem structures) to influence the timing of locomotor cycles (Rho et al., 1999; Bretzner and Drew, 2005). The adults that we previously studied had only partial lesions of cerebral motor areas, whereas the children and adolescents studied here had complete lesions. Therefore, we may not have seen the deficit in adults due to spared cerebral function.
Alternatively, the subjects with hemispherectomy might have crossed cerebellar degeneration, or diaschisis (i.e. metabolic depression, Baron et al., 1980). We would expect this to be more substantial in hemispherectomized subjects versus adults with stroke due to the difference in lesion size. In our prior work, cerebellar damage caused deficits in adaptation of both spatial and temporal parameters (Morton and Bastian, 2006). Thus, if secondary cerebellar damage were the mechanism, we would expect similar deficits; this did not occur. Anatomical studies would also predict crossed degeneration to be localized to the cerebellar hemisphere, whereas our previous study suggests that midline cerebellar structures are more crucial for split-belt adaptation (Morton and Bastian 2006). Taken together, these observations suggest that crossed cerebellar degeneration is not the cause of the deficit.
Separable feedforward adaptation of spatial and temporal walking parameters
Even though spatial and temporal variables in walking are inherently related, we found here that they can be recalibrated separately. Most hemispherectomy subjects could adapt spatial features, but had trouble adapting temporal coordination. One possibility is that there are distinct neural mechanisms for modulating spatial and temporal variables. There is evidence that locomotor cycle timing and cycle pattern (i.e. spatial features) are controlled by different interneuronal populations in the spinal cord (Koshland and Smith, 1989; Lafreniere-Roula and McCrea, 2005; McCrea and Rybak, 2008). While both the motor cortex and red nucleus have access to the circuits controlling the cycle structure, only the motor cortex has access to the circuits controlling cycle timing (Rho et al., 1999; Bretzner and Drew, 2005). This may explain why timing adaptation may require additional cerebral involvement.
The fact that hemispherectomy subjects had baseline asymmetries in temporal features (i.e. double support time, interlimb phase and stance time), but not in spatial features (i.e. step length and stride length) provides additional evidence that there are separate mechanisms for spatial verses temporal control. Given that the subjects had some time to recover after surgery, it seems that the nervous system is better at re-establishing spatial parameters than temporal parameters in walking. It is possible that spatial modification requires a simpler adjustment, such as gain modulation, while temporal modification requires more complicated processes involving cortical control.
It should also be noted that, we saw an unusual effect in adaptation of double support in hemispherectomy subjects. In the paretic slow condition, there was no perturbation (i.e. error) in double support symmetry from baseline to the split-belt condition, possibly due to a ceiling effect, and yet a small after-effect occurred post-adaptation (Fig. 4B). An after-effect resulting from small or no errors suggest that adaptation was not used to merely cancel ‘performance errors’ in double support symmetry. Recent work suggests that a sensory ‘prediction error’ might also be important for driving adaptation. This error is the difference between an internal prediction about the outcome of a motor command, versus the actual sensed outcome—in other words, did the body move where the brain thought it would (Miall et al., 1993; Krakauer and Shadmehr, 2007; Tseng et al., 2007). Therefore, one plausible explanation for this finding is that a sensory prediction error, rather than a performance error, drove adaptation. It is also possible that the error in another parameter (e.g. inter-limb phase) drove adaptation.
Table 2 summarizes cerebellum and cerebrum involvement in feedback control and feedforward adaptation during split-belt walking. Taken together, these findings suggest that intact cerebellar function is required to adapt spatial parameters; additional interactions between cerebellum and cerebral structures are involved to adapt timing. While we cannot rule out extensive plastic changes in many brain regions of these children and adolescents that may underlie the pattern and compensations observed, what is clear is that the structures remaining following hemispherectomy are not always capable of recalibrating and storing interlimb timing mechanisms for walking.
Table 2.
Summary of feedback control and feedforward adaptation during split-belt walking.
| Lesion | Feedback control |
Feedforward adaptation |
||
|---|---|---|---|---|
| Spatial (i.e. stride length) | Temporal (i.e. stance time) | Spatial (i.e. step length) | Temporal (i.e. interlimb phase) | |
| Cerebellum | Good | Good | Bad | Bad |
| Cerebrum (focal) | Good | Good | Good | Good |
| Cerebrum (entire hemisphere) | Good | Good | Good | Bad |
Comparison with adult stroke subjects
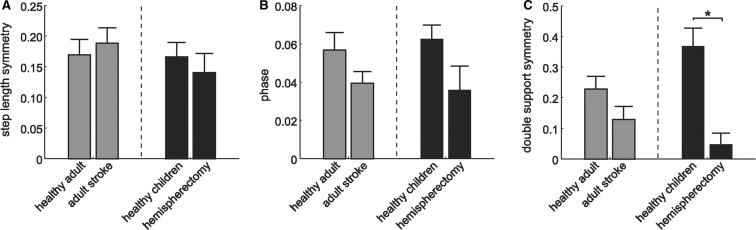
There are two key differences in the hemispherectomy subjects studied here and the stroke survivors studied in Reisman et al. (2007): lesion size and age at which the lesion occurred. One hypothesis is that hemispherectomy subjects would be more impaired because their lesion is bigger. An alternate hypothesis is that hemispherectomy subjects may have better recovery since the lesion occurred at an earlier age when the brain might be more plastic. Figure 6 shows a post hoc comparison of the after-effects in step length, double support and phasing from split-belt adaptation in adult stroke survivors (Reisman et al., 2007) and children and adolescents with hemispherectomy with their respective matched controls. Step length adaptation was intact in both stroke (P ≥ 0.5) and hemispherectomy (P ≥ 0.5) subjects compared with matched controls (Fig. 6A). The ability to adapt interlimb phasing was also largely intact in both stroke (P ≥ 0.5) and hemispherectomy (P = 0.2) subjects compared with controls (Fig. 6B). However, double support after-effects (Fig. 6C) were reduced in hemispherectomy subjects compared with control subjects (P < 0.001), while stroke subjects were comparable with healthy adults (P = 0.1). These results suggest that the retention of adaptive ability is worse in patients with larger cortical lesions, and that the overall younger age at the time of the lesion was not the more critical factor.
Figure 6.
Comparison between the after-effects from split-belt training in stroke and hemispherectomy subjects. After-effects in step length symmetry (A), interlimb phase (B) and double support symmetry (C) for stoke subjects (left) and hemispherectomy subjects (right) with their respective matched controls. Error bars indicate ± SE. *P < 0.05.
Clinical correlates and implications for functional recovery
We were somewhat surprised to find no correlation between sensorimotor functions (i.e. Fugl-Meyer scores, sensory thresholds) and locomotor adaptive function. This suggests that the mechanisms underlying recovery of voluntary movement and sensory perception are not related to those underlying locomotor recovery. Studies in stroke survivors have also found that the magnitude of after-effects from split-belt treadmill (Reisman et al., 2007) and visuomotor (Patton et al., 2006) adaptation were not correlated with the degree of clinical impairment. Interhemispheric cerebral reorganization has been associated with residual sensorimotor functions in some hemispherectomy subjects (Benecke et al., 1991; Holloway et al., 2000; Shimizu et al., 2000). Locomotor adaptive capabilities may instead depend more heavily on other circuits, such as the cerebellum and its targets (i.e. contra-lesion cerebral motor areas, brainstem motor areas).
Previous studies of children with hemispherectomy have suggested that etiology and (related) age at onset may influence the degree of residual function (de Bode et al., 2005). We found that locomotor adaptive ability is not different between stroke and Rasmussen groups, and is not linearly related to age at surgery or time since surgery. Typically, the basic kinematics and muscle activation patterns of walking emerge by 3 years of age and the adult pattern of coordination is complete by 7 years of age (Sutherland et al., 1980; Berger et al., 1984; Breniere and Bril, 1998; Okamoto et al., 2003). Hence, we wonder whether gait flexibility would be better if surgery is performed after the basic locomotor pattern has emerged, but while the nervous system is still in the process of fine-tuning the resultant adult pattern. In this study, the subjects who showed the largest after-effects (i.e. best learning) of temporal coordination were the ones who had the surgery between 4 and 6 years of age, which would agree with the above hypothesis. However, a bigger sample is needed to determine whether there is an optimal timing for surgery in terms of gait flexibility.
Limitations of the study
It is likely that neuronal reorganization influenced locomotor performance across hemispherectomy subjects. From this lesion study, we know what the nervous system cannot do after losing a cerebral hemisphere. However, we cannot definitively rule out cerebral contributions to the aspects of walking that showed no deficit, because other brain regions could have compensated for the lesion. It is also possible that our small sample size limited what differences we could see. Nonetheless, our results clearly show that the cerebrum is required for temporal control of walking at baseline and for adapting feedforward temporal control of gait.
We also expected variability in the way residual neural networks are re-configured given heterogeneity in the demographics of our patient population. Even within a diverse population, we found that all hemispherectomy subjects had intact feedback control, and spatial feedforward adaptation was comparable with controls. On the other hand, temporal feedforward control was impaired and also most variable across patients. From this study, we can conclude that the cerebrum is important for temporal feedfoward control. But, with a small sample from each subgroup, it is unclear how etiology, age at surgery, or time since surgery affects temporal feedforward control.
Conclusions
In sum, we showed that hemispherectomy subjects retain the flexibility to use feedback control in response to perturbations. They also have the ability to adapt feedforward control of spatial parameters after a period of training with sustained perturbations. Hence, these subjects could potentially benefit from split-belt treadmill training to correct for asymmetric step length patterns.
Funding
National Institutes of Health (R01 HD048740 to A.J.B.).
Acknowledgements
We would like to thank R. Bunoski, A. Torrie and N. Zamora for their excellent assistance with data collection.
References
- Armstrong DM. Supraspinal contributions to the initiation and control of locomotion in the cat. Prog Neurobiol. 1986;26:273–361. doi: 10.1016/0301-0082(86)90021-3. [DOI] [PubMed] [Google Scholar]
- Baron JC, Bousser MG, Comar D, Castaigne P. Crossed cerebellar diaschisis in human supratentorial brain infarction. Ann Neurol. 1980;8:128. [PubMed] [Google Scholar]
- Batschelet E. London: Academic Press; 1981. Circular Statistics in Biology. [Google Scholar]
- Benecke R, Meyer BU, Freund HJ. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp Brain Res. 1991;83:419–26. doi: 10.1007/BF00231167. [DOI] [PubMed] [Google Scholar]
- Berger W, Altenmueller E, Dietz V. Normal and impaired development of children's gait. Hum Neurobiol. 1984;3:163–70. [PubMed] [Google Scholar]
- Breniere Y, Bril B. Development of postural control of gravity forces in children during the first 5 years of walking. Exp Brain Res. 1998;121:255–62. doi: 10.1007/s002210050458. [DOI] [PubMed] [Google Scholar]
- Bretzner F, Drew T. Contribution of the motor cortex to the structure and the timing of hindlimb locomotion in the cat: a microstimulation study. J Neurophysiol. 2005;94:657–72. doi: 10.1152/jn.01245.2004. [DOI] [PubMed] [Google Scholar]
- Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10:1055–62. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- de Bode S, Firestine A, Mathern GW, Dobkin B. Residual motor control and cortical representations of function following hemispherectomy: effects of etiology. J Child Neurol. 2005;20:64–75. doi: 10.1177/08830738050200011101. [DOI] [PubMed] [Google Scholar]
- Drew T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J Neurophysiol. 1993;70:179–99. doi: 10.1152/jn.1993.70.1.179. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res Brain Res Rev. 2002;40:178–91. doi: 10.1016/s0165-0173(02)00200-x. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J, Rossignol S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand. 1980;108:283–95. doi: 10.1111/j.1748-1716.1980.tb06534.x. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Holloway V, Gadian DG, Vargha-Khadem F, Porter DA, Boyd SG, Connelly A. The reorganization of sensorimotor function in children after hemispherectomy. A functional MRI and somatosensory evoked potential study. Brain. 2000;123(Pt 12):2432–44. doi: 10.1093/brain/123.12.2432. [DOI] [PubMed] [Google Scholar]
- Koshland GF, Smith JL. Mutable and immutable features of paw-shake responses after hindlimb deafferentation in the cat. J Neurophysiol. 1989;62:162–73. doi: 10.1152/jn.1989.62.1.162. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Vining EP, Pillas DJ, Pyzik PL, Avellino AM, Carson BS, et al. Hemispherectomy for intractable unihemispheric epilepsy etiology vs outcome. Neurology. 2003;61:887–90. doi: 10.1212/01.wnl.0000090107.04681.5b. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Towards a computational neuropsychology of action. Prog Brain Res. 2007;165:383–94. doi: 10.1016/S0079-6123(06)65024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol. 2005;94:1120–32. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- Lam T, Anderschitz M, Dietz V. Contribution of feedback and feedforward strategies to locomotor adaptations. J Neurophysiol. 2006;95:766–73. doi: 10.1152/jn.00473.2005. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–46. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a smith predictor? J Mot Behav. 1993;25:203–16. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–16. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Okamoto K, Andrew PD. Electromyographic developmental changes in one individual from newborn stepping to mature walking. Gait Posture. 2003;17:18–27. doi: 10.1016/s0966-6362(02)00049-8. [DOI] [PubMed] [Google Scholar]
- Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp Brain Res. 2006;168:368–83. doi: 10.1007/s00221-005-0097-8. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Neural adaptation in the generation of rhythmic behavior. Annu Rev Physiol. 2000;62:723–53. doi: 10.1146/annurev.physiol.62.1.723. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–15. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–72. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho MJ, Lavoie S, Drew T. Effects of red nucleus microstimulation on the locomotor pattern and timing in the intact cat: a comparison with the motor cortex. J Neurophysiol. 1999;81:2297–315. doi: 10.1152/jn.1999.81.5.2297. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Philos Trans R Soc Lond B Biol Sci. 2006;361:1647–71. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Nariai T, Maehara T. Enhanced motor cortical excitability in the unaffected hemisphere after hemispherectomy. Neuroreport. 2000;11:3077–84. doi: 10.1097/00001756-200009280-00009. [DOI] [PubMed] [Google Scholar]
- Sutherland DH, Olshen R, Cooper L, Woo SL. The development of mature gait. J Bone Joint Surg Am. 1980;62:336–53. [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Halligan P. Development of a behavioral-test of visuospatial neglect. Arch Phys Med Rehab. 1987;68:98–102. [PubMed] [Google Scholar]
- Yang JF, Lam T, Pang MY, Lamont E, Musselman K, Seinen E. Infant stepping: a window to the behaviour of the human pattern generator for walking. Can J Physiol Pharmacol. 2004;82:662–74. doi: 10.1139/y04-070. [DOI] [PubMed] [Google Scholar]
- Yang JF, Lamont EV, Pang MY. Split-belt treadmill stepping in infants suggests autonomous pattern generators for the left and right leg in humans. J Neurosci. 2005;25:6869–76. doi: 10.1523/JNEUROSCI.1765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]