Abstract
Recent studies have shown that after partial spinal-cord lesion at the mid-cervical segment, the remaining pathways compensate for restoring finger dexterity; however, how they control hand/arm muscles has remained unclear. To elucidate the changes in dynamic properties of neural circuits connecting the motor cortex and hand/arm muscles, we investigated the cortico- and inter-muscular couplings of activities throughout the recovery period after the spinal-cord lesion. Activities of antagonist muscle pairs showed co-activation and oscillated coherently at frequencies of 30–46 Hz (γ-band) by 1-month post-lesion. Such γ-band inter-muscular coupling was not observed pre-lesion, but emerged and was strengthened and distributed over a wide range of hand/arm muscles along with the recovery. Neither the β-band (14–30 Hz) cortico-muscular coupling observed pre-lesion nor a γ-band oscillation was observed in the motor cortex post-lesion. We propose that a subcortical oscillator commonly recruits hand/arm muscles, via remaining pathways such as reticulospinal and/or propriospinal tracts, independent of cortical oscillation, and contributes to functional recovery.
Keywords: spinal cord injury, motor recovery, hand, rehabilitation, monkey
Introduction
Understanding the neural mechanisms of functional recovery after partial lesion of the CNS will contribute to establishing a roadmap of neuro-rehabilitational therapy for patients with brain damage or spinal cord injury. The macaque monkey models of brain/spinal cord injury are valuable, because the structures of their CNS and motor apparatus are similar to those of humans (Courtine et al., 2007). To date, Lawrence and Kuypers (1968) showed that after the bilateral pyramidotomy at the brain stem level, the monkeys could reach for an object, but the ability of precision grip was permanently impaired, which suggested the importance of the corticospinal tract (CST) in the control of dexterous finger movements. More recent studies using monkeys have shown that after spinal hemi-section at the upper or lower cervical level, the ability of grasping recovered, but dexterous finger movements resulted in rather poor recovery (Galea and Darian-Smith, 1997; Schmidlin et al., 2004; Freund et al., 2006). In contrast, dexterity of finger movements, such as precision grip and independent control of individual fingers, was found to recover within 1–3 months after lesion of the lateral corticospinal tract (l-CST) at the C4/C5 cervical spinal segments (Sasaki et al., 2004; Nishimura et al., 2007). In these animals, not only the direct cortico-motoneuronal (CM) pathway but also the rubrospinal pathway is considered to be damaged. Recent studies have shown the existence of disynaptic pathways from the motor cortex (M1) to hand/arm motoneurons via propriospinal and reticulospinal neurons (Alstermark et al., 1999; Sasaki et al., 2004; Isa et al., 2006). The strength and functional significance of these indirect pathways are still the topic of ongoing arguments (Alstermark and Isa, 2002; Lemon and Griffiths, 2005; Alstermark et al., 2007; Isa et al., 2007; Pettersson et al., 2007; Lemon, 2008), but these remaining indirect pathways are considered to be the candidates of the neural systems that take over the function of the direct CM pathway, in addition to the uncrossed corticospinal pathways, after the lesion of the l-CST. We have been using this animal model to clarify the neural mechanisms of functional compensation after the spinal-cord lesion (SCL). In a recent study, we investigated the role of cortical motor-related regions and found that the recovery was contributed by increased activity in the bilateral primary M1 at the early stage (around one month postoperatively) and expanded activation of the contralateral M1 and bilateral ventral premotor cortex (PMv) at later stages (after 3 months postoperatively) (Nishimura et al., 2007; Nishimura and Isa, in press). However, how the remaining neuronal systems drive the muscles participating in dexterous finger movements is still unclear. We hypothesized that, in addition to plastic changes of neural circuits in the spinal cord (Sasaki et al., 2004) and the cerebral cortex (Nishimura et al., 2007; Nishimura and Isa, in press), adaptive learning would occur in the functional coupling between the cortical or subcortical motor centers and motoneurons. In this study, to characterize changes in the dynamic properties of the neural network for the control of finger movements, we simultaneously recorded local field potentials (LFPs) in bilateral M1, PMv and S1, and electromyography (EMG) of various hand/arm muscles in two monkeys and analysed the cortico-muscular coupling (CMC) and inter-muscular coupling (IMC) during a force-tracking precision grip task both before the SCL and during the recovery course.
Methods
Subjects
Two monkeys (Macaca mulatta, Monkey Be: male 9.1 kg; Monkey Mu: male 5.7 kg) were used in this study. The experimental procedures were subjected to prior review by the ethical committee of the National Institute of Natural Sciences and performed in accordance with the NIH Guideline for the Care and Use of Laboratory Animals and the guideline of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Surgeries
All surgeries were performed under general anaesthesia, starting with a combination of ketamine (10 mg/kg, i.m.) and xylazine (1 mg/kg, i.m.) and followed by sodium pentobarbital (25 mg/kg, i.v.). Supplemental doses of anaesthetics were given as needed during surgery. Diclofenac sodium (Voltaren, Novartis, Tokyo) was routinely applied to the anus for analgesia after surgery.
Spinal cord injury
A full description of the method for transecting the l-CST has been published previously (Sasaki et al., 2004; Nishimura et al., 2007). Briefly, the border between the C4 and C5 spinal cord segments was exposed by laminectomy of the C3 and C4 vertebrae, and a transverse opening was made in the dura mater. The l-CST lesion was made under a surgical microscope using fine forceps. The dorsal part of the lateral funiculus was transected from the dorsal root entry zone ventrally to the convexity of the spinal cord. Then the lesion was extended ventrally at the most lateral part of the lateral funiculus. The opening of the dura mater was closed, and the skin and back muscles were sutured with nylon or silk threads. Both monkeys received the l-CST lesion on left side.
Surgery for LFP recording
The skull over the bilateral frontal cortices was widely exposed for recording LFPs in bilateral M1, PMv and S1. After partial removal of the skull, the cortex around the precentral gyrus was exposed bilaterally, and a chamber was attached to cover the opening on the right hemisphere (contralateral to the lesion). Movable electrodes were chronically implanted in the digit, wrist and arm areas of the M1 and the digit area of the PMv on the left hemisphere (ipsilateral to the lesion). Small titanium-steel screws were attached to the skull as anchors. Two stainless-steel tubes were mounted in parallel over the frontal and occipital lobes for head fixation. The chamber and stainless steel tubes were fixed to the screws with acrylic resin.
Surgery for EMG recording
EMG activities of the left forelimb muscles were recorded through chronically implanted pairs of multi-stranded stainless steel wires (Cooner Wire, Chatsworth, CA, USA), which were subcutaneously tunneled to their target muscles. Circular connectors (MCP-12, Omnetics, Minneapolis, MN, USA) were anchored to the skull.
Behavioural test for evaluation of functional recovery
To assess functional recovery of dexterous finger movements before and after transection of the l-CST, the monkeys were trained to reach, grasp and retrieve a small piece of sweet potato (about 7 mm cubic) through a narrow vertical slit using both the index finger and thumb. The food piece was positioned in the centre of a vertical slit, and the success ratio of food retrieval from the pin was evaluated as indicating dexterity of the finger control. A success trial was defined as any trial that resulted in the successful precision grip and removal of the food from the pin without dropping it. Each session consisted of 30 trials.
Behavioural task for electrophysiological study
Monkeys were trained to perform the force-tracking precision grip task (Fig. 1) for juice reward with the left hand, which is ipsilateral side to the lesion. The task required two independent levers to be pinched between the index finger and the thumb and held within their respective, electronically defined isometric force windows. Both levers had to be held correctly for 3.5 s, after which a tone signalled that the levers could be released for the animals to obtain a reward. Monkeys were over-trained in this task for longer than 6 months before lesion.
Figure 1.
An example of unprocessed recordings of local field potentials (LFPs) from hand territory of primary motor cortex in both hemispheres and rectified EMGs in a variety of forearm muscles in ipsilesional side, together with the force applied to the levers by the index finger and thumb during the force-tracking precision grip task. Note that oscillatory activity in the LFP appears in both hemispheres during the hold phase. Data were obtained in Monkey Mu before lesion. coM1 = primary motor cortex in the contralesional hemisphere; ipM1 = primary motor cortex in the ipsilesional hemisphere.
Rehabilitative training
During postoperative days 1–14, the monkeys were trained intensively every day with manual assistance of movements for both the force-tracking precision grip task and reach-grasp (food retrieval) task. Usually, the intact hand was restricted, not to be used. The daily session lasted for 30 min in the force-tracking precision grip task and 30 min in food retrieval task. From postoperative day 15 to the last day of recording session (approximately 3 months postoperative), the animal was trained every week day. During this stage, both monkeys could produce enough force in the force-tracking precision grip task and therefore were trained mainly with this task. The daily session lasted until the monkey stopped the task. It lasted for 0.5–2 h and consisted of about 100–1000 successful trials.
Data collection
The cortical LFPs were recorded through glass-coated Elgiloy electrodes (0.9–1.4 MΩ at 1 kHz) in the contralesional hemisphere and with chronically implanted electrodes (polyurethane-coated tungsten electrode, 0.8–1.2 MΩ at 1 kHz, Unique Medical, Osaka, Japan) in the ipsilesional hemisphere. For the contralesional hemisphere, electrode penetrations were made systematically in precentral and postcentral gyri at 1-mm grid intervals. Somatotopic representation in the M1 and S1 was defined with intracortical microstimulation technique and neuronal responses to tactile stimulation. Electrode penetrations covered the digit and wrist areas of M1 and S1, and lateral part of the arm area and medial part of face area were also covered. Electrode penetrations were also made in the convexity and bank of the PMv. One to five electrode penetrations were made per experiment on each day. Following preamplification, the signals from each electrode were filtered at 5–500 Hz. EMGs were recorded from selected forearm muscles. In Monkey Be, EMGs were recorded from two intrinsic hand muscles: adductor pollicis (ADP) and first dorsal interosseus (FDI); four digit muscles: extensor digitorum communis (EDC), extensor digitorum 2 and 3 (ED23), extensor digitorum 4 and 5 (ED45) and flexor digitorum superficialis (FDS); three wrist muscles: flexor carpi radialis (FCR), flexor carpi ulnaris (FCU) and palmaris longus (PL); and one elbow muscle: biceps brachii (BB). In Monkey Mu, EMGs were recorded from two intrinsic hand muscles: ADP and FDI; three digit muscles: EDC, ED23, FDS; two wrist muscles: FCU and extensor carpi ulnaris (ECU); and one elbow muscle: BB. EMG signals were amplified, filtered (5 Hz to 3 kHz) and rectified. All signals (LFPs, EMGs and finger forces) were sampled at 4 kHz and recorded through a 1401 A-D converter (Cambridge Electronic Design, Cambridge, UK) onto a computer using Spike2 software (Cambridge Electronic Design, Cambridge, UK).
Analysis of functional coupling
For coherence and cross-correlation analyses of LFP and EMG signals, a Spike2 software script was used. Coherence and cross-correlation estimates for CMC and IMC (Figs 3 and 5) were calculated during the hold phase of force production. Coherence and power spectra were calculated by averaging across segments, using non-overlapping segments composed of 2048 sample points. CMC or IMC was considered significant when it was >95% confidence limits computed from the number of epochs as described elsewhere (Halliday et al., 1995). As 240 epoch data were used for calculating coherence in this study, 95% confidence limit was 0.0125, which was indicated with a grey horizontal line in each coherence plot. The penetration track with the highest CMC was selected for further analyses. Cross-correlation estimates of long-term synchronization in Fig. 4 were calculated from 0.5 s before the increase of thumb force to the end of force production. We excluded records from muscle pairs demonstrating cross-talk between the EMG records as evidenced by high, narrow peaks in the cross-correlogram at the zero-lag time and a high degree of coherence at all frequencies. In Monkey Be, the cross-talk was detected from a muscle pair, ADP-FDI in all the recording sessions. In Monkey Mu, the cross-talk was detected from muscle pair ADP-FDI during all the recording sessions, ED23-FCU, ED23-BB, ADP-FCU on postoperative day 9, ED23-FCU, ED23-BB, ADP-FDS and ADP-BB on postoperative day 13. These data were not used for further analysis.
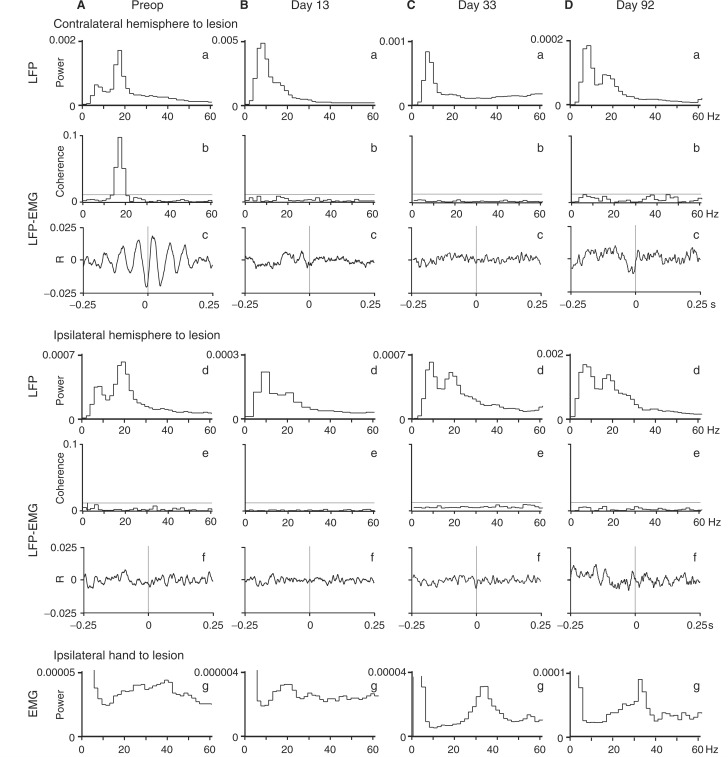
Figure 3.
Cortico-muscular coupling recorded at various times before and after the l-CST lesion. (A) preoperatively; (B) postoperative day 13; (C) postoperative day 33; (D) postoperative day 92. (a) Power spectra of LFP in contralesional M1 hand area. (b) Coherence between LFP in contralesional M1 hand area and EMG of ADP in ipsilesional side. (c) Cross-correlogram between LFP in contralesional M1 hand area and EMG of ADP in ipsilesional side. (d) Power spectra of LFP in ipsilesional M1 hand area. (e) Coherence between LFP in ipsilesional M1 hand area and EMG of ADP in ipsilesional side. (f) cross-correlogram between LFP in ipsilesional M1 hand area and EMG of ADP in ipsilesional side. (g) Power spectra of EMG of ADP in ipsilesional side. Coherence and cross-correlation estimates in this figure were calculated during the hold phase of force production. In the coherence plots of (a) and (d), the grey horizontal lines represent the 95% confidence limit of 0.0125. The vertical grey lines in the cross-correlograms represent the zero-lag time. Data in this figure were obtained in Monkey Mu.
Figure 5.
Oscillatory coupling of muscle pair (ED23 and ADP) activities recorded at various times before and after the l-CST lesion. (A–D) preoperatively (A), postoperative day 14 (B), postoperative day 34 (C) and postoperative day 92 (D). (a) The rectified EMG activity from ED23 (top) and ADP (bottom). Data obtained during the hold phase of precision grip in Monkey Be. (b and c) power spectra of ED23 and ADP, respectively. (d) Coherence between ED23 and ADP. In the coherence plots, the grey horizontal lines represent the 95% confidence limit (0.0125). (e) Cross-correlograms between ED23 and ADP. In cross-correlograms, the y-axes on the right and left sides indicate the absolute and relative values, respectively. The grey vertical lines in the cross-correlograms represent the zero-lag time. (E and F) Changes in the 30–46-Hz IMC during recovery in Monkey Be (E) and Monkey Mu (F). (a) Coupling between ED23 and a variety of other muscles (see inset). (b) Coupling between ADP and a variety of other muscles (see inset). n.s. in (a) and (b) indicates coherence values that were not significant. The grey horizontal lines in (a) and (b) represent the 95% confidence limit of coherence.
Figure 4.
Long-term synchronization of muscles (BB, FCR, ED23 and ADP) recorded at various times before and after the l-CST lesion. Data were obtained from 0.5 s before the increase of force in the thumb to the end of force production. (A–D) From preoperatively (A), postoperative day 14 (B), postoperative day 34 (C) and postoperative day 92 (D). (a) the EMG activity from BB (top), FCR (second row), ED23 (third row), ADP (forth row) and force trajectory of thumb (bottom row). (b) Cross-correlograms between ED23 and ADP. The grey vertical lines represent zero-lag time in the cross-correlograms. These data were obtained from Monkey Be. (E and F) Changes in long-term synchronization of muscle pairs during recovery in Monkey Be (E) and Monkey Mu (F). Correlation coefficients of the activities of muscle pairs at zero-lag time, indicated with vertical grey lines in each cross-correlograms, are plotted. (a) Correlations between ED23 activity and that of a variety of other muscles (see inset). (b) Correlations between ADP activity and that of a variety of other muscles (see inset).
Electrophysiological assessment of the lesion extent
After the behavioural sessions were terminated, the extent of CST lesion was estimated in the acute electrophysiological experiment under anaesthesia. Details of the experimental arrangement have been described elsewhere (Sasaki et al., 2004; Nishimura et al., 2007). In brief, the animals were first anaesthetized with intramuscular injection of xylazine and ketamine and then anesthetized with inhalation of isoflurane (1.0–2.0%) during surgery. During the recording session, they were anaesthetized with intravenous injection of α-chloralose (75–100 mg/kg), paralyzed with pancuronium bromide and artificially ventilated. The depth of anaesthesia was routinely checked by papillary reflex. The brain stem pyramid was stimulated by Tungsten needle electrodes on both sides, and conduction volley of the CST was recorded by the cord dorsum potential at the cervical segments with silver ball electrodes and by monopolar recordings from the spinal halves at the lower thoracic segment (Th12). In addition, intraspinal field potentials were recorded with a glass microelectrode (2 M K-citrate, impedance 3–7 MΩ), especially from the motor nucleus innervating the deep radial (DR) nerve, where amplitude of the antidromic field potential induced by peripheral stimulation of the DR nerve exceeded 0.5 mV. In the present experiments, the electrophysiological assessment was successfully conducted only in Monkey Mu, but not in Monkey Be, because of failure in the control of good systemic condition of the animal during the acute experiment.
Histological assessment of lesion
At the end of the experiments, the monkeys were deeply anaesthetized with an overdose of sodium pentobarbital (>70 mg/kg) and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) pH 7.3. The spinal cords were removed immediately, saturated with 30% sucrose in 0.1 M PBS (pH 7.3) and then cut serially into 50 µm-thick coronal sections on a freezing microtome. The sections were either processed with Klüver-Barrera Nissl-staining with 1% Cresyl Violet or immunostained with anti-calmodulin-dependent protein kinase IIα (CaMKIIα) antibody (Affinity BioReagents, Golden, CO, USA) according to the manufacturer's instructions to examine the existence of remaining CST fibres.
Results
Extent of the spinal cord lesion
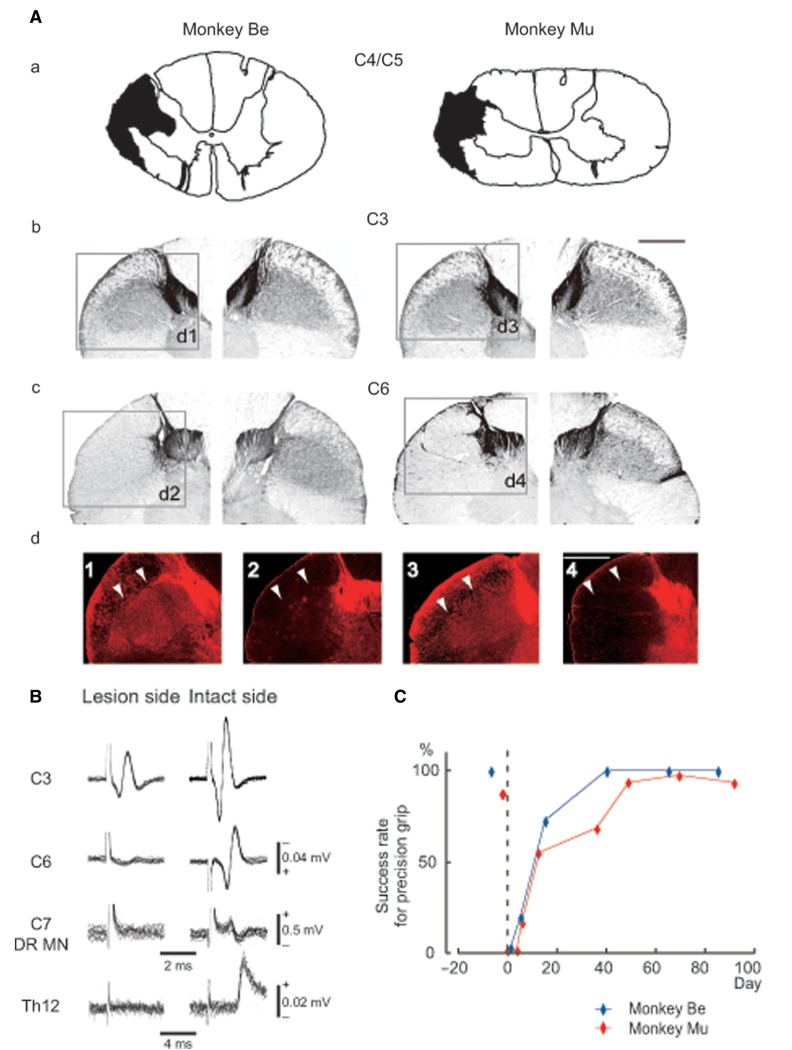
Figure 2Aa shows the extent of the SCLs at the C4/C5 border in the two monkeys. Figure 2Ab and Ac indicates normal distribution of the l-CST in sections rostral (C3, Fig. 2Ab, d1 and d3) and caudal (C6, Fig. 2Ac) to the lesion, revealed by CaMKIIα immunohistochemistry. Lesions in both monkeys appeared to encompass the normal distribution of the l-CST (compare the blackened areas in Fig. 2Aa with the areas where labelled axons are distributed in Fig. 2Ab on both sides and only on the right side in Fig. 2Ac). CaMKIIα immunohistochemistry demonstrated robust staining of immunopositive axons rostral to lesion on both sides (C3, Fig. 2Ab, d1 and d3), whereas no CaMKIIα-positive axons caudal to lesion on the lesioned side (C6, Fig. 2Ac, d2 and d4). Thus, lesion of the l-CST was regarded as complete in both the monkeys.
Figure 2.
Extent of spinal cord lesion and recovery of hand dexterity. (A, a) Drawings of the C4/C5 segments showing the extent of the l-CST lesion (labelled in black) in two monkeys. (b) CaMKIIα staining of spinal sections rostral to the lesion site (C3) visualized by diaminobenzidine. A scale bar in the inset indicates 1 mm. (c) CaMKIIα staining of spinal sections caudal to the lesion site (C6). Because CaMKIIα is known to be transported in CST axons, absence of labelled axons caudal to the lesion site indicates a complete lesion of unilateral l-CST. (d1—4) CaMKIIα immunostaining of the areas indicated in Ab and Ac in the adjacent sections of Ab and Ac, visualized by Alexafluor 555. White arrows indicate the CST rostral to lesion (d1and d3) and loss of the CST caudal to lesion (d2 and d4). Scale bars indicate 1 mm. (B) Cord dorsum potentials at the C3 (top row) and C6 (second row) segments, field potentials in the DR motor nucleus at the C7 segments (third row) and conduction volleys recorded in the spinal halves at the Th12 segment (monopolar recordings, bottom row), in response to the electrical stimulation of the medullary pyramid on the contralateral side to the recording at 200 μA (pulse width 100 μs), respectively. Recordings from the lesion side (left) of the spinal cord are indicated on the left column and those from the intact side (right) of the spinal cord are indicated on the right column. (C) Recovery time course of hand dexterity in two monkeys. Performance of precision grip was plotted against the time after lesion.
Completeness of lesion was also confirmed in electrophysiological experiments in Monkey Mu, by observing loss of the corticospinal volley in the cord dorsum potential recordings at the C6 segment, which is caudal to lesion, and in the spinal halves at the Th12 segment and loss of monosynaptic field potential in the DR motor nucleus at C7 in response to the electrical stimulation of the brain stem pyramid (Fig. 2B).
Recovery of finger dexterity
Independent control of digits and performance of precision grip showed deficits shortly after the lesion. In both the monkeys, the success ratio of precision grip dropped to zero on the first day; however, performance in the food retrieval task gradually recovered and their success ratio reached nearly 100% approximately 5–7 weeks after the lesions (Fig. 2C) as shown in previous studies (Sasaki et al., 2004; Nishimura et al., 2007).
Cortico-muscular coupling
LFPs in bilateral M1 hand area and EMGs of various hand/arm muscles were simultaneously recorded during a preoperative force-tracking precision grip task and throughout the recovery (Fig. 1). In Monkey Mu, Fourier analysis revealed that the LFPs in the hand area of bilateral M1 included peaks of power at both α- (8–13 Hz) and β- (14–30 Hz) frequency bands (Fig. 3Aa, d) preoperatively. The peak at the β-band component was higher than that at the α-band in both hemispheres. During this time, the EMG activity of an intrinsic hand muscle (ADP) contained frequency components over a broad range (Fig. 3Ag). Coherence analysis of the LFP and the EMG showed clear CMC between the contralateral M1 and ADP at the β-band with a peak at 17 Hz (Fig. 3Ab, c), whereas no coherence was found between the ipsilateral M1 and ADP (Fig. 3Ae, f). During the first month after the l-CST lesion, the β-band oscillatory component of the LFP in M1 decreased, and the α-band component predominated instead (Fig. 3Ba, Ca). But by postoperative day 92, the β-band oscillation recovered (Fig. 3Da, d) on both sides. The EMG activities of ADP started showing oscillatory activity around 33 Hz, which is in γ-band range during recovery (Fig. 3Cg, Dg); however, the CMC disappeared completely and did not recover at all by postoperative day 92 (Fig. 3Bb, Cb, Db), when the ability of precision grip had already recovered (Fig. 2C). By comparison, in Monkey Be, although the M1 LFP showed oscillations at both the α- and β-bands before the lesion and during recovery (data not shown), no coupling was observed between LFP and EMG activity before lesion, as previously reported in some healthy human subjects (Kilner et al., 2000; Baker and Baker, 2003) and never emerged during recovery.
Inter-muscular coupling
We then analysed the activation patterns of various arm/hand muscles during the force-tracking precision grip task. Figure 4 shows the EMG activity of BB (elbow flexor), FCR (wrist flexor), ED23 (an antagonist of the ADP) and ADP (a primary mover of the precision grip) in Monkey Be. Preoperatively, ED23 was activated just before the grip, and ADP was activated during the grip. Thus, these muscles showed reciprocal activation (Fig. 4Aa) and as a consequence, long-term synchrony (co-activation) was not observed, as evidenced by the absence of a positive correlation peak at the zero-lag time in the cross-correlogram (Fig. 4Ab). However, on postoperative day 14, these two muscles started showing co-activation (Fig. 4Ba), which resulted in the appearance of a positive broad correlation peak at the zero-lag time (Fig. 4Bb). The co-activation became more evident on postoperative day 34 (Fig. 4Ca), as evidenced by an increase in the positive correlation peak at the zero-lag time (Fig. 4Cb). On postoperative day 92, the positive correlation peak was slightly smaller, but still prominent (Fig. 4Db). These results indicate that muscle pairs that were antagonistic preoperatively became agonistic, developing long-term synchrony during the recovery. Correlation coefficients between each muscle pair at the zero-lag time, indicated by a vertical grey line in individual cross-correlograms, were plotted in Fig. 4E and F. These indicate long-term synchrony of the ED23 (Fig. 4Ea and 4Fa) and ADP (Fig. 4Eb and Fb) muscles with other hand/arm muscles, respectively, during the recovery course. Preoperatively, these correlation coefficients of muscle pairs varied considerably from negative to positive values. In Monkey Be, most of the correlation coefficients increased during the first month and then those of some pairs increased or decreased slightly later (Fig. 4Ea, b). In Monkey Mu, most correlation coefficients between muscles paired with ED23 and with ADP increased during the first or second postoperative month, respectively, but decreased slightly later (Fig. 4Fa, b). Correlation coefficients between all muscle pairs were positive after lesion in both monkeys. Thus, the co-activation pattern of antagonist muscles was widespread among various muscle pairs over both proximal and distal joints of the upper extremity.
In addition to the postoperative emergence of long-term synchrony, the EMG activities of muscles ADP and ED23 also became oscillatory and coherent. Prior to spinal cord lesions, the EMG activity of the ED23 muscle contained frequency components over a broad range (Fig. 5Ab) and that of the ADP had some small peaks at specific frequencies (Fig. 5Ac) of the power spectra, but there was no peak for coherence above the β frequency (Fig. 5Ad, e). By postoperative day 14, however, both ADP and ED23 started showing oscillatory activity at around 30 Hz (Fig. 5Ba, b, c), although there was no coherence between them at this stage (Fig. 5Bd, e). Then on postoperative day 34–92, the 30-Hz oscillatory activities of these muscles were coupled (Fig. 5C and D), and the coherence between them increased further (Fig. 5Cd, e and 5Dd, e). Such IMC was widespread, occurring among a variety of hand/arm muscles, and not only between synergistic muscle pairs but also between antagonistic muscle pairs and between muscle pairs of various joints, including distal and proximal ones (Fig. 5E and F). The peak frequency of coherence was detected at 33.24 ± 0.34 Hz (mean ± SD; range, 30–46 Hz). In Monkey Be, the coupling between most muscle pairs continuously increased through postoperative day 92 and decreased in only two pairs (Fig. 5Ea, b). In Monkey Mu, by contrast, the coupling between most muscle pairs increased for some time during the first postoperative 1–2 months, but then decreased during the third month. Only the coupling between the ADP and ED23 muscles maintained a high degree of coherency throughout the recovery (Fig. 5Fa, b). Such γ-band oscillatory LFP activities and coherence with muscle activities were not found in either the contra- or ipsilesional M1 (see Fig. 3B–Da, b and d, e), or the contralesional PMv or S1 (data not shown). Cortical activity displayed oscillatory components at either the α-band (8–13 Hz) or β-band (14–30 Hz) frequencies; both are lower than 30 Hz. Therefore, the IMC at frequencies of 30–46 Hz, which emerged during the course of recovery, was highly likely independent of cortical oscillation.
Discussion
The present results revealed that γ-band IMC, independent of cortical activity, was gradually strengthened and distributed over arm and hand muscles throughout the recovery of finger dexterity after spinal cord injury, which completely transected the direct CM connection at the mid-cervical spinal segment. This result implies that this adaptive change in oscillatory coupling between subcortical structures and spinal motoneuron pools of hand/arm muscles, bypassing SCL via the remaining subcortical pathways, contributes to functional recovery after spinal cord injury.
In a previous study, we demonstrated a time-dependent change in activation of cortical networks during the functional recovery of finger dexterity after lesion of the l-CST (Nishimura et al., 2007; Nishimura and Isa, 2009). However, because we used brain imaging and reversible inactivation techniques, both of which have low temporal resolution, changes in the dynamic properties of the neural circuits were not visible. In this study, electrophysiological analyses were used to clarify the change in dynamic properties of the neural networks connecting the motor-related cortices and hand/arm muscles. Cross-correlation and coherence analyses of EMG and cortical LFPs provide valuable information on the organization of networks responsible for driving spinal motoneurons during voluntary movement (Farmer et al., 1993a, b; Halliday et al., 1998; Hansen et al., 2005).
Previous studies showed that oscillations in the motor cortex of monkeys and humans are dominated by activity in the frequency range of 15–30 Hz (Murthy and Fetz, 1992; Sanes and Donoghue, 1993; Conway et al., 1995; Murthy and Fetz, 1996a, b; Baker et al., 1997; Halliday et al., 1998; Hari and Salenius, 1999; Kilner et al., 2000; Baker and Baker, 2003). Moreover, coherence was detected between cortical and muscle activities with a peak at 15–30 Hz in humans using electro- and magnetoencephalography (Conway et al., 1995; Salenius, 1997; Halliday et al., 1998; Hari and Salenius, 1999; Kilner et al., 1999, 2000; Gross et al., 2000; Mima et al., 2000, 2001; Baker and Baker, 2003) and in monkeys using LFP recordings (Baker et al., 1997), especially during the hold phase of tasks. It was proposed that such CMC is mediated by the monosynaptic CM pathway, which has a particularly pronounced influence over distal limb function (Farmer et al., 1993a, b). In support of this assumption, phase lags between cortex and muscle were consistent with conduction over the fast corticospinal pathway (Gross et al., 2000; Mima et al., 2000), and additionally, the coupling was reduced in patients with internal capsule lesions (Mima et al., 2001; Braun et al., 2007). However, in these studies, it was not clear whether the direct CM connection was required for generating the coherence, or the coherence could be mediated by indirect CM pathways via subcortical or spinal interneuronal systems, because in the patients with internal capsule lesions, most of the indirect CM pathways, mediated via those interneurons, were also damaged. The present study demonstrated that after lesions of the l-CST at the C4/C5 segments, the CMC did not recover, even though the monkeys showed prominent recovery of precision grip, and β-band oscillation recovered in the cortical LFP (Fig. 3D). A large proportion of indirect CM pathways, except for those mediated by segmental interneurons, remained intact in the present preparation (Sasaki et al., 2004; Isa et al., 2006; Nishimura et al., 2007). Therefore, the results suggest that recovery of finger dexterity is not related to β-band CMC. On the other hand, the present results also suggest that the direct CM connection is necessary for the generation of the coherence as previously proposed (Mima et al., 2000, 2001; Jackson et al., 2002; Hansen and Nielsen, 2004; Braun et al., 2007). However, it was also proposed that feedback from peripheral afferents is involved in generating the β-band CMC (Baker et al., 2006; Riddle and Baker, 2005). Therefore, the possibility cannot be excluded that disappearance of this coupling after the l-CST lesion was due to the interruption of the sensory-motor loop between the periphery and spinal motoneurons via motor cortices or loss of the cortical control of sensory feedback at the level of the lower cervical spinal cord.
Even preoperatively, Monkey Be did not display CMC at any frequency band. This is not surprising, as previous studies reported that such coupling was significantly smaller when the task was performed under an isometric condition than under a compliant condition in which subjects moved the levers against a spring-like load (Kilner et al., 2000); moreover, some healthy human subjects do not exhibit such coupling even under compliant conditions (Baker and Baker, 2003).
During the recovery period, both monkeys showed marked changes in the motor strategy for precision grip. Apparently, they could control their fingers independently and became successful in precision grip with just the index finger and thumb, while other fingers were kept flexed, as previously reported (Sasaki et al., 2004; Nishimura et al., 2007). However, as the recovery progressed, preoperatively antagonist muscles started working as agonists, showing long-term synchrony indicative of co-activation of a variety of forearm muscles, from proximal to distal. Such a strategy to increase stiffness might play a role during the learning of novel motor strategies in intact subjects (Osu et al., 2002).
Surprisingly, as the recovery progressed, the coupling between pairs of a variety of hand/arm muscles increased, with a coherence peak at 30–46 Hz, which corresponds to the γ-band. This was a robust finding, observed in both Monkey Be and Mu. In addition, although not reported in this study, we observed IMC in another monkey (Monkey S in a previous report, Nishimura et al., 2007) later than 3 months postoperatively during a reaching and grasping task that did not include a static phase, as in the present experiments. The β-band coherence (15–30 Hz) of EMG activity between pairs of muscles was reported previously and thought to originate from the cerebral cortex (Farmer et al., 1993a, b; Kilner et al., 1999; Hansen et al., 2005). This assumption derived from the observation that β-band coherence between muscle pairs was reduced in patients with internal capsule lesions (Fermer et al., 1993b) and spinal cord injury (Hansen et al., 2005), suggesting that pyramidal tract activity is involved in its generation. The 24–40-Hz IMC in antagonist muscle pairs has not been shown in normal subjects, but was observed in patients with incomplete injury of the CST (Norton and Gorassini, 2006). This finding is similar to that of the present study, but the authors’ interpretation of how the coupling emerges is different. They suggested that because the coherence value for the coupling was correlated with the amplitude of corticospinal conduction (assessed from motor evoked potential in response to transcranial magnetic stimulation) the 24–40-Hz coupling represented an increase in a common corticospinal drive to the antagonist muscle pair (Thomas and Gorassini, 2005; Norton and Gorassini, 2006). However, the frequency of such a 24–40-Hz IMC in patients with spinal cord injury appears to be higher than either the classical β-band CMC (Conway et al., 1995; Baker et al., 1997; Salenius, 1997; Halliday et al., 1998; Hari and Salenius, 1999; Kilner et al., 1999, 2000; Gross et al., 2000; Mima et al., 2000) or cortical oscillation (Murthy and Fetz, 1992; Sane and Donoghue, 1993; Conway et al., 1995; Murthy and Fetz, 1996a, b; Baker et al., 1997; Halliday et al., 1998; Hari and Salenius, 1999). This study demonstrates that the γ-band (30–46 Hz) IMC in both agonist and antagonist muscle pairs was generated in monkeys in which the direct CM connection was completely transected. Moreover, the coherence increased along with the functional recovery. In addition, after the recovery, the preoperatively recorded cortical oscillation at around 17 Hz was not coupled with EMG activity. It has also been reported that γ-band CMC was observed in normal subjects during static maximum voluntary contraction or phasic movements (Salenius et al., 1996; Brown et al., 1998; Marsden et al., 2000; Omlor et al., 2007), which suggested that γ-band oscillatory activity in muscles is actually driven by the oscillatory activity in M1. However, the cortical oscillation and CMC at γ-band frequency were not observed in both monkeys in the present study. As described in the ‘Methods’ section, LFP recordings were systematically performed in contralesional M1, PMv and S1 and ipsilesional M1, which indicated that this was the case not only for the contralateral M1, but also for the ipsilateral M1, and the contralateral PMv and S1.
On the basis of these findings, as shown in Fig. 6, we propose that the γ-band-IMC, which emerges during recovery, represents an increasing common drive from subcortical neuronal systems and/or peripheral afferents to various spinal motoneuron pools. The neuronal substrate of such a ‘common subcortical oscillator’ is not clear at this moment. The interneuronal systems mediating indirect CM drive and/or reflex afferents from the periphery, which remained after the lesion, may possibly contribute to its generation. Because the coherence appears only when movements are voluntarily driven, the common subcortical oscillator might also be driven by descending inputs from the cerebral cortex and/or by peripheral feedback associated with the movement. They might include (i) propriospinal neurons with cell bodies in the C3–C4 segments and with axons passing through the ventral part of the lateral funiculus towards hand/arm muscle motoneurons (Alstermark et al., 1999; Isa et al., 2006), (ii) reticulospinal neurons that control both proximal and distal hand/arm muscles (Davidson and Buford, 2006; Davidson et al., 2007) and (iii) the subcortical reflex pathways through the brainstem or spinal cord.
Figure 6.

Schematic illustrations of the proposed mechanisms underlying functional recovery after the l-CST lesion, proposed from the present results. (A) In the intact state, a direct CM connection (black) or peripheral feedback (green) contributes to generate the cortico-muscular coupling at the frequency of the β-band. (B) During the recovery from l-CST lesion, subcortical neural systems (red) that mediate cortical command or peripheral feedback (green) to motoneurons might be involved in generating the 30–46-Hz IMC that emerged in a variety of hand/arm muscles. Dotted lines indicate polysynaptic connections.
Conclusion
Finally, we conclude that after spinal cord injury, reorganization of neuronal circuits occur in specific neuronal networks, including not only those in the spinal cord (Sasaki et al., 2004) and cerebral cortex (Nishimura et al., 2007; Nishimura and Isa, 2009), but also remaining subcortical descending pathways downstream of cerebral cortex, presumably reticulospinal (Davidson and Buford, 2006; Davidson et al., 2007) and/or propriospinal pathways (Alstermark et al., 1999; Isa et al., 2006), contributes for functional recovery, and its oscillation and coupling with motoneruons provide a beneficial means for the limited neuronal resources available to effectively compensate for the motor ability.
Funding
Core Research for Evolutional Science and Technology; Japan Science and Technology Agency; Ministry of Education, Culture, Sports, Science and Technology of Japan (the grant for higher priority area—the Integrative Brain Research—project No. 18200027).
Acknowledgements
We thank Masahiro Mori and Kaoru Isa for technical assistance and Junichi Ushiba for assistance in experimental setup.
Glossary
Abbreviations:
- ADP
adductor pollicis
- BB
biceps brachii
- CaMKIIα
calmodulin-dependent protein kinase IIα
- CM
cortico-motoneuronal
- CMC
cortico-muscular coupling
- coM1
primary motor cortex in the contralesional hemisphere
- CST
corticospinal tract
- DR
deep radial
- ECU
extensor carpi ulnaris
- ED23
extensor digitorum 2 and 3
- ED45
extensor digitorum 4 and 5
- EDC
extensor digitorum communis
- EMG
electromyography
- FCR
flexor carpi radialis
- FCU
flexor carpi ulnaris
- FDI
first dorsal interosseus
- FDS
flexor digitorum superficialis
- IMC
inter-muscular coupling
- l-CST
lateral corticospinal tract
- ipM1
primary motor cortex in the ipsilesional hemisphere
- LFPs
local field potentials
- MI
motor cortex
- PL
palmaris longus
- PMv
ventral premotor cortex
- SCL
spinal-cord lesion
- Th12
12th thoracic segment
References
- Alstermark B, Isa T. Premotoneuronal and direct corticomotoneuronal control in the cat and macaque monkey. Adv Exp Med Biol. 2002;508:281–97. doi: 10.1007/978-1-4615-0713-0_34. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Ohki Y, Saito Y. Disynaptic pyramidal excitation in forelimb motoneurons mediated via C3-C4 propriospinal neurons in the Macaca fuscata. J Neurophysiol. 1999;82:3580–5. doi: 10.1152/jn.1999.82.6.3580. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Pettersson LG, Sasaki S. The C3-C4 propriospinal system in the cat and monkey: a spinal pre-motoneuronal centre for voluntary motor control. Acta Physiol. 2007;189:123–40. doi: 10.1111/j.1748-1716.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- Baker MR, Baker SN. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studies in man. J Physiol. 2003;546:931–42. doi: 10.1113/jphysiol.2002.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Chie M, Fetz EE. Afferent encoding of central oscillations in the monkey arm. J Neurophysiol. 2006;95:3904–10. doi: 10.1152/jn.01106.2005. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol. 1997;501:225–41. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C, Staudt M, Schmitt C, Preissl H, Birbaumer N, Gerloff C. Crossed cortico-spinal motor control after capsular stroke. Eur J Neurosci. 2007;25:2935–45. doi: 10.1111/j.1460-9568.2007.05526.x. [DOI] [PubMed] [Google Scholar]
- Brown P, Salenius S, Rothwell JC, Hari R. Cortical correlate of the Piper rhythm in humans. J Neurophysiol. 1998;80:2911–17. doi: 10.1152/jn.1998.80.6.2911. [DOI] [PubMed] [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, et al. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol. 1995;489:917–24. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13:561–6. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006;173:25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci. 2007;27:8053–8. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol. 1993a;470:127–55. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. J Physiol. 1993b;463:83–105. doi: 10.1113/jphysiol.1993.sp019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, et al. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–2. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Manual dexterity and corticospinal connectivity following unilateral section of the cervical spinal cord in the macaque monkey. J Comp Neurol. 1997;381:307–19. [PubMed] [Google Scholar]
- Gross J, Tass PA, Salenius S, Hari R, Freund HJ, Schnitzler A. Cortico-muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography. J Physiol. 2000;527:623–31. doi: 10.1111/j.1469-7793.2000.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process datatheory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–78. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hansen NL, Conway BA, Halliday DM, Hansen S, Pyndt HS, Biering-Sorensen F, et al. Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J Neurophysiol. 2005;94:934–42. doi: 10.1152/jn.00082.2005. [DOI] [PubMed] [Google Scholar]
- Hansen NL, Nielsen JB. The effect of transcranial magnetic stimulation and peripheral nerve stimulation on corticomuscular coherence in humans. J Physiol. 2004;561:295–306. doi: 10.1113/jphysiol.2004.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Salenius S. Rhythmical corticomotor communication. Neuroreport. 1999;10:1–10. [PubMed] [Google Scholar]
- Isa T, Ohki Y, Alstermark B, Pettersson L-G, Sasaki S. Direct and indirect cortico-motoneuronal pathways and control of hand/arm movements. Physiology. 2007;22:145–52. doi: 10.1152/physiol.00045.2006. [DOI] [PubMed] [Google Scholar]
- Isa T, Ohki Y, Seki K, Alstermark B. Properties of propriospinal neurons in the C3-C4 segments mediating disynaptic pyramidal excitation to forelimb motoneurons in the macaque monkey. J Neurophysiol. 2006;95:3674–85. doi: 10.1152/jn.00103.2005. [DOI] [PubMed] [Google Scholar]
- Jackson A, Spinks RL, Freeman TC, Wolpert DM, Lemon RN. Rhythm generation in monkey motor cortex explored using pyramidal tract stimulation. J Physiol. 2002;541:685–99. doi: 10.1113/jphysiol.2001.015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20:8838–45. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN. Task-dependent modulation of 15-30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol. 1999;516:559–70. doi: 10.1111/j.1469-7793.1999.0559v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–79. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Werhahn KJ, Ashby P, Rothwell J, Noachtar S, Brown P. Organization of cortical activities related to movement in humans. J Neurosci. 2000;20:2307–14. doi: 10.1523/JNEUROSCI.20-06-02307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Steger J, Schulman AE, Gerloff C, Hallett M. Electroencephalographic measurement of motor cortex control of muscle activity in humans. Clin Neurophysiol. 2000;111:326–37. doi: 10.1016/s1388-2457(99)00229-1. [DOI] [PubMed] [Google Scholar]
- Mima T, Toma K, Koshy B, Hallett M. Coherence between cortical and muscular activities after subcortical stroke. Stroke. 2001;32:2597–601. doi: 10.1161/hs1101.098764. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA. 1992;89:5670–4. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol. 1996a;76:3949–67. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. J Neurophysiol. 1996b;76:3968–82. doi: 10.1152/jn.1996.76.6.3968. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Isa T. Compensatory changes at the cerebral cortical level after spinal cord injury. Neuroscientist, 2009 doi: 10.1177/1073858408331375. in press. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–5. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- Norton JA, Gorassini MA. Changes in cortically related intermuscular coherence accompanying improvements in locomotor skills in incomplete spinal cord injury. J Neurophysiol. 2006;95:2580–9. doi: 10.1152/jn.01289.2005. [DOI] [PubMed] [Google Scholar]
- Omlor W, Patino L, Hepp-Reymond MC, Kristeva R. Gamma-range corticomuscular coherence during dynamic force output. Neuroimage. 2007;34:1191–8. doi: 10.1016/j.neuroimage.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Osu R, Franklin DW, Kato H, Gomi H, Domen K, Yoshioka T, et al. Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. J Neurophysiol. 2002;88:991–1004. doi: 10.1152/jn.2002.88.2.991. [DOI] [PubMed] [Google Scholar]
- Pettersson LG, Alstermark B, Blagovechtchenski E, Isa T, Sasaski S. Skilled digit movements in feline and primate—recovery after selective spinal cord lesions. Acta Physiol. 2007;189:141–54. doi: 10.1111/j.1748-1716.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Manipulation of peripheral neural feedback loops alters human corticomuscular coherence. J Physiol. 2005;566:625–39. doi: 10.1113/jphysiol.2005.089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci USA. 1993;90:4470–4. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77:3401–5. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Salenius S, Salmelin R, Neuper C, Pfurtscheller G, Hari R. Human cortical 40 Hz rhythm is closely related to EMG rhythmicity. Neurosci Lett. 1996;213:75–8. doi: 10.1016/0304-3940(96)12796-8. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, et al. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–7. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- Schmidlin E, Wannier T, Bloch J, Rouiller EM. Progressive plastic changes in the hand representation of the primary motor cortex parallel incomplete recovery from a unilateral section of the corticospinal tract at cervical level in monkeys. Brain Res. 2004;1017:172–83. doi: 10.1016/j.brainres.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol. 2005;94:2844–55. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]