Abstract
Grape seed extract (GSE) is a widely consumed dietary supplement that has antitumor activity. Here, we have investigated the inhibitory effect of GSE on the expression of vascular endothelial growth factor (VEGF) and the mechanism underlying this action. We found that GSE inhibited VEGF messenger RNA (mRNA) and protein expression in U251 human glioma cells and MDA-MB-231 human breast cancer cells. GSE inhibited transcriptional activation of the VEGF gene through reducing protein but not mRNA expression of hypoxia-inducible factor (HIF) 1α. The inhibitory effect of GSE on HIF-1α expression was mainly through inhibiting HIF-1α protein synthesis rather than promoting protein degradation. Consistent with this result, GSE-suppressed phosphorylation of several important components involved in HIF-1α protein synthesis, such as Akt, S6 kinase and S6 protein. Furthermore, in the MDA-MB-231 tumor, we found that GSE treatment inhibited the expression of VEGF and HIF-1α and the phosphorylation of S6 kinase without altering the subcellular localization of HIF-1α, correlating with reduced vessel density and tumor size. Depletion of polyphenol with polyvinylpyrrolidone abolished the inhibitory activity of GSE, suggesting a water-soluble fraction of polyphenol in GSE is responsible for the inhibitory activity. Taken together, our results indicate that GSE inhibits VEGF expression by reducing HIF-1α protein synthesis through blocking Akt activation. This finding provides new insight into the mechanisms of anticancer activity of GSE and reveals a novel molecular mechanism underlying the antiangiogenic action of GSE.
Introduction
Vascular endothelial growth factor (VEGF) is one of the most critical and specific factors that stimulate angiogenesis (1). Inhibition of VEGF has demonstrated efficacy in the treatment of several cancers including colorectal cancer and renal cancer. Expression of VEGF is regulated by hypoxia, growth factors and oncogenes. The primary regulator of VEGF expression in response to hypoxia is hypoxia-inducible factor (HIF) (1).
HIF-1 is a heterodimeric transcription factor that consists of HIF-1β and HIF-1α, which is highly regulated. The level of HIF-1α expression is determined by the rate of protein synthesis, which is oxygen independent, and the rate of protein degradation, which is oxygen dependent. HIF-1 activates the expression of VEGF genes by binding to the hypoxia response element in the VEGF promoter region. In the presence of oxygen (2), HIF-1α protein is rapidly degraded via ubiquitination and subsequent degradation by proteasome. HIF-1α degradation is dependent on the hydroxylation of Pro-564 and Pro-402 via an enzymatic process that requires O2 and iron. The hydroxylated HIF-1α then binds rapidly to von hippel-lindau (VHL) tumor suppressor protein, which directs HIF-1α for proteasomal degradation through its E3 ubiquitin ligase activity. Under hypoxia, HIF-1α is not hydroxylated in the absence of oxygen and therefore cannot bind to von hippel-lindau to be degraded. Consequently, HIF-1α accumulates in the nucleus, forms an active complex with HIF-1β and activates transcription of target genes (3).
In addition to hypoxia, HIF-1α level can also be stimulated by growth factors, cytokines and other signaling molecules by increasing HIF-1α protein synthesis via activation of phosphoinositide-3-kinase (PI3K)/Akt or mitogen-activated protein kinase (MAPK) pathways (2,4,5) and activation of Stat3-signaling pathway (6–9). VEGF expression can also be regulated in HIF-1α-independent manner. Multiple transcription factor binding sites including Stat3, activated protein 1 (AP-1), Sp-1 and cAMP response element binding have been identified within the VEGF promoter to regulate VEGF expression (10,11).
HIF-1α plays a central role in tumor progression and angiogenesis in vivo. Tumor xenografts of HIF-1β-deficient hepatoma cells (12), HIF-1α-deficient H-ras-transformed cell lines or embryonic stem cells from HIF-1α−/− mice showed reduced growth rate and vascularization compared with the tumor xenograft of wild-type cells (13–15). More importantly, overexpression of HIF-1α has been demonstrated in many common human cancers including human breast cancer. There is a positive link between increased patient mortality and elevated HIF-1α levels (16–18).
While many agents targeting VEGF have been developed for cancer treatment, the efficacy of these anti-VEGF agents could be improved if they can be used chronically. However, most of the current anti-VEGF agents result in some side effects such as hypertension, bleeding, gastrointestinal perforation, etc. and therefore cannot be used chronically (19). The diet-based anti-angiogenesis approach is being actively explored (20,21), as it has a major advantage due to the proven safety in human use. It is evident that consumption of a plant-based diet can prevent the development and progression of cancer; however, the underlying mechanisms remain largely unclear (22).
Grapes and red wines are consumed world widely and have been reported to be associated with reduced risk of cancer (23). Grapes are rich in polyphenols, of which ∼60–70% is found in grape seeds. Commercial preparations of grape seed extract (GSE) contain 70–95% standardized procyanidins. GSE is marketed as a dietary supplement in the USA, due to their powerful antioxidant activity. It is also being explored for its cancer preventive properties. There are a number of studies showing cancer preventative potential of GSE against breast, prostate, lung, skin and gastrointestinal cancers (24–30). Several biochemical characteristics of GSE, such as anti-aromatase, anti-proliferation, pro-apoptosis and anti-oxidation, have been proposed as possible mechanism for its anticancer activity (24,25,31–34). Recently, GSE has been shown effective in inhibiting angiogenesis, suggesting a strong possibility that growth inhibitory effects of GSE on tumor are, in part, contributed via the inhibition of tumor angiogenesis (27,35,36).
GSE has also been shown previously to suppress VEGF production in prostate cancer (27); however, the mechanism is not well known. In this study, we found that GSE could inhibit VEGF expression through inhibiting HIF-1α protein expression in human breast cancer cells (MDA-MB-231) and human glioma cells (U251), but had no apparent inhibitory effects on HIF-1α messenger RNA (mRNA) expression. We further found that the inhibition of HIF-1α and VEGF by GSE appeared to involve blockage of HIF-1α protein synthesis via inhibiting PI3/Akt-signaling pathway.
Materials and methods
Grape seed extract
GSE-standardized preparation, constituting of at least 85% (wt/wt) procyanidins, was provided by San Joaquin Valley Concentrates (Fresno, CA) and was dissolved in water and incubated in a boiling water bath for 30 min. The solution was centrifuged at 13 000 r.p.m. for 10 min to remove any insoluble ingredients. Same lot of GSE was used for all experiments. The GSE preparation contains ∼19, 36, 8 and 22 ng/μg of procyanidins B1, B2, B3 and B4, respectively.
Reagents and antibodies
Cycloheximide (CHX), deferoxamine mesylate (DFX), MG132 and antibody for β-actin were from Sigma (St. Louis, MO). The antibodies against phospho-Akt (Ser473), phospho-S6K, S6K, phospho-S6 and S6 were from Cell Signaling Technology (Danvers, MA). Antibody against total Akt was from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against HIF-1α was from BD Bioscience (Lexington, KY). Antibodies for β-tubulin were from Thermo Fisher Scientific (Fremont, CA). Small interfering RNA (siRNA) of HIF-1α and control siRNA were from Santa Cruz.
Cell culture
The MDA-MB-231 human breast cancer cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (Gibco BRL, Grand Island, NY), 100 U/ml penicillin and 100 μg/ml streptomycin, 1 mM sodium pyruvate, 2.5 g/l glucose, 10 μg/ml insulin, 2 mM L-glutamine. U251 human glioma cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin in 5% CO2 incubator at 37°C. For cell culture under hypoxia, the cells were grown in a chamber containing 1% O2, 5% CO2 and 94% N2 at 37°C. Hypoxia can also be induced in normoxia using the hypoxia mimetic agent DFX (Sigma) at a concentration of 250 μM. Human umbilical vascular endothelial cells (Clonetics, Lonza, Allendale, NJ) were cultured in endothelial cell growth medium 2 (Lonza) containing 10% fetal bovine serum.
Construction of plasmids
A 2135 bp fragment of the human VEGF gene promoter (−2080 to +54), encompassing the HIF-1-binding site at position −985 and −939, was amplified from human peripheral blood mononuclear cells genomic DNA by polymerase chain reaction (PCR) using a KpnI restriction site primer (5′-CGGGGTACCTCAGAGCCTCCATCCTGCCCCAAG-3′) and a HindIII restriction site primer (5′-CCCAAGCTTGACCGGTCCACCTAACCGCT-3′). The amplicon was cloned into the upstream of the luciferase (Luc) gene of pGL4.14/[luc2/Hygro] plasmid (Promega, Madison, WI) with KpnI and HindIII restriction enzymes to construct the Luc reporter plasmid (pGL4/VEGF-Luc). The plasmid (pGL4/VEGF-Luc) was validated by sequencing.
Reporter cell line
Stable transfection was used to generate U251/VEGF-Luc reporter cell line using construct pGL4/VEGF-Luc. Human glioma U251 cells were transfected with VEGF-Luc plasmid. Stably transfected single cell lines were cloned from cells resistant to 250 μg/ml of hygromycin B (Calbiochem, San Diego, CA). One of the cell lines (U251-VEGF-Luc) with stable Luc activity and high response to hypoxia treatment was chosen for the study.
Luc reporter assay
U251/VEGF-Luc cells were seeded at a concentration of 2.5 × 104 cells per well in 48-well plate or 1.5 × 104 cells per well in 96-well plate in growth medium the day before treatment. Cells were then washed with phosphate-buffered saline and incubated with serum-free medium for 16 h in the presence of GSE. There is no add-back of complete media with GSE. All experiments were done in triplicates. Cells were washed once with phosphate-buffered saline and lysed with reporter lysis buffer from Promega (Madison, WI). Luc activities were determined according to the manufacturer's instruction and were normalized to protein concentrations or cell numbers. The experiment was repeated three times. Data were reported as an average ± SD.
Preparation of conditioned medium
U251/VEGF-Luc cells and MDA-MB-231 cells were seeded in 10 cm dishes in 10 ml of growth medium to reach ∼90% confluency. Cells were washed with phosphate-buffered saline three times and then treated with GSE or water (<1% in volume in culture media) for 24 h under normoxic or hypoxic condition in 6 ml of serum-free medium at 37°C. The media were collected and subjected to low-speed centrifugation. The remaining cells were used in parallel for real-time PCR (RT-PCR).
Quantitative real-time PCR
Total RNA was extracted from cell lines using Qiagen RNeasy Mini Kit. Residual genomic DNA was removed by incubating the RNA with DNase (Qiagen, Valencia, CA). Complementary DNA was synthesized from 1.0 μg of total RNA using the Superscript III first-strand complementary DNA synthesis kit (Invitrogen, Carlsbad, CA) in a final volume of 20 μl with 0.25 μg random hexamer and 200 U of Superscript RNase H reverse transcriptase. The reaction mixture was first incubated at 25°C for 5 min and followed by incubation at 50°C for 50 min. Quantitative real-time PCR was carried out in ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The reaction mixture consisted of 1× ABI SYBR Green PCR Master Mix, 0.25 μl complementary DNA and 0.2 μM of each primer. The PCR protocol was 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The following primers were used: HIF-1α, 5′-TGAGGAAATGAGAGAAATGCTTACA-3′ (forward) and 5′-ACACTGAGGTTGGTTACTGTTGGT-3′ (reverse); VEGF, 5′-CCAGCACATAGGAGAGATGAGCTT-3′ (forward) and 5′-TCTTTCTTTGGTCTGCATTCACAT-3′ (reverse) and β-actin, 5′-ATCTGGCACCACACCTTCTACAA-3′ (forward) and 5′-GTACATGGCTGGGGTGTTGAAG-3′ (reverse).
β-Actin was amplified as an internal control. Samples were loaded in triplicates, and results of each sample were normalized to β-actin. Comparative quantitative method was used to calculate the fold change of mRNA expression according to the formula of 2−(ΔΔCT).
Quantification of VEGF
An enzyme-linked immunosorbent assay-based bead multiplex assay (Luminex Corp., Austin, TX) was used to measure VEGF level. Human VEGF was determined using a human cytokine kit from Invitrogen.
Immunoblot
Total cell extract was prepared in Laemmli sample buffer and electrophoresed on sodium dodecyl sulfate gels. Separated proteins were transferred to polyvinylidene difluoride (Millipore, Billerica, MA) membrane and incubated with primary antibody. Binding of primary antibody was detected using a horseradish peroxidase-conjugated secondary antibody and chemiluminescent substrate (Thermo Fisher Scientific). Densitometric analysis was performed using the AlphaEase FC imaging system (Alpha Innotech Corporation, San Leandro, CA).
Immunohistochemistry
HIF-1α staining was performed as described previously (37). In brief, antigen retrieval was performed (45 min at 96°C) in target retrieval solution (Dako, Glostrup, Denmark). The primary mouse antibody (anti-HIF-1α; 1:500 dilution; BD Transduction Laboratories, Lexington, KY) was incubated for 30 min at room temperature. Tyramide signal amplification system (NEN Life Science Products, Boston, MA) was used to detect HIF-1α staining.
Migration assay
Endothelial cell migration was assessed using a modified Boyden chamber assay (38). Human umbilical vascular endothelial cells (1 × 105) were plated in endothelial cell basal medium 2 containing 0.05% fetal calf serum in the upper chamber of the transwell (8 μm pore, Corning Costar, Corning, NY), which were precoated with 200 μg/ml Matrigel (Becton-Dickinson, Bedford, MA). Serum-free Dulbecco's modified Eagle's medium, containing various amount of tumor-conditioned medium, was added to the lower chamber of the transwell. After 5 h, non-migrated cells were removed by cotton swap and migrated cells were stained and examined under microscope. The number of migrated cells was quantified by counting the cells at ×40 objectives.
Statistical analysis
Data were expressed as mean ± SD. Student's t-test is used to determine statistical significance between control and test group. P < 0.05 is considered to be statistically significant.
Results
Effect of GSE on VEGF expression in human tumor and human cancer cells
We have previously found that GSE could suppress MDA-MB-231 breast cancer growth in mice partly though inhibiting vascular endothelial cell growth factor receptor 2 (VEGFR2) signaling pathway (36). To better understand the mechanism by which GSE inhibit tumor growth, we further investigated whether GSE could have an impact on the expression of VEGF. Real-time PCR was used to compare the level of VEGF mRNA in tumors derived from mice treated with GSE versus controls treated with water. As shown in Figure 1A, we found that the mRNA level of human VEGF produced in MDA-MB-231 tumor was significantly reduced in GSE-treated mice. Similar result was obtained with VEGF protein level assessed by enzyme-linked immunosorbent assay (Figure 1B). These results were consistent with our previous report that GSE was able to reduce breast cancer growth and the blood vessel density in the same MDA-MB-231 tumor xenograph model (36).
Fig. 1.
GSE inhibits VEGF expression in tumor and cultured cancer cells. (A and B) GSE inhibits VEGF mRNA and protein expression in human breast tumors. Severe combined immunodeficiency (SCID) mice carrying MDA-MB-231 human tumor grown subcutaneously in the back was treated either with GSE (50 mg/kg) or water. Tumor tissues were collected at the end of the treatment and measured for human VEGF mRNA and protein expression by RT-PCR or enzyme-linked immunosorbent assay as described. The data were expressed as a percentage of control treated with water. Each bar represents the mean ± SD of four tumors. *P < 0.05 versus tumor fed with water alone. (C and D) GSE inhibits VEGF production in human cancer cells. MDA-MB-231 cells (C) were incubated with GSE (10 μg/ml) in serum-free medium under normoxia and hypoxia, either in the presence of 1% oxygen or 250 μM DFX. Glioma U251 cells (D) were incubated with GSE (10 μg/ml) in the presence of DFX. After 24 h incubation, conditioned media were collected and analyzed for the presence of VEGF by enzyme-linked immunosorbent assay. Data were normalized to cell numbers and medium volume. **P < 0.005 versus control treated with water (<1% in culture media).
To determine whether GSE can directly inhibit VEGF expression in cancer cells, we treated human breast cancer cell line MDA-MB-231 with GSE under normoxia or hypoxia condition, either in the presence of 1% O2 or 250 μM DFX that mimic hypoxia condition. The conditioned media were collected and measured for the protein level of VEGF by enzyme-linked immunosorbent assay. VEGF level was normalized to cell numbers and medium volume. As shown in Figure 1C, GSE treatment led to a decrease of VEGF production in MDA-MB-231 cells under both normoxia and hypoxia. In addition, GSE also inhibited VEGF expression in U251 glioma cells in the presence of DFX (Figure 1D).
To determine whether the reduced level of VEGF protein expression in cancer cells might occur at the level of mRNA, real-time PCR was used to compare VEGF mRNA expression. As shown in Figure 2A and B, GSE could inhibit VEGF mRNA level under normoxia, as well as the elevated VEGF mRNA level in cells cultured under hypoxia, either in the presence of 1% oxygen or 250 μM DFX. Time course analysis showed that the inhibitory effect of GSE on VEGF mRNA production increased with time and peaked at 16 h (Figure 2C). These results suggest that GSE can inhibit VEGF mRNA expression.
Fig. 2.
GSE inhibits VEGF expression in human cancer cells. Human MDA-MB-231 breast cancer cells (A) and glioma U251 cells (B and C) were incubated with GSE (10 μg/ml) under both normoxia and hypoxia, either in the presence of 1% oxygen or 250 μM DFX that mimic hypoxia condition. RT-PCR was used to determine the effect of GSE on VEGF mRNA expression in human breast cancer MDA-MB-231 cells (A) and in human glioma U251 cells (B) after 16 h incubation. Data were expressed as a ratio to the control treated with water under normoxia. (C) U251 cells were incubated with GSE (10 μg/ml) for various times in the presence of DFX. Each sample was measured for mRNA by RT-PCR. Data were expressed as a ratio to the control treated with water at each time point. (D and E) GSE inhibits Luc activity of VEGF reporter. U251 cells expressing Luc reporter containing human VEGF gene were incubated with GSE (10 μg/ml) for 16 h under both normoxia and hypoxia. Each sample was measured for Luc activity (D) and cell numbers (E) as described. Data were represented as a ratio to control treated with water under normoxia. *P < 0.05, **P < 0.005 versus control treated with water alone.
We next examined the effect of GSE on VEGF promoter reporter containing a 2.1 kb fragment of human VEGF gene promoter in U251 cells. This Luc reporter could test whether GSE inhibited transcriptional activation of the VEGF gene and thereby affected VEGF mRNA production. As shown in Figure 2D, GSE was found to inhibit the VEGF reporter activity under both normoxia and hypoxia. At this concentration, GSE had little effect on cell numbers (Figure 2E).
Effect of GSE on HIF-1α protein expression
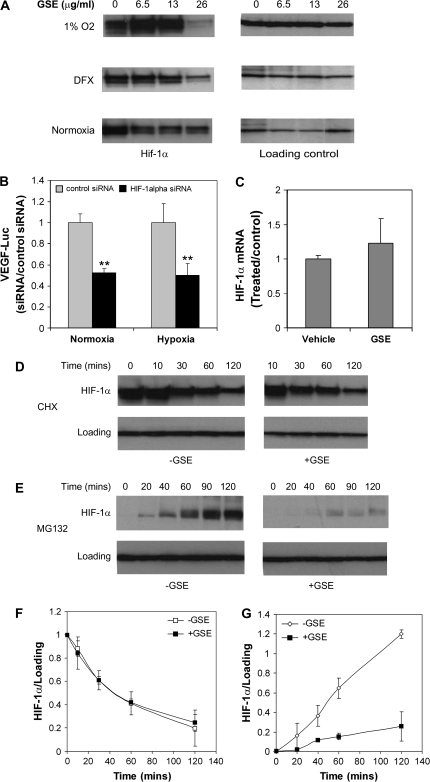
To explore the possible mechanism of inhibition of VEGF production by GSE, we asked whether GSE could modulate the level of HIF-1α, a major regulator of VEGF expression. Overexpression of HIF-1α and VEGF is commonly observed in many human cancers including prostate, breast and ovarian cancer cells (16–18). We treated U251 cells with GSE and then examined the effect of GSE on HIF-1α protein level. As shown in Figure 3A, we found that HIF-1α protein expression in U251 cells were suppressed by the increasing amount of GSE under both normoxia and hypoxia conditions. GSE showed an effective inhibition of HIF-1α protein expression at a concentration of 26 μg/ml. This concentration was higher than what was needed (10 μg/ml) for an effective inhibition of VEGF protein (Figure 1) and mRNA expression (Figure 2), perhaps the smaller inhibition of HIF-1α level seen at a lower concentration (e.g. 13 μg/ml) may be sufficient to elicit an inhibition of VEGF expression. Alternatively, GSE might also modulate factors or pathways that work in co-ordination with HIF-1α in regulating VEGF expression, thus a small inhibition of individual factors could lead to a synergistic effect on VEGF expression.
Fig. 3.
GSE inhibits HIF-1α expression. (A) GSE inhibits HIF-1α protein expression. U251 cells were incubated under normoxia, 1% oxygen or treated with DFX for 16 h, in the absence or presence of increasing concentrations of GSE (6.5–26 μg/ml). Whole-cell lysates were analyzed by immunoblotting with antibody against HIF-1α. β-Actin level was used as a loading control. (B) Silence of HIF-1α expression resulted in a decrease in VEGF expression. HIF-1α siRNA and control siRNA were transfected into U251 cells expressing VEGF reporter gene. Cells were incubated under normoxia and hypoxia (1% oxygen) for 24 h and then measured for Luc activity. Data were represented as a ratio to control treated with water in each condition, **P < 0.005. (C) GSE has no apparent effect on HIF-1α mRNA expression. U251 cells were incubated with GSE (10 μg/ml) in the presence of DFX for 16 h. Cells were harvested for HIF-1α mRNA expression by RT-PCR. Data were normalized to β-actin and expressed as a ratio to the control treated with water alone. (D) GSE has little effect on HIF-1α degradation. Cells were first incubated in the presence of DFX (250 μM) for 4 h and then treated with CHX (10 μM) in the presence or absence of GSE (26 μg/ml) for indicated times. Cells were harvested and whole-cell lysates were analyzed for HIF-1α protein level by immunoblotting. (E) GSE inhibits HIF-1α protein synthesis. Cells were pretreated with GSE (26 μg/ml) for 1 h prior to the addition of proteasome inhibitor MG-132 (10 μM). Cells were then incubated for various times as indicated. Cells were harvested and whole-cell lysates were analyzed by immunoblotting for the presence of HIF-1α. (F and G) Relative levels of HIF-1α protein in (D) and (E) were determined by measuring the density of the HIF-1α protein band and normalized to that of β-actin (F) or β-tubulin (G). Data were expressed as a ratio to control at time zero (F) and were the mean ± SD of three experiments. *P < 0.05 versus control treated with water.
To address whether HIF-1α is involved in the regulation of VEGF expression in U251 cells, we performed RNA interference of HIF-1α using siRNA under both normoxia and hypoxia. As shown in Figure 3B, VEGF Luc reporter expression was significantly reduced in the cells transfected with siRNA but not in the cells transfected with control siRNA.
We next asked whether inhibition of HIF-1α protein accumulation by GSE could be a result of transcriptional inhibition. RT-PCR of HIF-1α showed no significant changes in HIF-1α mRNA levels in U251 cells after exposure to hypoxia for 16 h (Figure 3C). Similar results were obtained in MDA-MB-231 cells under the same experimental conditions (data not shown). These results suggest that GSE suppresses HIF-1α protein accumulation probably via a posttranscriptional mechanism.
One possible posttranscriptional mechanism of the inhibitory activity of GSE is increased degradation and/or reduced synthesis of HIF-1α protein. To determine the effect of GSE on the stability of HIF-1α, protein translation inhibitor CHX was used to prevent de novo HIF-1α protein synthesis. We first induced HIF-1α accumulation by exposing the cells to DFX to mimic hypoxia condition for 4 h followed by addition of CHX alone or together with GSE. In the presence of CHX, HIF-1α levels declined rapidly as expected. The degradation rate of HIF-1α in the presence or absence of GSE appeared comparable (Figure 3D and F), suggesting that the inhibitory activity of GSE on VEGF/HIF-1α is less probably mediated through directly promoting HIF-1α degradation.
To determine the effect of GSE on HIF-1α protein synthesis, we examined the accumulation of HIF-1α in U251 cells with the use of proteasome inhibitor MG-132 to prevent HIF-1α degradation. HIF-1α rapidly accumulated over a period of 2 h in the presence of MG-132 under normoxia. However, accumulation of HIF-1α protein was markedly impaired in the presence of GSE (Figure 3E and G). As a control, little effect of GSE on β-tubulin synthesis was observed. These results suggest that the inhibitory effect of GSE on VEGF and HIF-1α protein expression is mainly mediated by suppressing the synthesis of HIF-1α protein.
Effect of GSE on PI3K/Akt pathway
The PI3K/Akt pathway has been implicated in regulation of HIF-1α protein synthesis at the translational level (39). It has been shown that many growth factors and mitogens induce the activation of p70 S6 kinase, which in turn phosphorylates the S6 ribosomal protein of the 40S subunit of the ribosome. Phosphorylation of S6 correlates with an increase in translation, particularly of mRNAs with an oligopyrimidine tract in their 5′ untranslated region (3,40,41). To address whether the inhibition of HIF-1α protein synthesis was mediated by downregulation of the PI3K/Akt pathway, we tested the effect of GSE on phosphorylation of Akt, S6 kinase and ribosomal S6 protein, the major components involved in regulating HIF-1α protein synthesis. We found that GSE could inhibit the phosphorylation of Akt, S6 kinase and S6 protein (Figure 4A). A slight inhibition of the total protein level was seen for Akt and S6K, but not in the case of S6. GSE showed little effect on the phosphorylation of p42/p44 MAPK, which was relatively low in U251 cells (data not shown).
Fig. 4.
GSE inhibits phosphorylation of Akt, S6K and S6 proteins. (A) U251 cells were incubated in the presence of DFX and increasing concentrations of GSE for 16 h. Cells were harvested and whole-cell lysates were analyzed by immunoblotting. The blots were reprobed with additional antibodies. β-Actin level was used as a loading control. (B and C) The effect of GSE on VEGF-Luc expression in the presence of Myr-Akt (B) and HDM2 (C). Myr-Akt (B) and HDM2 (C) were co-transfected with renilla expression vector into U251 cells expressing VEGF reporter gene. Empty vector was added to adjust to the same amounts of plasmids. After 24 h, cells were treated with GSE (13 μg/ml). The cells were incubated for 16 h and then measured for the Luc activity. Data were represented as percentage of control that was treated with water alone in each condition. *P < 0.05 versus control treated with water alone.
To further address whether inhibition of PI3K/Akt pathway contribute, at least in part, to the inhibitory effect of GSE on HIF-1α expression, we transfected an active form of Akt, Myr-Akt, into U251 cells. In the presence of Myr-Akt, inhibition of VEGF reporter activity by GSE was blocked (Figure 4B). Taken together, our results suggest that GSE can suppress VEGF expression through inhibiting HIF-1α protein translation regulated by the PI3/Akt pathway.
HDM2 is an oncogene that is regulated by PI3K/Akt signaling and has been shown to regulate HIF-1α expression by mediating p53 degradation. P53 is known to reduce HIF-1α level by promoting HIF-1α degradation. Therefore, we tested the effect of overexpression of HDM2 on GSE-induced inhibition. As shown in Figure 4C, expression of HDM2 protein in U251 cells did not significantly alter the GSE-inhibited VEGF transcription. Similarly, HDM2 expression had no significant effect on GSE-inhibited HIF-1α expression (data not shown). This suggests that HDM2 is unlikely the major factor involved in the inhibition by GSE in U251 cells.
Effect of GSE on HIF-1α expression in tumors
To explore whether GSE can affect HIF-1α expression in tumors, we compared HIF-1α level in tumors derived from mice treated with GSE versus controls treated with water. As shown in Figure 5A, HIF-1α expression was significantly reduced in the GSE-treated tumor. Consistent with the in vitro findings, phosphorylation of S6K and S6 in the tumor was also significantly reduced. The phosphorylation of Akt was relatively low and was hard to evaluate.
Fig. 5.
GSE inhibits HIF-1α expression in tumor. (A) GSE inhibited HIF-1α expression and phosphorylation of S6K and S6 ribosome proteins in tumors implanted in the mice. Tumor tissue lysates were analyzed by immunoblotting for the expression of HIF-1α and phosphorylation of S6K and S6 proteins. β-tubulin was used as loading control. (B) GSE treatment had little effect on HIF-1α localization in tumor by immunohistochemistry. MDA-MB-231 tumor sections were stained with antibody against HIF-1α. Bar, 20 μM. (C and D) Effect of MDA-MB-231-conditioned medium (C) and U251-conditioned medium (D) on endothelial cell migration. Human umbilical vascular endothelial cells were placed in the top chamber of a transwell, whereas serum-free medium containing various amount of tumor-conditioned medium was placed in the bottom of chamber. Migrated cells were quantified 5 h after incubation under ×40 objective. Data were expressed as number of migrated cells per high power field (HPF). *P < 0.05 versus control treated with water alone.
As the activity of HIF-1α can also be regulated by its localization, we further asked whether the inhibitory effect of GSE on HIF-1α expression could be mediated by affecting HIF-1α nuclear localization. As shown in Figure 5B, in tumors derived from the mice treated with GSE, HIF-1α was mainly found in the nucleus by immunohistochemistry, suggesting that GSE had little effect on HIF-1α subcellular localization. Similar result was obtained in cultured cells (data not shown). Taken together, our in vitro and in vivo results support that GSE inhibits VEGF expression by preventing HIF-1α protein expression.
Anti-angiogenesis activity of GSE
The ability of GSE to block VEGF protein expression is consistent with our previous report that GSE can suppress both tumor growth and blood vessel density in MDA-MB-231 xenograph model (36). To further address whether GSE inhibits angiogenesis by targeting angiogenesis factors such as VEGF, we used endothelial cell migration assay to examine the effect of conditioned media derived from tumor cells treated with either GSE or water. As shown in Figure 5C and D, conditioned medium derived from tumor cells was able to induce endothelial cell migration. However, the ability of conditioned medium to induce endothelial cell migration was substantially suppressed when tumor cells treated with GSE (Figure 5C and D). This result is consistent with our observation that GSE could block VEGF expression and further supports the idea that one mechanism of the antitumor angiogenesis activity of GSE is mediated by reducing the expression of angiogenesis factors produced by tumors.
Water-soluble polyphenol of GSE in anti-VEGF activity
GSE is rich in polyphenols. To test whether polyphenol in GSE is responsible for its inhibitory activity on VEGF expression, we used polyvinylpyrrolidone (PVPP) to remove the polyphenol from GSE. PVPP forms hydrogen bonds with phenolic compounds, yielding a PVPP–phenolic precipitate that can be removed by centrifugation. As shown in Figure 6, PVPP-treated GSE has little effect on VEGF reporter activity, suggesting that a water-soluble polyphenol in GSE is probably responsible for the inhibitory activity on VEGF expression in GSE.
Fig. 6.
PVPP treatment depletes the inhibitory activity of GSE on VEGF expression. U251 cell expressing VEGF reporter was incubated with increasing amount of GSE with or without PVPP treatment under hypoxia condition (250 μM DFX). PVPP was used to remove polyphenol. After incubation for 16 h, cells were subjected to Luc assay. Data were represented as percentage of control that was treated with water alone in the presence of DFX. **P < 0.005 versus control treated with water alone.
Discussion
Angiogenesis, the formation of new blood vessel, plays a critical role in tumor progression. Angiogenesis inhibitors that suppress blood vessel formation have emerged as a new class of drugs that can be used to treat cancer (42). While many of the inhibitors are currently being tested at various stages of clinical development, dietary-based anti-angiogenesis approaches are being actively explored for cancer prevention and treatment. Proven safety for human use is a major merit that strengthens this approach. The long-known preventive effect of plant-based diet on tumorigenesis and other chronic disease is well documented. These data indicate that certain plant-derived dietary groups might contain phytochemicals that exert antitumor and anti-angiogenesis activity, thereby offering anticancer protection (20,21).
GSE is a popular dietary supplement that has been shown to have a wide variety of beneficial actions including antioxidant and antitumor activity. We have recently studied the anti-angiogenesis activity of GSE and found that GSE could suppress tumor growth and reduce microvessel density in MDA-MB-231 xenograph model (36). To better understand the mechanism by which GSE inhibit tumor growth and angiogenesis, here we investigated the idea that GSE could inhibit tumor angiogenesis partly by blocking expression of angiogenesis factors in tumors. We (43) have looked at the effect of GSE on the expression of VEGF, one of the most critical factors that stimulate angiogenesis and found that GSE could reduce VEGF expression in both U251 human glioma cells and MDA-MB-231 human breast cancer cells, which are in good agreement with previous report showing that GSE can inhibit VEGF secretion from DU145 prostate cancer cells (27). Red wine, containing both GSE and reseveratrol, has also been reported to suppress VEGF expression in vascular smooth muscle cells (44). Consistent with these observations, endothelial cell migration assay showed that the angiogenesis potential of conditioned medium from tumor cells was significantly reduced when cells were treated with GSE, supporting that GSE is a natural anti-angiogenesis inhibitor by suppressing VEGF expression in tumor cells.
We found that GSE could inhibit HIF-1α expression in U251 glioma cells. RNA interference of HIF-1α resulted in reduced expression of VEGF under both normoxia and hypoxia conditions, suggesting that HIF-1α is one of the major factors regulating VEGF expression in U251 cells. The possible mechanisms by which GSE inhibits HIF-1α protein expression could conceivably include (i) blocking HIF-1α mRNA expression; (ii) promoting HIF-1α protein degradation or (iii) reducing HIF-1α protein translation. The first possibility can be ruled out because GSE treatment did not change the level of HIF-1α mRNA (Figure 3C). The second possibility is unlikely to be a major one, as under our experimental condition, comparable level of HIF-1α protein was observed regardless of GSE treatment in the presence of protein synthesis inhibitor (CHX) (Figure 3D and F). On the other hand, inhibition of HIF-1α protein synthesis appears to be a possible mechanism of the GSE action, as GSE treatment led to an impaired accumulation of HIF-1α protein in the presence of proteasomal inhibitor MG132 (Figure 3E and G). GSE is not a general translation inhibitor, as the expression level of ribosomal S6 protein, actin and tubulin was not inhibited by GSE. Although our results indicate that GSE can suppress VEGF expression through inhibition of HIF-1α, our data do not rule out the possibility that GSE may modulate other pathways also important for regulating VEGF expression.
Many cellular signaling pathways that regulate translation factors have been elucidated, including MAPK pathway and Akt pathway (5,39,45). The phosphorylation level of MAPK is low in U251 cells, and addition of GSE did not seem to reduce the MAPK phosphorylation, suggesting that MAPK is not a major pathway regulating HIF-1α involved in the U251 cells. Consistent with this result, GSE had little effect on HIF-1α cellular localization, which could be regulated by MAPK pathway.
On the other hand, phosphorylation of Akt is significantly reduced upon treatment with GSE. Given that PI3K/Akt pathway plays an important role in hypoxia-mediated HIF-1α stabilization (3), one anticipated outcome of GSE treatment would be reduced stability of HIF-1α under hypoxia. However, under our experimental condition, significant inhibition was observed in HIF-1α protein synthesis but not in HIF-1α protein stabilization. The fact that this effect of GSE was also observed under the condition of normoxia implies that GSE can affect HIF-1α protein synthesis in cancer cells activated by growth factors and oncogenes, which can be regulated by the Akt pathway as reported previously (5,39,43,46). Therefore, a reduction in phosphorylation of Akt could be a possible mechanism for GSE-induced inhibition of HIF-1α protein synthesis in cancer cells.
Many natural products display an inhibitory effect on VEGF production, for example, green tea extract, epigallocatechins-3-gallate, soy, resveratrol, apigenin and chrysin (47–51). These agents have been shown to suppress VEGF expression in cancer cells through promoting HIF-1α protein degradation in cell culture or suppressing HIF-1α protein synthesis in the case of apigenin (43). Here, our data indicate that GSE inhibits VEGF expression mediated in part by blocking HIF-1α protein synthesis. It thus appears that the mechanism of the action of GSE or other natural products is cellular context dependent, for example depending on the relative ratio between MAPK and Akt in individual cell type, the expression of their downstream signaling molecules and the mechanisms regulating HIF-1α and VEGF expression in the cell.
Human tumors can remain dormant for years owing to the balance between cell proliferation and apoptosis. It is thought that systemic concentration of angiogenesis inhibitors exceeding that of stimulator could prevent tumor from growing as well as spreading to other organs. In this study, we have shown that GSE inhibits VEGF expression in human cancer cells and we present one possible mechanism underlying this action. Our data indicate that the effect of GSE can be mediated through inhibition of Akt activation and HIF-1α protein synthesis. This finding adds new insight into the potential mechanisms of the anticancer activity of GSE and offers a novel molecular mechanism underlying the antiangiogenic action of GSE. These results would help in the design of future strategies of developing GSE as a chemopreventive agent for a potential clinical therapy in combination with current anticancer drugs.
Funding
National Institutes of Health (CA44735, ES08258 to S.C.); Stop Cancer Foundation and Concern Foundation to W.W.
Acknowledgments
We thank Dr Judah Folkman (1933–2008) for his mentorship and vision. We thank Dr Kunxin Luo (University of California, Berkley) for the gift of Myr-Akt expression plasmid, Dr Andrew Kung (Dana-Farber Cancer Institute, Boston) for the gift of HIF-1α expression plasmid and Dr Maureen Murphy (Fox Chase Cancer Center) for the gift of HDM2 expression plasmid. We also like to thank Dr Tim Synold and Binxin Xi for analyzing the composition of GSE and for their suggestions, Drs Susan Kane and Edward Newman for their valuable discussions and Dr Michael Kalos and Shu Mi for the Bioplex assay.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CHX
cycloheximide
- DFX
deferoxamine mesylate
- GSE
grape seed extract
- HIF
hypoxia-inducible factor
- Luc
luciferase
- MAPK
mitogen-activated protein kinase
- mRNA
messenger RNA
- PCR
polymerase chain reaction
- PI3K
phosphoinositide-3-kinase
- PVPP
polyvinylpyrrolidone
- RT-PCR
real-time PCR
- siRNA
small interfering RNA
- VEGF
vascular endothelial growth factor
References
- 1.Ferrara N, et al. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 2.Mizukami Y, et al. Hypoxia inducible factor-1 independent pathways in tumor angiogenesis. Clin. Cancer Res. 2007;13:5670–5674. doi: 10.1158/1078-0432.CCR-07-0111. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Targeting Hif-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 4.Karni R, et al. Activated pp60c-Src leads to elevated hypoxia-inducible factor (HIF)-1alpha expression under normoxia. J. Biol. Chem. 2002;277:42919–42925. doi: 10.1074/jbc.M206141200. [DOI] [PubMed] [Google Scholar]
- 5.Laughner E, et al. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1{alpha} (HIF-1{alpha}) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Q, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, et al. The STATs of cancer—new molecular targets come of age. Nat. Rev. Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 8.Niu G, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 9.Wei LH, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 10.Pore N, et al. Sp1 is involved in Akt-mediated induction of VEGF expression through an HIF-1-independent mechanism. Mol. Biol. Cell. 2004;15:4841–4853. doi: 10.1091/mbc.E04-05-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizukami Y, et al. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res. 2004;64:1765–1772. doi: 10.1158/0008-5472.can-03-3017. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell PH, et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc. Natl Acad. Sci. USA. 1997;94:8104–8109. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, et al. Role of HIF-1[alpha] in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 14.Ryan HE, et al. Hypoxia-inducible factor-1{{alpha}} is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 15.Ryan HE, et al. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindl M, et al. Overexpression of hypoxia-inducible factor 1{alpha} is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin. Cancer Res. 2002;8:1831–1837. [PubMed] [Google Scholar]
- 17.Bachtiary B, et al. Overexpression of hypoxia-inducible factor 1{alpha} indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin. Cancer Res. 2003;9:2234–2240. [PubMed] [Google Scholar]
- 18.Hui EP, et al. Coexpression of hypoxia-inducible factors 1{alpha} and 2{alpha}, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin. Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- 19.Kamba T, et al. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhat TA, et al. Tumor angiogenesis—a potential target in cancer chemoprevention. Food Chem. Toxicol. 2008;46:1334–1345. doi: 10.1016/j.fct.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Albini A, et al. Molecular pathways for cancer angioprevention. Clin. Cancer Res. 2007;13:4320–4325. doi: 10.1158/1078-0432.CCR-07-0069. [DOI] [PubMed] [Google Scholar]
- 22.Adlercreutz H, et al. Dietary phytoestrogens and cancer: in vitro and in vivo studies. J. Steroid Biochem. Mol. Biol. 1992;41:331–427. doi: 10.1016/0960-0760(92)90359-q. [DOI] [PubMed] [Google Scholar]
- 23.Zheng T, et al. A case-control study of oral cancer in Beijing, People's Republic of China. Associations with nutrient intakes, foods and food groups. Eur. J. Cancer B Oral Oncol. 1993;29B:45–55. doi: 10.1016/0964-1955(93)90010-c. [DOI] [PubMed] [Google Scholar]
- 24.Eng ET, et al. Suppression of estrogen biosynthesis by procyanidin dimers in red wine and grape seeds. Cancer Res. 2003;63:8516–8522. [PubMed] [Google Scholar]
- 25.Kijima I, et al. Grape seed extract is an aromatase inhibitor and a suppressor of aromatase expression. Cancer Res. 2006;66:5960–5967. doi: 10.1158/0008-5472.CAN-06-0053. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, et al. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation–promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20:1737–1745. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]
- 27.Singh RP, et al. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int. J. Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 28.Kaur M, et al. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin. Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 29.Raina K, et al. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–5982. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 30.Arii M, et al. Chemopreventive effect of grape seed extract on intestinal carcinogenesis in the APCMin mouse. Proc. Am. Assoc. Cancer Res. 1998;39:20. [Google Scholar]
- 31.Agarwal C, et al. A polyphenolic fraction from grape seeds causes irreversible growth inhibition of breast carcinoma Mda-Mb468 cells by inhibiting mitogen-activated protein kinases activation and inducing G1 arrest and differentiation. Clin. Cancer Res. 2000;6:2921–2930. [PubMed] [Google Scholar]
- 32.Agarwal C, et al. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2002;23:1869–1876. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- 33.Tyagi A, et al. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene. 2003;22:1302–1316. doi: 10.1038/sj.onc.1206265. [DOI] [PubMed] [Google Scholar]
- 34.Dhanalakshmi S, et al. Inhibition of NF-kappaB pathway in grape seed extract-induced apoptotic death of human prostate carcinoma DU145 cells. Int. J. Oncol. 2003;23:721–727. [PubMed] [Google Scholar]
- 35.Agarwal C, et al. Anti-angiogenic efficacy of grape seed extract in endothelial cells. Oncol. Rep. 2004;11:681–685. [PubMed] [Google Scholar]
- 36.Wen W, et al. Grape seed extract (GSE) inhibits angiogenesis via suppressing VEGFR signaling pathway. Cancer Prev. Res. 2008;1:554–561. doi: 10.1158/1940-6207.CAPR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vleugel MM, et al. Differential prognostic impact of hypoxia induced and diffuse HIF-1{alpha} expression in invasive breast cancer. J. Clin. Pathol. 2005;58:172–177. doi: 10.1136/jcp.2004.019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kisker O, et al. Continuous administration of endostatin by intraperitoneally implanted osmotic pump improves the efficacy and potency of therapy in a mouse xenograft tumor model. Cancer Res. 2001;61:7669–7674. [PubMed] [Google Scholar]
- 39.Semenza GL. Signal transduction to hypoxia-inducible factor 1. Biochem. Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 40.Gingras AC, et al. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 41.Berven LA, et al. Cellular function of p70S6K: a role in regulating cell motility. Immunol. Cell Biol. 2000;78:447–451. doi: 10.1046/j.1440-1711.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 42.Folkman J. Angiogenesis-dependent diseases. Semin. Oncol. 2001;28:536–542. doi: 10.1016/s0093-7754(01)90021-1. [DOI] [PubMed] [Google Scholar]
- 43.Mirzoeva S, et al. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol. Carcinog. 2008;47:686–700. doi: 10.1002/mc.20421. [DOI] [PubMed] [Google Scholar]
- 44.Oak M-H, et al. Red wine polyphenolic compounds inhibit vascular endothelial growth factor expression in vascular smooth muscle cells by preventing the activation of the p38 mitogen-activated protein kinase pathway. Arterioscler. Thromb. Vasc. Biol. 2003;23:1001–1007. doi: 10.1161/01.ATV.0000070101.70534.38. [DOI] [PubMed] [Google Scholar]
- 45.Fukuda R, et al. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J. Biol. Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 46.Zhong H, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/Akt/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 47.Fu B, et al. Chrysin inhibits expression of hypoxia-inducible factor-1{alpha} through reducing hypoxia-inducible factor-1{alpha} stability and inhibiting its protein synthesis. Mol. Cancer Ther. 2007;6:220–226. doi: 10.1158/1535-7163.MCT-06-0526. [DOI] [PubMed] [Google Scholar]
- 48.Fang J, et al. Apigenin inhibits tumor angiogenesis through decreasing HIF-1{alpha} and VEGF expression. Carcinogenesis. 2007;28:858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q, et al. Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol. Cancer Ther. 2006;5:1227–1238. doi: 10.1158/1535-7163.MCT-05-0490. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, et al. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1{alpha} and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol. Cancer Ther. 2005;4:1465–1474. doi: 10.1158/1535-7163.MCT-05-0198. [DOI] [PubMed] [Google Scholar]
- 51.Guo Y, et al. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J. Nutr. Biochem. 2007;18:408–417. doi: 10.1016/j.jnutbio.2006.08.006. [DOI] [PubMed] [Google Scholar]