Abstract
NDRG (N-Myc downstream-regulated gene)-2 is a member of the NDRG family. Although it has been suggested that NDRG2 is involved in cellular differentiation and tumor suppression, its intracellular signal and regulatory mechanism are not well known. Here, we show the differential expression of NDRG2 in human colon carcinoma cell lines and tissues by reverse transcription–polymerase chain reaction and immunohistochemical analyses with monoclonal antibody against NDRG2. NDRG2 was strongly expressed in normal colonic mucosa and colonic adenomatous tissues (25 of 25) but not in all invasive cancer tissues [44 of 99 (44%)]. Most distinctive results indicated that the high expression level of NDRG2 has a positive correlation with tumor differentiation and inverse correlation with tumor invasion depth and Dukes’ stage of colon adenocarcinoma. To investigate the roles of NDRG2 in tumorigenesis, we used in vitro cell culture system. SW620 colon cancer cell line with a low level of intrinsic NDRG2 protein was transfected with NDRG2-expressing plasmid. TOPflash luciferase reporter assay showed that the transcriptional activity of T-cell factor (TCF)/lymphoid enhancer factor (LEF) was reduced by NDRG2 introduction, but not by the introduction of mutant NDRG2 generated by deletion or site-directed mutagenesis. Intracellular β-catenin levels were slightly reduced in the NDRG2-transfected SW620 cells and this regulation of β-catenin stability and TCF/LEF activity were mediated through the modulation of glycogen synthase kinase-3beta activity by NDRG2 function. Our results suggest that NDRG2 might play a pivotal role as a potent tumor suppressor by the attenuation of TCF/β-catenin signaling for the maintenance of healthy colon tissues.
Introduction
N-Myc downstream-regulated gene (NDRG)-2, which belongs to the NDRG family, comprised of four identified members; NDRG1–4 have been implicated in the regulation of cell differentiation and proliferation. NDRG1 is the most widely observed member of the NDRG family in various tissues of different species, whereas NDRG2–4 have been reported to be restricted to specialized organs or tissues including brain and heart, suggesting their different but related specific functions in different tissues and organs (1–3). In addition to NDRG2 expression in normal human tissues, differential expression of NDRG2 was reported in some human tumor tissues or cell lines including meningioma (4), liver and pancreatic cancer (5) and glioblastoma (6). In these studies, the expression of NDRG2 was significantly downregulated in a variety of different malignant neoplasms compared with the corresponding normal or benign tissues, which suggested that it might have a role in the modulation of the aggressive behavior of tumor progression. Indeed, accumulated evidence suggests that NDRG2 plays a role as a tumor suppressor, but the exact intracellular functional role of the NDRG2 remains to be clarified further, although much effort has been devoted to the investigation of NDRG2 function in cultured cells and animal systems.
NDRG2 was identified as a novel target molecule of antidepressants and electroconvulsive treatment in the central nervous system (7). Furthermore, Nichols et al. (8) confirmed that NDRG2 has a putative role in neural differentiation, synapse formation and axon survival in response to glucocorticoids. It was also reported that NDRG2 was upregulated with disease pathogenesis in the human brain disease, Alzheimer disease (9). Interestingly, Choi et al. (10) reported that NDRG2 is involved in the process of dendritic cells differentiation of monocytes, CD34 precursor and leukemia cells by any maturation-inducing stimuli. NDRG2 might have specific functions in the regulation of cellular differentiation and the maintenance of the status of various tissues or organs.
The structure of NDRG2 is composed of an α/β-hydrolase domain in the N-terminal region and several potential phosphorylation sites in the C-terminal region that contains three consensus Akt phosphorylation sites. Burchfield et al. (11) reported that NDRG2 is regulated by insulin-dependent phosphorylation in C2C12 skeletal muscle cells. It has been reported that NDRG2 is induced by mineralocorticoid hormones such as aldosterone (12) and has been identified as a physiological substrate phosphorylated by serum- and glucocorticoid-induced kinase 1 in vitro (13). However, the precise physiological role of NDRG2 phosphorylation is not yet known.
Human colorectal cancer is the leading cause of cancer death in the USA when smoking-related cancers are excluded. Colorectal cancer frequently involves genetic inactivation of the Wnt signal transduction system. Wnt ligands initiate a signaling cascade that reaches the nucleus via rapid movement of cytoplasmic β-catenin through nuclear pores. To effect this nuclear localization, the Wnt signal inhibits the activity of serine/threonine kinase glycogen synthase kinase-3beta (GSK-3β) (14–16). Under normal conditions, free β-catenin is rapidly phosphorylated by GSK-3β and subsequently degraded in the ubiquitin–proteasome pathway. When the Wnt signal is activated or the tumor suppressor adenomatous polyposis coli (APC) is non-functional, which occurs in many cases of colorectal cancer, GSK-3β activity is blocked. As a result, high levels of β-catenin accumulate in the cytoplasm and subsequently translocate into the nucleus after forming a complex with the T-cell factor (TCF) or lymphoid enhancer factor (LEF), which leads to the activation of Wnt target genes including cyclin D1 and c-Myc (17–21). Wnt/β-catenin signaling is one of the key signaling pathways in tumorigenesis, cell growth, motility and differentiation (22–26). Stability and intracellular localization of β-catenin is critical in the regulation of TCF/LEF activities.
Although NDRG2 expression in brain, breast, liver and pancreatic cancer have been reported, differential expression and intracellular function of NDRG2 in human colon cancer have not yet been investigated. We first investigated the localization and expression pattern of NDRG2 in colon tissues by immunohistochemical analysis as well as the expression level of NDRG2 in human colon cancer cell lines. We also looked at the function of NDRG2 in tumorigenesis, especially the engagement of NDRG2 in Wnt signal by examining the modulation of β-catenin. In NDRG2-overexpressing cells, we found a decrease in levels of intracellular β-catenin and a subsequent decrease in TCF/LEF transcription activities.
Materials and methods
Patient samples and cell lines
Human colorectal carcinoma samples were obtained from patients who underwent routine surgery for colorectal cancer at the Department of Surgery, Eulji University Hospital, between January 2002 and December 2005. For the immunohistochemical study, 25 colorectal adenomatous tissues, 99 colorectal carcinoma tissues and paired normal mucosal tissues taken from a site distant from the tumorous lesion were fixed in 10% neutralized-buffered formalin solution for 24 h. Some of the tissue specimens were immediately kept frozen after resection and stored in liquid nitrogen until further use. Each patient's clinical status was classified according to the pathological grade of the tumor size, lymph node and metastasis classification system. All cell lines used in our studies were purchased from American Type Culture Collection (Rockville, MD); KM12c, Colo205, HCT116, HT29, SW480 and SW620 cells. The cells were cultured in Dulbecco's Modified Eagle's Medium (Gibco BRL, Grand Island, NY) supplemented with 2 mM glutamine, 1% penicillin–streptomycin and 10% fetal bovine serum (Hyclon, Logan, UT) and kept at 37°C in a humidified incubator that was maintained with 5% CO2. Plasmid-containing wild-type or mutant NDRG2 coding region was transfected into the SW620 cell line using Lipofectamine plus reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For selection of the positive clones, 100 μg/ml of G418 (Sigma, St Louis, MO) was applied 48 h after transfection. Three weeks later, the colonized cells were selected and then cultured for further selection. After single cellular selection, the established cell lines were identified by western blotting with anti-human NDRG2 antibody and maintained with normal growth medium containing 100 μg/ml of G418.
Antibodies, western blotting and immunoprecipitation
Anti-β-catenin monoclonal antibody (mAb) was obtained from BD Biosciences (San Jose, CA). Anti-α-tubulin mAb and horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G (IgG) were purchased from Sigma. The anti-phospho-β-catenin, anti-phospho-Akt, anti-Akt, anti-GSK-3β and anti-phospho-GSK-3β were acquired from Cell Signaling Technology (Beverly, MA). Anti-His mAb was purchased from SantaCruz Biotechnology (Pasadena, CA). mAb to NDRG2 protein was generated from a hybridoma cell line established in house by fusing the splenocytes of Balb/c mice immunized with the recombinant NDRG2 protein with the SP2/0-Ag14 murine myeloma cells as described in USA patent 11/3938979. During western blot analysis, the cells were washed with phosphate-buffered saline (PBS) and lysed with cell lysis buffer [20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM ethylene glycol tetraacetic acid, 1 mM ethylenediaminetetraacetic acid, 1% NP-40, 2.5 mM sodium pyrophosphate, 1 mM Na3VO4, 1 mM NaF and protease inhibitors cocktail (Sigma)] on ice for 30 min. The lysates were then clarified by centrifugation and total protein content in the cell lysates was quantified by Bradford assay. The lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 10 or 12% polyacrylamide gels and transferred to polyvinylidene difluoride membranes, and the membranes were blocked with 5% non-fat dry skim milk in Tris-buffered saline (20 mM Tris–HCl, pH 7.4, 150 mM NaCl) at room temperature. After incubation with the appropriate primary antibodies for 2 h at room temperature, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h and visualized with an ECL® detection system (Amersham Pharmacia Biotech, Piscataway, NJ). To detect the interaction of β-catenin and NDRG2, the lysates from NDRG2-overexpressing SW620 and Colo205 cells were immunoprecipitated with an anti-NDRG2 antibody. The immunocomplexes were captured by the protein G agarose (BD Biosciences), the precipitates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and NDRG2 and β-catenin were analyzed by western blotting. The inhibition of NDRG2 expression in NDRG2-overexpressed SW620 cells was achieved using 1 μg of NDRG2-specific SMARTPool small interfering RNA (siRNA) (Dharmacon, Lafayette, CO). Cells were transfected by electroporation and immediately transferred to supplemented medium in 6-well plates and incubated at 37°C for 16 h. Cells were then harvested and further cultured for experiments
Plasmids construction and transfection
Full-length complementary DNA (cDNA) for human NDRG2 (NM_201536) was obtained from a cDNA library via polymerase chain reaction (PCR) amplification and cloned into the BamHI/XhoI site of pcDNA3.1 (+) (Invitrogen). Amino acid substitution or deletion of human NDRG2 was introduced into the constructed pcDNA3.1-NDRG2 plasmid using QuikChange™ Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) following the instruction manual. The PCR mixture contained 20 ng of template plasmid DNA, 125 ng of each mutagenic primer, 5 μl of 10× reaction buffer, 1 μl of dNTPs and 2.5 U PfuTurbo® DNA polymerase (Stratagene) in a total volume of 50 μl. PCR was performed under the following conditions: denaturation at 95°C for 2 min; followed by 16 cycles of denaturation at 95°C for 40 s; annealing at 58°C for 50 s and extension at 68°C for 15 min. An aliquot of the PCR mixture was analyzed by agarose gel electrophoresis and was used to transform DH5α competent cells after treatment with 10 U of DpnI restriction enzyme (Roche, Indianapolis, IN) at 37°C for 2 h. The integrity of the mutant NDRG2 sequences was confirmed by DNA sequencing. Cells at 80% confluence were transfected with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. The amount of DNA used for transfection was 1.5 μg per well in a 6-well plate. After 1–4 days, the transfected cells were washed and harvested for downstream experiments. The nucleotide sequences for the site-directed mutagenesis are as follows: NDRG2Δ302—5′-GCAAGGCATGGGCTAAATGGCCTCATCCTGC-3′, 5′-GCAGGATGAGGCCATTTAGCCCATGCCTTGC-3′ and NDRG2T334A—5′-CGGTCCCGCTCTCGCGCCCTGTCCCAGAGCA-3′, 5′-TGCTCTGGGACAGGGCGCGAGAGCGGGACCG-3′.
Luciferase reporter assay
The cells were transfected with TOPflash (or FOPflash, which harbors mutant TCF-binding sites) luciferase reporter plasmid (Upstate Biotechnology, Lake Placid, NY) and the appropriate protein-expressing vectors. β-Galactosidase was used to normalize the transfection efficiency of the cells. Following transfection, 20 mM of LiCl (Sigma) was added to the cells for 6–12 h. After 2 days, the cells were lysed with lysing buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100) on ice for 30 min, and the cleared lysates were then transferred to a 96-well plate, followed by the addition of luciferase assay reagent (Promega, Madison, WI). The light intensity of the reaction was determined using a plate-reading luminometer (Turner Designs, Sunnyvale, CA) and luciferase intensity was calculated relative to β-galactosidase activity. All experimental and control groups contained at least three wells, and the results were reported as mean absorption ± standard error.
Confocal microscopy
Cells were cultured on coverslips, rinsed three times in cold PBS, fixed with 4% paraformaldehyde at room temperature for 20 min and permeabilized with 0.1% Triton X-100 in PBS for 10 min. Next, the cells were blocked with 1% bovine serum albumin in PBS for 30 min and then stained with anti-β-catenin and/or anti-NDRG2 mAbs for 2 h. Finally, the cells were incubated with Alexa Fluor 488 or 594-conjugated rabbit anti-mouse IgG (Molecular Probe, Eugene, OR) in dark for 1 h and the nuclei were stained with 4′,6-diamidino-2-phenylindole. The coverslips containing the cells were mounted on glass slides using VectaShield mounting medium (Vector Laboratories, Burlingame, CA) and visualized using a Zeiss confocal microscope LSM510META (Carl Zeiss, Jena, Germany) at ×40 magnification. The confocal images were captured using the Zeiss LSM Image Browser program.
RT–PCR analysis
Several groups of tissue pairs including normal and cancerous region from patients with colon cancer were obtained from the tumor tissue bank at Eulji University hospital. Prepared colon cancer tissues were lysed and total RNA was extracted using TRIZOL reagent (Invitrogen) according to manufacturer's instructions. Five microgram of total RNA was used for reverse transcription to generate cDNAs using a ProSTAR first-strand reverse transcription–polymerase chain reaction (RT–PCR) kit (Stratagene). PCR reaction for each target gene was executed using the cDNA as template with specific primer pairs. β-Actin was used as a reaction standard. The primer pairs that were used are as follows: NDRG2—5′-GGACATCTTTTCAGCCAGGA-3′ (F), 5′- CCCATGCCTTGCAGGAAGT-3′ (R); β-actin—5′-AGCCGTGGCCATCTCTTGCTCGAAG-3′ (F), 5′-GCCATGTACGTTGCTATCCAGGCTG-3′ (R); GAPDH—5′-CCATCACCATCTTCCAGGAG-3′ (F), 5′-ACAGTCTTCTGGGTGGCAGT-3′ (R); Cyclin D1—5′-AACTACCTGGACCGCTTCCT-3′ (F), 5′-CCACTTGAGCTTGTTCACCA-3′(R) and Fibronectin—5′-CGGGAATCTTCTCTGTCAGC-3′ (F), 5′-GCCATGACAATGGTGTGAAC-3′.
Specimen preparation and immunohistochemistry
Tissue specimens obtained from therapeutic procedures were fixed in neutral-buffered formalin (10% vol/vol formalin in water; pH 7.4) and embedded in paraffin wax. Serial sections of 4 μm thickness were cut and mounted on glass slides (Superfrost Plus; Fisher Scientific, Rochester, NY). Immunohistochemistry conditions for NDRG2 were optimized and evaluated by two independent pathologists (J.H.K. and H.J.S.). In brief, tissue sections were microwaved twice for 10 min in citrate buffer (pH 6.0) for antigen retrieval. The sections were then treated with 3% hydrogen peroxide in methanol to quench the endogenous peroxidase activity followed by incubation with 1% bovine serum albumin to block non-specific binding. Mouse mAb against NDRG2 (clone# 18c12) was used at a dilution of 1:200 in PBS. The avidin–biotin detection method was used and tissue sections were immersed in 3-amino-9-ethyl carbazole as a substrate and then counterstained with 10% Mayer's hematoxylin, dehydrated and mounted by crystal mount. An unrelated mouse IgG was used as a negative control.
Assessment of immunostaining and statistical analysis
Each slide was evaluated for NDRG2 immunoreactivity using a semiquantitative scoring system for both the intensity of the stain and the percentage of positive neoplastic cells. NDRG2 immunoreactivity was observed primarily in the cytosolic membrane, although cytosolic expression of NDRG2 was noted in some colorectal mucosal and malignant cells. The intensity of membrane or cytosolic staining was scored using the following system: 0, lower than the adjacent normal-appearing mucosal epithelium; 1, similar to the adjacent mucosal epithelium; 2, stronger than the adjacent mucosal epithelium. The percentage of cells displaying a stronger staining intensity than the adjacent mucosal epithelium was scored as 1 (0–24% tumor cells stained); 2 (25–49% tumor cells stained); 3 (50–74% tumor cells stained); 4 (75–100% tumor cells stained). For the purpose of statistical analysis, the median of this series (25% of malignant cells showing a stronger intensity than adjacent mucosal epithelium) was used as a cutoff value to distinguish tumors with a low (<25%) or high (≥25%) level of NDRG2 expression. The relationship between the results of the immunohistochemical study and the clinicopathologic parameters was determined using the SAS® software package (version 9.01; SAS Institute, Cary, NC). The correlation between staining index scores and other categorical factors was analyzed using the Pearson's chi-square test of independence. Results were considered statistically significant at P < 0.05.
Results
NDRG2 mRNA was expressed differentially in human colon carcinoma tissues and colon cancer cell lines
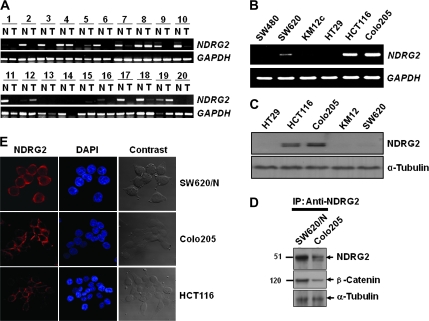
To compare NDRG2 expression levels in colon cancer tissues, we examined the messenger RNA (mRNA) level of NDRG2 by performing RT–PCR analysis on pairs of tissue containing normal and tumor tissues from the same donor. β-Actin was used as a reference gene to correct for the variations in the amount of mRNA in individual samples. As shown in Figure 1A, 20 cases of colon cancer tissues randomly selected from clinically diagnosed patients showed significant decrease of NDRG2 mRNA expression compared with normal tissue from the same patients. RT–PCR analyses also showed differential expression of NDRG2 in human colon cancer cell lines, which was especially high in HCT116 and Colo205 cell lines (Figure 1B). The levels of NDRG2 mRNA in colon cancer cell lines correlated with those of NDRG2 protein as shown by western blot analysis (Figure 1C). SW620 cell was shown very slightly expressed NDRG2 protein.
Fig. 1.
Differential expression of NDRG2 in colon tumor tissues and colon cancer cell lines. (A) NDRG2 mRNA levels in colon tumor tissues were evaluated by RT–PCR. T indicates colon tumor tissues and N represents normal mucosa tissues adjacent to tumor. Quality of total RNA used for RT was evaluated by gel electrophoresis on 1% denaturing agarose gel and the amount of RT products was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B) Endogenous expression of NDRG2 in colon cancer cell lines was also examined by RT–PCR. (C) Expression level of NDRG2 protein in colon cancer cell lines was analyzed by western blot analysis. α-Tubulin was used as a loading control. (D) NDRG2 was immunoprecipitated with an anti-NDRG2 antibody, and the precipitant was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western Blot analysis using anti-β-catenin antibody. (E) Intracellular localization of NDRG2 was determined using a confocal microscope. For nuclear staining, 4′,6-diamidino-2-phenylindole (DAPI) was used. NDGR2 introduced SW620 (SW620/N), HCT116 and Colo205 showed high expression levels of the protein, which localized mainly in the plasma membrane and cytosol.
NDRG2 was localized mainly on plasma membrane and cytosol of colon cell lines
Analysis using confocal microscopy gave evidence that the endogenous form of the intracellular NDRG2 protein was also recognized by the generated mAb. Western blot analysis in colon cancer cell lines showed that intracellular NDRG2 was localized mainly in the plasma membrane of the colon cancer cells, HCT116 and Colo205, which exhibited high expression level of NDRG2 protein (Figure 1C and E). Additionally, the exogenously introduced NDRG2 was localized mainly in the plasma membrane and cytosol of the SW620 colon cancer cell line, although a small proportion of the NDRG2 was also observed in the nucleus (Figures 1E and 3C).
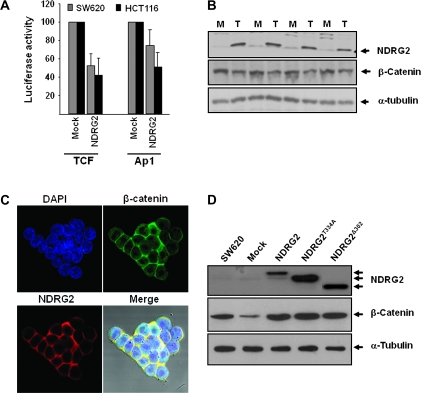
Fig. 3.
Modulation of TCF/β-catenin signaling by NDRG2. TOPflash luciferase reporter assay system containing a luciferase reporter plasmid with three copies of the optimal TCF-/LEF-binding sites upstream of the minimal thymidine kinase promoter was used to elucidate the role of NDRG2 in modulation of Wnt/β-catenin signaling. (A) Transiently transfected NDRG2 induced the decrease of TCF/LEF transcription activity in SW620 and HCT116. NDRG2 was overexpressed in SW620 cells, which originally expressed low levels of NDRG2. (B and C) Significant reduction in β-catenin expression was observed in the four selected clones, but its localization was not changed. (D) Mutant forms of NDRG2, NDRG2T334A and NDRG2Δ302 generated by site-directed mutagenesis did not induce downregulation of β-catenin. M and T represent cells transfected with empty vector or NDRG2-expressing vector, respectively.
NDRG2 expression in diverse colon cancer tissues
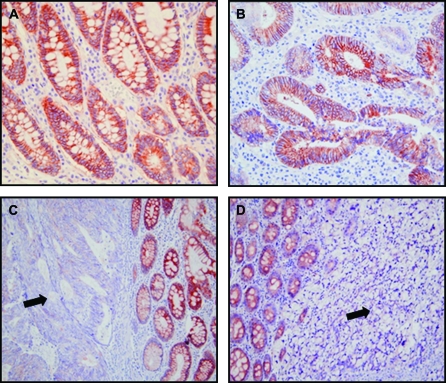
Expression levels and patterns of NDRG2 in human colorectal carcinomas were evaluated by immunohistochemical analysis (Figure 2). NDRG2 was highly expressed in normal colonic mucosa (100%) from patients both in the cytosol and plasma membrane (Figure 2A). In addition, staining was well preserved in the dysplastic epithelial cells in all adenomatous polyps that were examined. A high level of NDRG2 expression was observed in 44 (44%) of 99 patient's tissues with colorectal cancer. NDRG2 was distributed primarily in the cytosolic membrane and partly in the cytosol of tumor cells. The variations of staining intensity were dependent on tumor differentiation. NDRG2 was distinctively expressed in well-differentiated colorectal carcinomas (Figure 2B) compared with moderately and poorly differentiated tumor tissues (Figure 2C and D) in all of the cases examined. In some moderately or poorly differentiated cases, staining was heterogenous with faint labeling alternating with areas displaying no labeling. Interestingly, NDRG2 was more strongly expressed in the well-differentiated area than the less-differentiated area even in the same colon tissue of each patient. NDRG2 was expressed in colon cancer tissues in a tumor invasion depth-dependent manner; NDRG2 expression was significantly lower in advanced colorectal carcinomas compared with normal mucosal tissues or early invasive cancer tissues from the same cancer patients. Notably, normal dendritic cells exhibited cytologic labeling for NDRG2 in the tumor areas.
Fig. 2.
NDRG2 expression in patients with colon carcinoma. NDRG2 was highly expressed in the normal colonic mucosa, but its expression was decreased in colon cancer tissues as shown by immunohistochemical staining with anti-NDRG2 antibody. (A) Mucosal epithelial cells exhibited strong immunoreaction with a progressive increase along the crypt toward the upper part from the deeper part (×40 magnification). (B) In well-differentiated adenocarcinoma, NDRG2 was strongly expressed primarily on the cytosolic membrane of tumor cells (×40 magnification). The variations of staining in neoplastic cells correlated with the differentiation state of the tumor. (C) The staining signals in moderately differentiated adenocarcinomatous tissue (arrowhead) were very weak or null (×20 original magnification). (D) In poorly differentiated adenocarcinoma (arrowhead), tumor cells did not show any staining, whereas the adjacent normal epithelium showed strong signals (×20 original magnification).
Association of NDRG2 expression levels and clinicopathological characteristics in colorectal cancer
We first examined whether the expression level of NDRG2 correlated with clinicopathological prognostic parameters. The analysis showed that the expression level of NDRG2 positively correlated with histologic tumor differentiation but inversely correlated with tumor invasion depth and clinical Dukes’ stage in univariate analysis (Table I). We carried out multivariate logistic analyses to assess the predictive value of NDRG2 expression status for clinicopathologic significance by adjusting other potentially prognostic parameters. The results corroborated that tumor differentiation and the expression level of NDRG2 was a significant covariate; however, clinical Dukes’ stage and high level of NDRG2 expression was a reverse covariate, respectively (Table II). A possible inverse correlation between high NDRG2 expression and tumor size (P = 0.054) or the presence of nodal metastasis (P = 0.0719) was notable, although the values did not reach statistical significance at the level of 0.05. These findings suggest the possibility that NDRG2 protein might play a role in the maintenance of differentiation of colorectal epithelial cells and the delay of development and progression of human colorectal adenocarcinoma.
Table I.
Clinicopathologic parameters and the expression status of NDRG2
| Characterstics | Total | NDRG2 expression level |
P | |||
| Negative/low |
High |
|||||
| n | % | n | % | |||
| Age (years) | 0.0561 | |||||
| <50 | 37 | 27 | 73.0 | 10 | 27.0 | |
| ≥50 | 62 | 28 | 45.2 | 34 | 54.8 | |
| Gender | 0.1795 | |||||
| Female | 51 | 25 | 49.0 | 26 | 51.0 | |
| Male | 48 | 30 | 62.5 | 18 | 37.5 | |
| Site | 0.5187 | |||||
| Right/transverse colon | 28 | 17 | 60.7 | 11 | 39.3 | |
| Left colon and rectum | 71 | 38 | 53.5 | 33 | 46.5 | |
| Size | 0.4251 | |||||
| <5 cm in diameter | 60 | 32 | 53.3 | 28 | 46.7 | |
| ≥5 cm in diameter | 39 | 23 | 59.0 | 16 | 41.0 | |
| Differentiation | <0.0001 | |||||
| Well | 28 | 5 | 17.9 | 23 | 82.1 | |
| Moderately | 46 | 25 | 54.3 | 21 | 45.7 | |
| Poorly | 25 | 25 | 100 | 0 | 0 | |
| Invasion depth | 0.0015 | |||||
| T1 | 3 | 0 | 0 | 3 | 100 | |
| T2 | 18 | 4 | 22.2 | 14 | 77.8 | |
| T3 | 72 | 48 | 66.7 | 24 | 33.3 | |
| T4 | 6 | 3 | 50.0 | 3 | 50.0 | |
| Nodal status | 0.0719 | |||||
| N0 | 44 | 20 | 45.5 | 24 | 54.5 | |
| N1 | 55 | 35 | 63.6 | 20 | 36.4 | |
| Distant metastasis | 1.00 | |||||
| M0 | 90 | 50 | 55.6 | 40 | 44.4 | |
| M1 | 9 | 5 | 55.6 | 4 | 44.4 | |
| Dukes’ stage | 0.0004 | |||||
| A | 17 | 2 | 11.8 | 15 | 88.2 | |
| B | 27 | 18 | 66.7 | 9 | 33.3 | |
| C | 55 | 35 | 63.6 | 20 | 36.4 | |
Table II.
Multivariate logistic regression analysis with the expression status of NDRG2
| Categories | Odds ratio | 95% Confidence limits | P-value |
| Differentiation | <0.0001 | ||
| Moderately versus well | 0.150 | 0.047–0.477 | |
| Poorly versus well | 0.017 | 0.002–0.200 | |
| Dukes' stage | 0.002 | ||
| Dukes A versus C | 10.195 | 2.092–49.69 | |
| Dukes B versus C | 0.698 | 0.210–2.314 |
TCF/β-catenin signaling was reduced in NDRG2 overexpressing cells
To elucidate the effect of NDRG2 on the differentiation of colon cancer cells, reporter assays for several transcription factor activities were developed via transient transfection in SW620 and KM12c colon cancer cell lines. Introduction of full-length NDRG2 induced the decrease of TCF/LEF transcription activity in HCT116 and SW620 cell lines (Figure 3A), whereas the activities of activator protein-1 (AP-1) were slightly reduced in both cell lines (Figure 3A). The reduced activity of AP-1 induced growth retardation (27) and colonocyte differentiation (25). To further study the regulation of TCF/β-catenin signaling by NDRG2, we established SW620 cell lines that express NDRG2 protein constitutively (SW620/N). Interestingly, β-catenin expression was decreased in all of the four selected cell clones (Figure 3B), although there was no significant change in its localization induced by NDRG2 introduction (Figure 3C). Immunoprecipitation with anti-NDRG2 antibody was carried out to verify interacting of NDRG2 with β-catenin. β-Catenin was detected in the precipitate (Figure 1D). Western blot analysis of colon cancer cell lines showed that NDRG2 was separated into two bands (Figure 1C). We hypothesized that the slow migrating band is a phosphorylated form of the faster migrating band because the slow migrating band disappeared when the threonine residue at 334 amino acid (which matches with the 348th amino acid in the longer form of NDRG2, NDRG2a) was changed into alanine by site-directed mutagenesis. NDRG2T334A protein exhibited the same migration rate as the higher migrating band of wild-type NDRG2 and could not induce the decrease of β-catenin expression. The level of β-catenin protein was not changed by the overexpression of NDRG2Δ302 nor by the overexpression of NDRG2T334A (Figure 3D).
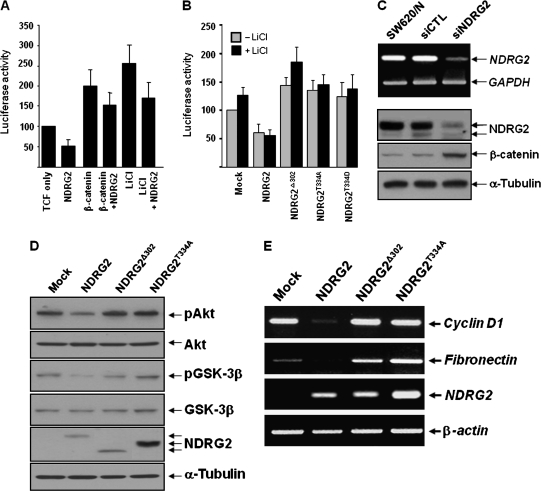
NDRG2 modulates TCF/β-catenin signaling via the regulation of GSK-3β phosphorylation
Protein stability of β-catenin is known to be regulated by the kinase activity of GSK-3β that seemed to be affected by NDRG2 function. TCF/LEF activity in SW620 cells was enhanced by treatment with LiCl, a well-known GSK-3β inhibitor, as well as by cotransfection of β-catenin. NDRG2-induced decrease of TCF/LEF activity was also detected under these enhanced conditions (Figure 4A). The data that NDRG2 attenuated the increase of TCF/LEF activity following LiCl treatment support the notion that NDRG2 may regulate the kinase activity of GSK-3β. Mutants NDRG2, NDRG2T334A, NDRG2T334D and NDRG2Δ302 showed no effect on the transcription activity of TCF/LEF (Figure 4B). Otherwise, as the expression of NDRG2 was downregulated by NDRG2 siRNA, the protein level of β-catenin was increased (Figure 4C). As shown in Figure 4D, the inhibitory phosphorylation of GSK-3β at Ser9 and Akt was reduced in SW620-NDRG2 cell line, which activated the kinase activity of GSK-3β and induced the degradation of β-catenin protein. The effect of NDRG2 on TCF/β-catenin signaling was verified by RT–PCR analysis of TCF/LEF target genes. Cyclin D1 and fibronectin are known to have TCF-/LEF-binding sites in their promoter regions and their transcription was decreased by NDRG2 expression (Figure 4E). However, their expression was not changed in SW620-NDRG2T334A and SW620-NDRG2Δ302, indicating that NDRG2 phosphorylation in its C-terminal region is critical to the regulation of the TCF/LEF transcription activity. As expected, NDRG2T334A and NDRG2Δ302 showed no effect on the phosphorylation status of GSK-3β or on the degradation of β-catenin (Figures 3D and 4D).
Fig. 4.
NDRG2 regulates the phosphorylation of GSK-3β. (A) NDRG2 introduction attenuated the increase of TCF/LEF activity following LiCl or β-catenin treatment. (B) Mutant NDRG2, NDRG2T334A and NDRG2Δ302 showed no effect on the transcription activity of TCF/LEF. (C) SW620/N was treated with NDRG2 siRNA. The reduction of NDRG2 mRNA and protein levels by siRNA was confirmed by RT–PCR and western blot analyses. The protein level of β-catenin was increased in the NDRG2 siRNA-introduced cells. (D) The suppression of LiCl-induced TCF activation by NDRG2 presented the possible function of NDRG2 in the regulation of GSK-3β activity, and the inhibitory phosphorylation of GSK-3β was reduced by NDRG2 overexpression. (E) Target genes of TCF/LEF transcription factor were downregulated following NDRG2 introduction.
Discussion
NDRG2 has been studied in a number of cell and animal systems, and it has been shown to influence diverse cellular processes such as differentiation and proliferation, although its exact molecular mechanism is not yet known. In this study, we discovered that NDRG2 expression decreases with tumor stages and modulates the transcriptional activity of TCF/LEF through the regulation of β-catenin stability, which relates NDRG2 function with tumorigenesis of colon cells. The TCF/β-catenin-signaling pathway has been implicated in the regulation of colonic epithelial cell proliferation and differentiation (19–21). For the first time, we showed that the upregulation of NDRG2 induced the downregulation TCF/β-catenin signaling in human colon cancer cells.
The intracellular concentration of β-catenin is regulated by its degradation, which occurs via interaction with APC tumor suppressor protein and phosphorylation at its N-terminus through the interaction with GSK-3β (28–31). In the majority of sporadic colorectal cancer cases, the rate-limiting event is either loss of APC function or oncogenic β-catenin mutations, which makes β-catenin resistant to proteolytic degradation. Intracellular β-catenin accumulation eventually results in the formation of β-catenin–TCF complex and its nuclear translocation, followed by the stimulation of tumor formation via increases in the expression of c-Myc and cyclin D1 (32,33). Our data show that NDRG2 introduction into SW620 cells induces a decrease in GSK-3β phosphorylation, which increases its kinase activity (Figure 4D) and subsequently induces β-catenin downregulation (Figures 3D and 4C). SW620 cell line is known to have a mutant APC type and wild-type β-catenin. Large proportion of β-catenin in wild-type SW620 cell line was shown to localize in the nucleus (Figure 3C). The decrease of intracellular β-catenin following the introduction of NDRG2 in this cell type induces the decrease of nuclear β-catenin, which then results in the attenuation of TCF/LEF activity.
We also showed that the phosphorylation of NDRG2 might be essential for the regulation of TCF/β-catenin signaling. The data from NDRG2 mutants showed that the phosphorylation of NDRG2 is crucial for activating TCF/LEF signaling. The mutant forms of NDRG2, including NDRG2Δ302, NDRG2T334A and NDRG2T334D, showed no effect on the transcription activity of TCF/LEF (Figure 4B). Additionally, the expression levels of fibronectin and cyclin D1, which are well known as being modulated by TCF/LEF transcriptional activity, were reduced only in wild-type NDRG2-overexpressing cells (Figure 4E). Threonine residue at the 334 amino acid position is known to be phosphorylated by Akt kinase. Further, examination of the (phosphoinositide 3-kinase/Akt) pathway in relation to NDRG2 function in TCF/β-catenin signaling is needed to elucidate its roles in tumor development.
The mAb that was generated with specific binding capability was used to determine NDRG2 expression in human colon cancer tissues. The data from immunohistochemical analysis are noteworthy in that NDRG2 is expressed in the well-differentiated tumor tissues and the earliest stage of colon cancer, but the more advanced and poorly differentiated tumor tissues showed lower or no NDRG2 expression levels (Figure 2, Table I). Normal colonic mucosa and premalignant lesions such as dysplasia showed well-defined membraneous expression of NDRG2 in colon cells (Figure 2). Recently, Lorentzen et al. (34) reported that NDRG2 expression was downregulated at a late stage in colorectal carcinogenesis. The biological role of NDRG2 as suppressor of metastasis was reported in liver cancer (35). Guan et al. (36) reported that DRG1 expressed primary colonic and metastatic tumors as well as eight cancer cell lines (including SW620 and Colo205 cells) and it might be an element in colonic epithelial cell differentiation because its expression was downregulated in metastatic lesions and cell lines, and overexpression induced differentiation and suppressed invasiveness like as those of NDRG2. Our data correlate with their RT–PCR data and present the same question, whether NDRG2 downregulation is a cause or a consequence of tumor cell malignancy.
The TCF/β-catenin inhibitory activities of NDRG2 suggest that colon cancer with higher NDRG2 levels is probably to correspond with better biologic behavior and clinical prognosis (Figure 2, Tables I and II). Considering the potential modulator of additional therapies, the TCF/β-catenin inhibitory activities of NDRG2 would also be important in providing a novel strategy for the treatment of cancer and its metastasis.
The role of Akt/GSK-3β/β-catenin transduction pathway has been investigated in muscle anti-atrophy action (37), in adipogenesis (38) and in cell cycle progression (39). Schakman et al. (37) reported that muscle atropy induced by dexamethasone administration occurred with decrease in Akt phosphorylation together with a decrease in β-catenin protein levels. Here, we suggest that NDRG2 might be crucial for TCF/β-catenin signaling and that this TCF/β-catenin signal regulation is mediated through the phosphorylation of NDRG2 as well as the phosphorylation of GSK-3β. However, the regulatory mechanism of NDRG2 phosphorylation and its role in the control of GSK-3β activity should be examined more deeply. Thus, further studies will be required in order to clarify and elucidate the mechanisms underlying NDRG2-mediated inhibition of TCF/β-catenin signaling in the tumorigenesis of human colorectal cancer.
Funding
21C Frontier Stem Cell Research Project (KGM1210821 to J.W.K.); Korea Science and Engineering Foundation, Research Center for Women's Diseases, Sookmyung Women's University to S.Y.Y. and D.C.; 2007 Eulji Research Grant (EJRG-07-002-12E05 to J.H.K.).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- APC
adenomatous polyposis coli
- cDNA
complementary DNA
- GSK-3β
glycogen synthase kinase-3beta
- LEF
lymphoid enhancer factor
- MAb
monoclonal antibody
- mRNA
messenger RNA
- NDRG
N-Myc downstream-regulated gene
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- RT–PCR
reverse transcription–polymerase chain reaction
- siRNA
small interfering RNA
- TCF
T-cell factor
References
- 1.Zhang J, et al. The repression of human differentiation-related gene NDRG2 expression by Myc via Miz-1-dependent interaction with the NDRG2 core promoter. J. Biol. Chem. 2006;281:39159–39168. doi: 10.1074/jbc.M605820200. [DOI] [PubMed] [Google Scholar]
- 2.Kovacevic Z, et al. The metastasis suppressor, Ndrg-1: a new ally in the fight against cancer. Carcinogenesis. 2006;27:2355–2366. doi: 10.1093/carcin/bgl146. [DOI] [PubMed] [Google Scholar]
- 3.Liu N, et al. Promoter methylation, mutation, and genomic deletion are involved in the decreased NDRG2 expression levels in several cancer cell lines. Biochem. Biophys. Res. Commun. 2007;358:164–169. doi: 10.1016/j.bbrc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 4.Lusis EA, et al. Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res. 2005;65:7121–7126. doi: 10.1158/0008-5472.CAN-05-0043. [DOI] [PubMed] [Google Scholar]
- 5.Hu XL, et al. NDRG2 expression and mutation in human liver and pancreatic cancers. World J. Gastroenterol. 2004;10:3518–3521. doi: 10.3748/wjg.v10.i23.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng Y, et al. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int. J. Cancer. 2003;106:342–347. doi: 10.1002/ijc.11228. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, et al. Ndrg2 promotes neurite outgrowth of NGF-differentiated PC12 cells. Neurosci. Lett. 2005;388:157–162. doi: 10.1016/j.neulet.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Nichols NR, et al. Glucocorticoid regulation of glial responses during hippocampal neurodegeneration and regeneration. Brain Res. Brain Res. Rev. 2005;48:287–301. doi: 10.1016/j.brainresrev.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Mitchelmore C, et al. NDRG2: a novel Alzheimer's disease associated protein. Neurobiol. Dis. 2004;16:48–58. doi: 10.1016/j.nbd.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Choi SC, et al. Expression and regulation of NDRG2 (N-myc downstream regulated gene 2) during the differentiation of dendritic cells. FEBS Lett. 2003;553:413–418. doi: 10.1016/s0014-5793(03)01030-5. [DOI] [PubMed] [Google Scholar]
- 11.Burchfield JG, et al. Akt mediates insulin-stimulated phosphorylation of Ndrg2: evidence for cross-talk with protein kinase C theta. J. Biol. Chem. 2004;279:18623–18632. doi: 10.1074/jbc.M401504200. [DOI] [PubMed] [Google Scholar]
- 12.Boulkroun S, et al. Characterization of rat NDRG2 (N-Myc downstream regulated gene 2), a novel early mineralocorticoid-specific induced gene. J. Biol. Chem. 2002;277:31506–31515. doi: 10.1074/jbc.M200272200. [DOI] [PubMed] [Google Scholar]
- 13.Murray JT, et al. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem. J. 2004;384:477–488. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodgett JR. Regulation and functions of the glycogen synthase kinase-3 subfamily. Semin. Cancer Biol. 1994;5:269–275. [PubMed] [Google Scholar]
- 15.Cook D, et al. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J. 1996;15:4526–4536. [PMC free article] [PubMed] [Google Scholar]
- 16.Harwood AJ. Regulation of GSK-3: a cellular multiprocessor. Cell. 2001;105:821–824. doi: 10.1016/s0092-8674(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 17.Peifer M, et al. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 18.Taipale J, et al. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 19.Bienz M, et al. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 20.Moon RT, et al. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 21.Henderson BR, et al. The ins and outs of APC and beta-catenin nuclear transport. EMBO Rep. 2002;3:834–839. doi: 10.1093/embo-reports/kvf181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelton DN, et al. Up-regulation of CYP26A1 in adenomatous polyposis coli-deficient vertebrates via a WNT-dependent mechanism: implications for intestinal cell differentiation and colon tumor development. Cancer Res. 2006;66:7571–7577. doi: 10.1158/0008-5472.CAN-06-1067. [DOI] [PubMed] [Google Scholar]
- 23.Liu JJ, et al. Repression of HIP/RPL29 expression induces differentiation in colon cancer cells. J. Cell. Physiol. 2006;207:287–292. doi: 10.1002/jcp.20589. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarty S, et al. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 25.Mariadason JM, et al. Down-regulation of beta-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res. 2001;61:3465–3471. [PubMed] [Google Scholar]
- 26.Rockman SP, et al. Id2 is a target of the beta-catenin/T cell factor pathway in colon carcinoma. J. Biol. Chem. 2001;276:45113–45119. doi: 10.1074/jbc.M107742200. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, et al. NDRG2 suppresses cell proliferation through down-regulation of AP-1 activity in human colon carcinoma cells. Int. J. Cancer. 2009;124:7–15. doi: 10.1002/ijc.23945. [DOI] [PubMed] [Google Scholar]
- 28.Cohen P, et al. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 30.Salic A, et al. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol. Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 31.Behrens J, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 32.Tetsu O, et al. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 33.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 34.Lorentzen A, et al. Expression of NDRG2 is down-regulated in high-risk adenomas and colorectal carcinoma. BMC Cancer. 2007;7:192. doi: 10.1186/1471-2407-7-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DC, et al. Functional and clinical evidence for NDRG2 as a candidate suppressor of liver cancer metastasis. Cancer Res. 2008;68:4210–4220. doi: 10.1158/0008-5472.CAN-07-5040. [DOI] [PubMed] [Google Scholar]
- 36.Guan RJ, et al. Drg-1 as a differentiation-related, putative metastatic suppressor gene in human colon cancer. Cancer Res. 2000;60:749–755. [PubMed] [Google Scholar]
- 37.Schakman O, et al. Role of Akt/GSK-3beta/beta-catenin transduction pathway in the muscle anti-atrophy action of insulin-like growth factor-I in glucocorticoid-treated rats. Endocrinology. 2008;149:3900–3908. doi: 10.1210/en.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu D, et al. The effect of pleiotrophin signaling on adipogenesis. FEBS Lett. 2007;581:382–388. doi: 10.1016/j.febslet.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 39.Liang J, et al. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]