Abstract
Neurofibrillary tangles composed of hyperphosphorylated and aberrantly cleaved microtubule-associated protein tau are a major neuropathological hallmark of Alzheimer's disease. Recent studies suggest that the predominant neurotoxic effect of pathologically processed tau is mediated by intermediate tau multimers rather than the mature tau tangles, thus underscoring the importance of studying tau self-association preceding tangle formation. However, experimental approaches for such studies are limited. Here, we describe a modification of the β-galactosidase (β-gal) complementation assay, which provides a simple, sensitive and quantitative system to monitor pre-tangle tau-tau interactions in a cell model. Full-length tau (T4) and tau truncated at D421 (C3, to mimic caspase-cleaved tau) were fused to one of a pair of weakly complementing β-gal mutants (Δα and Δω) and expressed in human embryonic kidney cells. The tau-tau interactions and the subsequent juxtapositioning of Δα and Δω led to β-gal complementation and an increase in β-gal activity which was detected by histochemical staining and quantified by chemiluminescent assays. After cross-linking with disuccinimidyl suberate, tau formed high molecular weight complexes which were detected on denaturing acrylamide gels, further confirming the close proximity among self-associated tau molecules. The self-association of C3 appeared to be less efficient than that of T4. Furthermore, treatment with lithium decreased β-gal complementation of both T4 and C3 indicating that the interaction of these proteins was attenuated. Overall, this study suggests that β-gal complementation assay can be a useful tool to monitor tau self-association.
Keywords: Alzheimer's disease, lithium, tangles, tau-tau interactions, β-galactosidase activity
The neuropathology of Alzheimer's disease (AD) is characterized by extracellular senile plaques and intracellular neurofibrillary tangles (NFTs), which are mainly composed of abnormally accumulated hyperphosphorylated microtubule-associated protein tau (Grundke-Iqbal et al. 1986; Kosik et al. 1986). Tau is predominantly expressed in neurons where its primary function is to promote tubulin polymerization and microtubule stability (Johnson and Bailey 2002). Hyperphosphorylation of tau has been suggested to impair its ability to bind and stabilize microtubules, and promote tau self-assembly and aggregation (Alonso et al. 1994, 2001). In addition to hyperphosphorylation, aberrant cleavage is another pathological post-translational modification of tau that has been shown to play an important role in tau aggregation. In vitro, tau truncated at D421 (C3) that mimics in vivo cleavage by caspase 3 aggregates more rapidly and to a greater degree than full-length tau (T4) (Gamblin et al. 2003b; Rissman et al. 2004).
Although the abundance of NFTs correlates positively with the severity of cognitive impairment in AD (Grober et al. 1999), NFTs themselves are not necessarily the tau species inducing neurotoxicity. Attenuation of tau over-expression in a transgenic mouse model prevented further neuronal loss and memory impairment without decreasing the number of NFTs (Santacruz et al. 2005). Also, inhibition of tau hyperphosphorylation in another mouse model prevented severe motor deficits without reducing NFTs counts (Le Corre et al. 2006). These studies suggest that it is the process of tau aggregation and the intermediate tau species preceding the formation of mature NFTs that are likely neurotoxic. Therefore, it is important to study the formation of pre-tangle tau complexes. However, currently, there is no easily accessible and sensitive approach for monitoring such tau-tau interactions in a cell model.
Valuable insights into the conformation of tau and the mechanisms involved in tau polymerization have been provided by examining purified recombinant tau protein in vitro with techniques such as thioflavin S staining, electron microscopy, spectroscopy, and laser light scattering (Gamblin et al. 2003a; von Bergen et al. 2005). However, the readout of these techniques is the formation of tau filaments, a relative late stage in tau aggregation, and also it is not feasible to model the regulation of tau polymerization by other cellular factors in these in vitro systems. When in situ or in vivo systems are used, most studies on tau-tau interactions focus on the formation of insoluble tau aggregates using sarkosyl fractionation or electron microscopy (DeTure et al. 2002; Andorfer et al. 2003; Ferrari et al. 2003). In addition, quantitative assays for the measurement of pre-tangle tau self-association in situ, such as fluorescence resonance energy transfer (FRET) (Chun and Johnson 2007), are largely very technically demanding and require technology that is not always readily available.
Given the importance of understanding the process of tau self-association, the purpose of this study was to establish a relatively easy and accessible assay for the analysis of pre-tangle tau-tau interactions in situ. To accomplish this goal we adapted the β-galactosidase (β-gal) complementation system (Mohler and Blau 1996; Rossi et al. 1997) to monitor tau-tau interactions in mammalian cells. This technique has been used to monitor protein interactions such as oligomerization of cell surface receptors and clustering of integrins (Blakely et al. 2000; Yan et al. 2002; Buensuceso et al. 2003), but has never been applied to the study of interactions of aggregation-prone proteins involved in neurodegenerative processes. In this study, tau was fused to one of a pair of inactive β-gal mutants (Δα and Δω). The tau-tau interactions brought Δα and Δω into proximity and reconstituted β-gal activity, whereas in the absence of interaction, Δα and Δω only displayed a low level of spontaneous complementation. Using this assay, we further examined the effects of C-terminal tau truncation and lithium on tau self-association. Our data suggest that β-gal complementation assay is a promising new tool for studying tau self-association and its modulation.
Materials and methods
Constructs, cell culture, and transfection
The pWZL-Δα and pWZL-Δω retroviral vectors containing β-gal mutants Δα and Δω, respectively, were obtained from Dr Helen Blau (Stanford University) (Mohler and Blau 1996; Rossi et al. 1997). pcDNA3.1(-)-T4 containing human tau with four microtubule binding repeats but without exon 2 and 3, and pcDNA3.1(+)-C3 containing T4 truncated at D421 thus mimicking caspase 3 cleavage have been described previously (Cho and Johnson 2003). Δα and Δω were amplified and subcloned into pcDNA3.1(+) to generate pcDNA-Δα and pcDNA-Δω. T4Δα, T4Δω, C3Δα, and C3Δω were also generated by fusing Δα or Δω to the C-terminus of T4 or C3 with three residues Leu-Glu-Ser in between as a linker. LacZ construct was obtained from Stratagene (La Jolla, CA, USA). Human embryonic kidney (HEK) cells were grown at 37°C in Dulbecco's modified Eagle's medium/F-12 medium (Cellgro, Manassas, VA, USA) supplemented with 5% fetal bovine serum (HyClone, Logan, UT, USA), 2 mM L-glutamine, 10 U/mL penicillin and 100 U/mL streptomycin (Cellgro). Twenty-four hours after cells were plated, different combinations of constructs were transiently transfected into HEK cells with Effectene Reagent (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions and processed as following.
Histochemical assay for β-gal activity
The assay was performed according to a protocol described previously (Mohler and Blau 1996; Rossi et al. 1997). After transfection with the β-gal constructs for 48 h in a 12-well plate, HEK cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 25°C for 5 min and rinsed twice with PBS for 5 min. X-gal (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside; Sigma, St Louis, MO, USA) was diluted to a final concentration of 1 mg/mL in 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2 in PBS, applied to cells, incubated at 37°C overnight. Cells were rinsed twice with PBS for 5 min. Images were captured by an AxioCamMR3 camera connected to a Zeiss Axio Observer D1 microscope with an A-Plan 10X/0.25 Ph1 objective and exported in TIFF format using AXIOVISION software (Thornwood, NY, USA).
Chemiluminescent detection of β-gal activity
HEK cells were transfected with the β-gal constructs in 96-well white plates with clear bottoms. Gal-screen assays were performed as described in the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). Briefly, Gal-Screen substrate was diluted 1 : 25 in Gal-Screen Buffer A to prepare the reaction mixture. One hundred microliters of reaction mixture was added to each well containing 100 μL of culture medium, and chemiluminescent signal was read at 26°C for 2 h with a Synergy HT multi-mode microplate reader (BioTek, Winooski, VT, USA).
Cell lysis and immunoblotting
Cells were collected in 2x sodium dodecyl sulfate (SDS) stop buffer (0.25 M Tris-Cl pH 6.8, 10% glycerol, 2% SDS, 5 mM EDTA, 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, and 10 μg/mL of leupeptin, aprotinin, and pepstatin), mixed with equal volume of 2x protein loading buffer (2x SDS stop buffer, 25 mM dithiothreitol, and 0.01% bromophenol blue), and boiled for 5 min. Equal volumes of protein lysates were separated on 8% SDS-polyacrylamide gels, transferred to nitrocellulose membrane and probed with antibodies against β-gal (Abcam, Cambridge, MA, USA) or tau (Dako, Carpinteria, CA, USA). After incubation with the horseradish peroxidase-conjugated goat anti-rabbit IgG (H + L) secondary antibody, the blots were developed using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA).
Protein cross-linking
HEK cells transfected with β-gal constructs in 6-well plates were washed with PBS three times and resuspended in PBS pH 8.0. The protein cross-linker disuccinimidyl suberate (DSS; Pierce, Rockford, IL, USA) was added to the cell suspension at a final concentration of 5 mM and incubated at 25°C for 30 min before quenched with 20 mM Tris pH 7.5 for 15 min. An equal volume of 2x protein-loading buffer was added to lyse cells. After boiled for 10 min, protein lysates were separated on 4-12% gradient SDS-polyacrylamide gels and immunoblotted with appropriate antibodies.
Lithium chloride treatment
Seventeen hours after transfection with β-gal constructs, HEK cells were treated with 20 mM LiCl for 25 h followed by chemiluminescent assays for β-gal activity.
Statistic analysis
Data were analyzed using Student t-test, and values were considered significantly different when p < 0.05.
Results
Expression of tau-β-gal fusion proteins for β-gal complementation
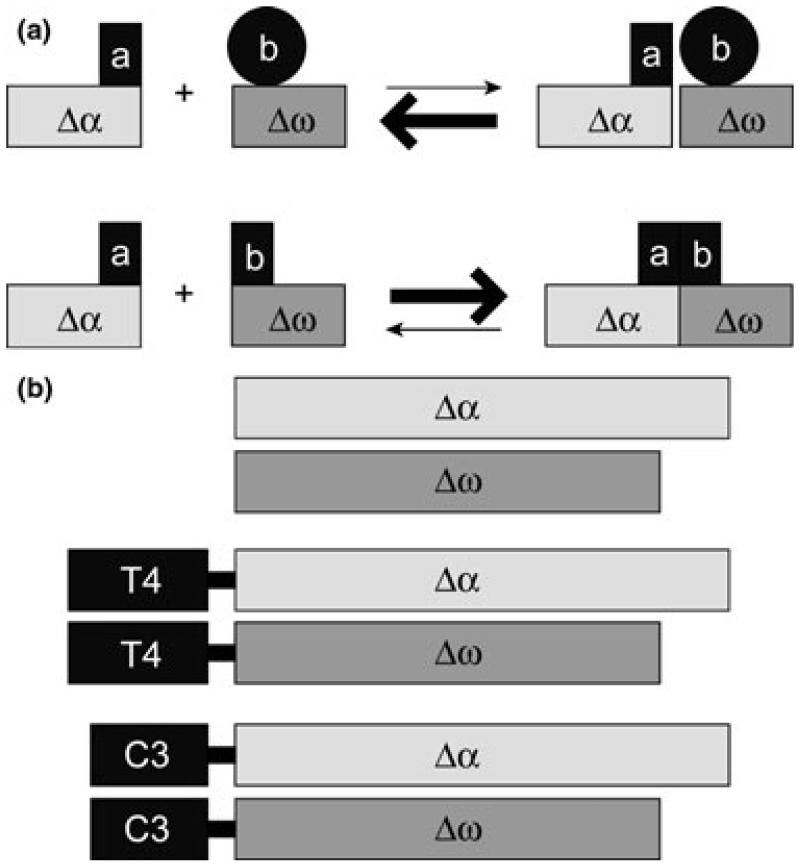
To monitor tau-tau interactions in cell model, we used a β-gal complementation system reported previously (Rossi et al. 1997). In this system, a pair of inactive β-gal mutants Δα and Δω complement spontaneously with very low efficiency. The interaction between the two proteins of interest fused to Δα and Δω drives β-gal complementation and reconstitutes β-gal activity (Fig. 1a). Thus, the enzyme activity based on complementing β-gal serves as a marker for protein-protein interactions. To test whether this system can be used to examine the interactions among tau proteins, we generated tau-β-gal fusion constructs by fusing Δα and Δω to the C-terminus of T4 and C3 (Fig. 1b). The approximately equivalent expression of Δα and various Δα fusion proteins or Δω and various Δω fusion proteins in HEK cells was confirmed by immunoblotting with antibodies against β-gal or tau (Fig. 2c). Δω and tau-Δω fusion proteins migrated faster than Δα and tau-Δα, respectively, because of the greater size of ω domain compared with α domain. Also as expected, there was a slight difference in migration between T4-β-gal fusion proteins and C3-β-gal fusion proteins because of the truncation in the C3 constructs.
Fig. 1.
Schematic diagram of β-galactosidase (β-gal) complementation and generation of tau-β-gal fusion constructs. (a) In the β-gal complementation assay, proteins of interest a and b are fused to the β-gal deletion mutants Δα or Δω. The interaction between a and b and the subsequent juxtapositioning of Δα and Δω lead to complementation and an increase in β-gal activity (lower panel), whereas in the absence of interaction, Δα and Δω only display a low level of spontaneous complementation (upper panel) (adapted from Rossi et al. 1997). (b) Tau-β-gal fusion constructs used in this study. Δα and Δω represent β-gal mutants lacking α and ω domains, respectively. Different fusion constructs were created by linking Δα or Δω to the C-terminus of full-length tau (T4) or tau truncated at D421 (C3) as indicated.
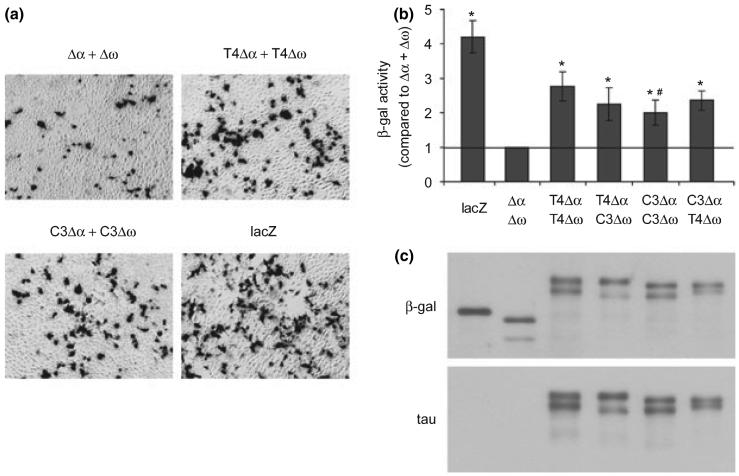
Fig. 2.
Tau-β-galactosidase (β-gal) fusion proteins complement. (a) Complementation detected by histochemistry. HEK cells were transfected with Δα + Δω, full-length tau (T4) Δα + T4Δω, or tau truncated at D421 (C3) Δα + C3Δω for 48 h followed by X-Gal reaction for β-gal activity. LacZ-transfected cells were used as positive controls. Shown is one representative experiment of three independent experiments with similar results. (b) Complementation quantified by chemiluminescent assay. HEK cells were transfected with β-gal proteins as indicated for 65 h followed by chemiluminescent assay for β-gal activity. LacZ-transfected cells were used as positive controls. The relative β-gal activities from three independent experiments with triplicates within each group (mean ± SEM) were expressed as fold increase relative to the negative control Δα + Δω. *p < 0.05 versus Δα + Δω. #p<0.05 versus T4Δα + T4Δω. (c) β-gal proteins in transfected HEK cells were expressed at approximately equivalent levels shown by immunoblotting with antibodies against β-gal and tau.
Tau-tau interactions monitored by β-gal complementation
To detect the β-gal complementation driven by tau-tau interactions, HEK cells transfected with three pairs of β-gal constructs (Δα + Δω, T4Δα + T4Δω, and C3Δα + C3Δω) were subjected to histochemical examination for β-gal activity (Fig. 2a). Cells transfected with lacZ were also stained to show efficient transfection and serve as a positive control for β-gal activity. The spontaneous complementation between Δα and Δω resulted in a very low percentage of X-Gal positive cells. But clearly, there were more X-Gal positive cells in cell cultures transfected with T4Δα + T4Δω or C3Δα + C3Δω than with Δα + Δω, suggesting that the interactions among T4 proteins or truncated tau proteins promoted β-gal complementation thus recreated β-gal activity. To quantify β-gal activity, we performed chemiluminescent assays on cells transfected with different pairs of β-gal constructs (Δα + Δω, T4Δα + T4Δω T4Δα + C3Δω, C3Δα + C3Δω, and C3Δα + T4Δω) (Fig. 2b). β-gal activities of all combinations of tau-β-gal fusion proteins were significantly higher than that of Δα + Δω. The β-gal proteins were all expressed at approximately similar levels (Fig. 2c), therefore, the increases of β-gal activity were indeed because of the complementation strengthened by tau-tau interactions. We also observed higher β-gal activity of T4Δα + T4Δω compared with that of C3Δα + C3Δω (Fig. 2b), suggesting that the intrinsic interactions among T4 are stronger than that of C3. Together, these data demonstrate that the self-association of tau can be detected and quantified with β-gal complementation assay.
Cross-linking of complementing tau-β-gal fusion proteins
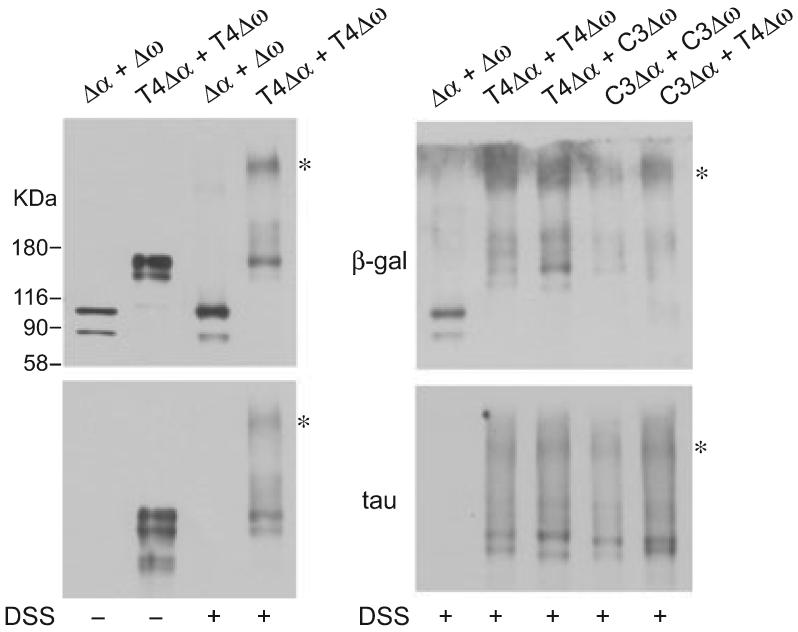
To further confirm the close proximity of the complementing tau-β-gal fusion proteins, transfected HEK cells were cross-linked intracellularly with the cell permeable cross-linker DSS and the protein lysates were separated on 4-12% gradient SDS-polyacrylamide gels. After cross-linking, T4Δα and T4Δω formed protein complexes of high molecular weight detected by both β-gal and tau antibodies, whereas protein complexes formed by Δα and Δω were barely detectable. Similar high molecular weight protein complexes were also detected with other combinations of tau-β-gal fusion proteins (Fig. 3).
Fig. 3.
Complementing tau-β-galactosidase (β-gal) fusion proteins were cross-linked by disuccinimidyl suberate (DSS). Human embryonic kidney (HEK) cells transfected with β-gal proteins as indicated were treated with dimethylsulfoxide or 5 mM DSS. Cell lysates were separated on 4-12% gradient sodium dodecyl sulfate-polyacrylamide gels and immunoblotted with antibodies against β-gal or tau. After cross-linking, full-length tau (T4) Δα and T4Δω formed protein complexes of high molecular weight detected by both β-gal and tau antibodies (asterisks), whereas protein complexes formed by Δα and Δω were barely detectable (left panel). Similar high molecular weight protein complexes were also detected with other combinations of tau-β-gal fusion proteins (right panel).
Lithium attenuates complementation of tau-β-gal fusion proteins
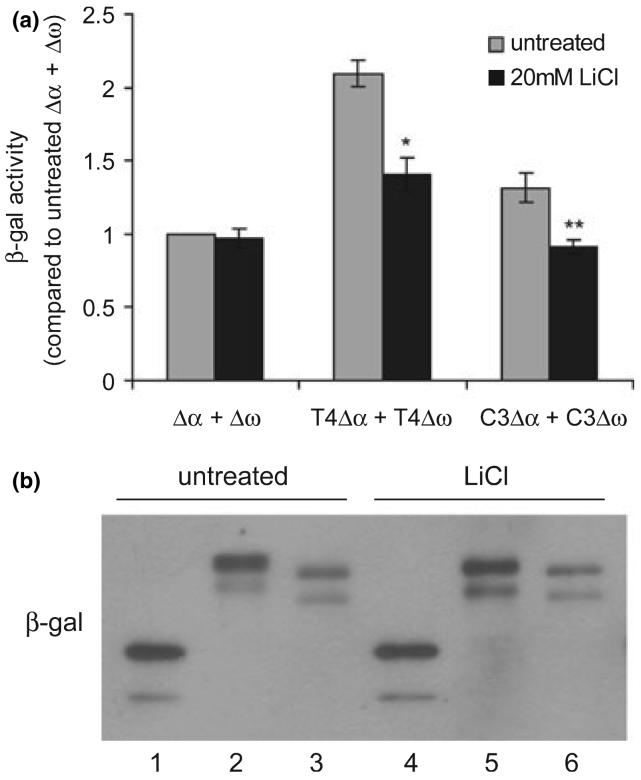
Lithium reduces tau phosphorylation by inhibiting glycogen synthase kinase 3β (GSK-3β) (Hong et al. 1997; Noble et al. 2005; Caccamo et al. 2007), a major kinase implicated in tau hyperphosphorylation in AD (Mazanetz and Fischer 2007). To test whether the tau-tau interactions and hence the complementation of tau-β-gal fusion proteins can be modulated, HEK cells transfected with Δα + Δω, T4Δα + T4Δω, or C3Δα + C3Δω were treated with lithium chloride and subjected to chemiluminescent assays for β-gal activity. Lithium treatment did not affect the spontaneous complementation of Δα and Δω; however, it significantly decreased the complementation of T4Δα and T4Δω and of C3Δα and C3Δω by 33% and 32%, respectively (Fig. 4a). To rule out the possibility that this decrease of complementation was due to the decrease of protein expression, we immunoblotted cell lysates from a duplicate experiment with an antibody against β-gal. The result shows that β-gal proteins were expressed at the similar levels in untreated and treated groups (Fig. 4b), suggesting that the decrease of complementation of tau-β-gal fusion proteins was caused by the attenuation of tau-tau interactions by lithium.
Fig. 4.
LiCl attenuates complementation of tau-β-galactosidase (β-gal) fusion proteins. Human embryonic kidney (HEK) cells were transfected with Δα + Δω, full-length tau (T4) Δα + T4Δω, or tau truncated at D421 (C3) Δα + C3Δω for 17 h and treated with or without 20 mM LiCl for 25 h. β-gal activities were measured by chemiluminescent assays. (a) Quantification of the relative β-gal activities from three independent experiments with triplicate within each group (mean ± SEM), expressed as fold increase relative to untreated Δα + Δω. LiCl treatment significantly attenuated β-gal activities of T4Δα + T4Δω and C3Δα + C3Δω but not that of Δα + Δω. *p<0.01 versus untreated T4Δα + T4Δω; **p<0.05 versus untreated C3Δα + C3Δω. (b) β-gal proteins in untreated and LiCl-treated cells were expressed at approximately equivalent levels shown by immunoblotting β-gal. Lane 1, 4: Δα + Δω; lane 2, 5: T4Δα + T4Δω; lane 3, 6: C3Δα + C3Δω.
Discussion
NFTs observed in AD mainly consist of paired helical filaments of aggregated tau. Given that there was a positive correlation between NFTs and cognitive impairment (Grober et al. 1999), it was originally hypothesized that NFTs are the tau species that cause neurotoxicity. However, in a mutant tau transgenic Drosophila model progressive neurodegeneration occurred without NFTs formation (Wittmann et al. 2001). More importantly, in an inducible tau transgenic mouse model for tauopathy, turning off tau expression rescued memory impairments and neuronal loss but NFTs continued to accumulate (Santacruz et al. 2005). Additionally, reduction of endogenous tau prevented behavioral abnormalities in an amyloid precursor protein transgenic mouse model, in which substantial neurofibrillary pathology is absent (Roberson et al. 2007). Thus, the accumulation of NFTs alone can be dissociated from neuronal toxicity and cognitive deficits. These findings stimulated interest in studying the formation of tau complexes in pre-tangle stage in which potentially toxic tau species are produced and how tau-tau interactions are modulated by various cellular factors.
Here, we report that the β-gal complementation system is a new tool that can be used in cell culture models to study tau-tau interactions preceding the formation of tau filaments. Although FRET microscopy is an elegant technique and has been used to study intermolecular tau association (Chun and Johnson 2007), it is technically demanding and requires extensive data analysis, as well as expensive and not readily available equipment. The β-gal complementation assay has advantages in signal amplification based on the enzymatic activity of β-gal, thus allowing interactions to be monitored without gross over-expression of complementing protein chimeras, and in straightforward signal quantification based on a chemiluminescent, or other readily measured signal. In addition, it is fairly easy to set up the β-gal complementation system. Once the parameters are established the tau-β-gal fusion constructs are transfected into cells and the β-gal enzyme activity, which reflects the strength of tau-tau interactions, can be examined with convenient assays such as chemiluminescence, fluorescence or colorimetric readouts depending on the substrate chosen (Mohler and Blau 1996; Rossi et al. 1997).
Using the β-gal complementation system, we were able to detect tau-tau interactions in a transfected cell culture model by histochemistry and quantify the relative extent of interactions with chemiluminescent assays (Fig. 2), strongly suggesting that wild-type tau can come into close physical proximity to form tau complexes in situ without the presence of any non-physiological inducers. This is further supported by the formation of high molecular weight tau-β-gal fusion protein complexes detected after chemical cross-linking (Fig. 3), which also validates β-gal complementation as a useful tool for studying tau self-association. The biological significance of pre-existing tau multimers is still not clear. But considering the previous studies showing that over-expression of tau alone is not enough to promote the formation of sarkosyl-insoluble or thioflavin S-positive tau aggregates (Cho and Johnson 2004; Ding et al. 2006), the multimeric state of soluble tau proteins might predispose tau to pathogenic conformational changes triggered by stressors associated with AD and tauopathies, which will eventually lead to tau aggregation. Although active β-gal is a tetramer and in bacteria, restoration of β-gal activity from complementing mutants results from formation of hetero-octamers, in mammalian cells the precise number of monomers in an active β-gal complex remains to be determined (Langley and Zabin 1976; Matthews 2005). Thus, the extent of the multimeric state of tau in this model needs to be further studied.
Interestingly, we detected higher β-gal activity from complementing T4-β-gal fusion proteins than from C3-β-gal proteins (Fig. 2), suggesting stronger interactions in situ of T4 compared to C3. This is probably not related to the fusion of the β-gal mutant to the C-terminus instead of N-terminus of tau because a previous study using fluorescent protein-labeled tau in FRET analysis showed a similar extent of tau self-association of both C-terminal and N-terminal labeled T4 or C3 tau proteins (Chun and Johnson 2007). Thus, deletion of the last 20 amino acids somehow weakens the in situ interactions of one truncated tau with itself. This seems to be contradictory to the in vitro studies showing that the C-terminal truncation actually enhances tau aggregation in the presence of polymerization inducers (Gamblin et al. 2003b; Rissman et al. 2004). However, it needs to be considered that it is possible that C3 displays different conformations in the cell than in vitro. Second, the self-association of tau detected in our study occurred spontaneously and relatively early without formation of tau filaments. Therefore, this apparent disparity could mean that the conformational changes related to the C-terminal truncation do not necessarily dictate the occurrence of tau aggregation (such as in our study) but can lower the critical concentration needed for aggregation to occur and enhance tau filament nucleation efficiency (such as in the in vitro studies) (Yin and Kuret 2006). Indeed, we have previously shown that over-expression of C3 together with GSK-3β, but not T4 with GSK-3β or C3 alone, resulted in sarkosyl insoluble and thioflavin S-positive tau aggregates in the cell (Cho and Johnson 2004; Ding et al. 2006).
Lithium reduces tau phosphorylation by inhibiting a major tau kinase GSK-3β (Hong et al. 1997) and is being explored as a therapeutic drug to reduce tau hyperphosphorylation and tau pathology in AD (Noble et al. 2005; Caccamo et al. 2007). Here, we show that the complementation of both T4-β-gal and C3-β-gal fusion proteins was significantly attenuated by lithium treatment (Fig. 4), indicating that the decrease of tau phosphorylation is associated with weakened tau-tau interactions. This is consistent with a previous study showing that increasing tau phosphorylation by expressing active GSK-3β enhanced tau self-association (Chun and Johnson 2007). As the tau-microtubule interactions are also regulated by tau phosphorylation, the attenuation of tau-β-gal complementation by lithium we observed could also be partially because of a decrease in the cytosolic pool of free tau resulting from increased association of tau with microtubules.
In summary, we have described a novel way to use β-gal complementation as a tool to detect tau-tau interactions in the cell and study the modulation of tau-tau interactions by tau truncation and phosphorylation. Further studies using this method will help elucidate the mechanisms of the regulation of tau self-association in transition to tau aggregation. With appropriate modifications, this method may have broader applications in studying self-association of aggregation-prone proteins in other neurodegenerative diseases.
Acknowledgement
We thank Dr Helen Blau of Stanford University for providing the pWZL-Δα and pWZL-Δω constructs. This work was supported by the National Institutes of Health grant NS051279 and a grant from the Alzheimer's Association.
Abbreviations used
- AD
Alzheimer's disease
- C3
tau truncated at D421
- DSS
disuccinimidyl suberate
- FRET
fluorescence resonance energy transfer
- GSK-3β
glycogen synthase kinase 3β
- HEK
human embryonic kidney
- NFTs
neurofibrillary tangles
- PBS
phosphate-buffered saline
- SDS
sodium dodecyl sulfate
- T4
full-length tau
- β-gal
β-galactosidase
References
- Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc. Natl Acad. Sci. USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl Acad. Sci. USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- von Bergen M, Barghorn S, Biernat J, Mandelkow EM, Mandelkow E. Tau aggregation is driven by a transition from random coil to beta sheet structure. Biochim. Biophys. Acta. 2005;1739:158–166. doi: 10.1016/j.bbadis.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Blakely BT, Rossi FM, Tillotson B, Palmer M, Estelles A, Blau HM. Epidermal growth factor receptor dimerization monitored in live cells. Nat. Biotechnol. 2000;18:218–222. doi: 10.1038/72686. [DOI] [PubMed] [Google Scholar]
- Buensuceso C, de Virgilio M, Shattil SJ. Detection of integrin alpha IIbbeta 3 clustering in living cells. J. Biol. Chem. 2003;278:15217–15224. doi: 10.1074/jbc.M213234200. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Tran LX, LaFerla FM. Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles. Am. J. Pathol. 2007;170:1669–1675. doi: 10.2353/ajpath.2007.061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Johnson GV. Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J. Biol. Chem. 2003;278:187–193. doi: 10.1074/jbc.M206236200. [DOI] [PubMed] [Google Scholar]
- Cho JH, Johnson GV. Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J. Biol. Chem. 2004;279:54716–54723. doi: 10.1074/jbc.M403364200. [DOI] [PubMed] [Google Scholar]
- Chun W, Johnson GV. Activation of glycogen synthase kinase 3beta promotes the intermolecular association of tau. The use of fluorescence resonance energy transfer microscopy. J. Biol. Chem. 2007;282:23410–23417. doi: 10.1074/jbc.M703706200. [DOI] [PubMed] [Google Scholar]
- DeTure M, Ko LW, Easson C, Yen SH. Tau assembly in inducible transfectants expressing wild-type or FTDP-17 tau. Am. J. Pathol. 2002;161:1711–1722. doi: 10.1016/S0002-9440(10)64448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Matthews TA, Johnson GV. Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation. J. Biol. Chem. 2006;281:19107–19114. doi: 10.1074/jbc.M511697200. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Hoerndli F, Baechi T, Nitsch RM, Gotz J. beta-Amyloid induces paired helical filament-like tau filaments in tissue culture. J. Biol. Chem. 2003;278:40162–40168. doi: 10.1074/jbc.M308243200. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Berry RW, Binder LI. Modeling tau polymerization in vitro: a review and synthesis. Biochemistry. 2003a;42:15009–15017. doi: 10.1021/bi035722s. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2003b;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, Lipton RB. Memory and mental status correlates of modified Braak staging. Neurobiol. Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl Acad. Sci. USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J. Biol. Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Bailey CD. Tau, where are we now? J Alzheimers Dis. 2002;4:375–398. doi: 10.3233/jad-2002-4505. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl Acad. Sci. USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley KE, Zabin I. beta-Galactosidase alpha complementation: properties of the complemented enzyme and mechanism of the complementation reaction. Biochemistry. 1976;15:4866–4875. doi: 10.1021/bi00667a018. [DOI] [PubMed] [Google Scholar]
- Le Corre S, Klafki HW, Plesnila N, et al. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc. Natl Acad. Sci. USA. 2006;103:9673–9678. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BW. The structure of E. coli beta-galactosidase. C. R. Biol. 2005;328:549–556. doi: 10.1016/j.crvi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug Discov. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- Mohler WA, Blau HM. Gene expression and cell fusion analyzed by lacZ complementation in mammalian cells. Proc. Natl Acad. Sci. USA. 1996;93:12423–12427. doi: 10.1073/pnas.93.22.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W, Planel E, Zehr C, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl Acad. Sci. USA. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Rossi F, Charlton CA, Blau HM. Monitoring protein-protein interactions in intact eukaryotic cells by beta-galactosidase complementation. Proc. Natl Acad. Sci. USA. 1997;94:8405–8410. doi: 10.1073/pnas.94.16.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Yan YX, Boldt-Houle DM, Tillotson BP, Gee MA, D'Eon BJ, Chang XJ, Olesen CE, Palmer MA. Cell-based high-throughput screening assay system for monitoring G protein-coupled receptor activation using beta-galactosidase enzyme complementation technology. J. Biomol. Screen. 2002;7:451–459. doi: 10.1177/108705702237677. [DOI] [PubMed] [Google Scholar]
- Yin H, Kuret J. C-terminal truncation modulates both nucleation and extension phases of tau fibrillization. FEBS Lett. 2006;580:211–215. doi: 10.1016/j.febslet.2005.11.077. [DOI] [PubMed] [Google Scholar]