Abstract
Small GTP-binding genes act as molecular switches regulating myriad of cellular processes including vesicle-mediated intracellular trafficking, signal transduction, cytoskeletal reorganization and cell division in plants and animals. Even though these genes are well conserved both functionally and sequentially across whole Eukaryotae, occasional lineage-specific diversification in some plant species in terms of both functional and expressional characteristics have been reported. Hence, comparative phyletic and correlative functional analyses of legume small GTPases homologs with the genes from other Metazoa and Embryophyta species would be very beneficial for gleaning out the small GTPases that could have specialized in legume-specific processes; e.g., nodulation. The completion of genome sequences of two model legumes, Medicago truncatula and Lotus japonicus will significantly improve our knowledge about mechanism of biological processes taking place in legume-rhizobia symbiotic associations. Besides, the need for molecular switches coordinating busy cargo-trafficking between symbiosis partners would suggest a possible subfunctionalization of small GTPases in Fabaceae for these functions. Therefore, more detailed investigation into the functional characteristics of legume small GTPases would be helpful for the illumination of the events initialized with the perception of bacteria by host, followed by the formation of infection thread and the engulfment of rhizobial bacteria, and end with the senescence of nitrogen-fixing organelles, nodules. In summary, a more thorough functional and evolutionary characterization of small GTPases across the main lineages of Embryophyta is significant for better comprehension of evolutionary history of Plantae, that is because, these genes are associated with multitude of vital biological processes including organogenesis.
Key words: small GTPases, Medicago truncatula, nodulation, symbiosis, embryophyta
Introduction
Small GTP-binding genes function as signaling molecules regulating vast number of biological processes including early and late secretory pathway,1–3 abiotic and biotic stress signal transduction pathways,4–7 and mitotic spindle assembly8,9 in both Metazoa and Plantae. Even though the functional characteristics of these genes have been widely studied in Metazoa species such as human and yeast and in Arabidopsis, there are still many unknowns, especially about Plantae homologs. These genes are generally grouped in four main subfamilies in plants: (1) ARF/SAR; (2) RAB; (3) d ROP (Rho-like proteins in Plants); and (4) RAN.10 Since these genes are essential for cell survival due to their housekeeping functions, they are for the most part strictly conserved with regards to their functional and sequential characteristics across whole eukaryotic kingdom.11–14 However, varying level of functional and expansional divergence has been reported in plants hinting a possible gain of new or related-functions.10,15 Thus, with the availability of sequence information from more plant species representing the main branches of Embryophyta, it will be interesting to look into the degree of divergence of these genes in different lineages.
Fabaceous plants are one of few lineages of plants with ability of converting atmospheric nitrogen into usable form by living organisms, ammonia, with specialized organelles in their roots, called nodules.16 Therefore, understanding of modus operandi of nodulation at molecular level is significant. Since the establishment and maintenance of symbiosis organelles involves heavy trafficking of exchange of meterials between the symbionts, Rhizobium and plant, comparative analyses of functional and phyletical characteristics of Fabaceae small GTPases would further our knowledge about nodulation.
Comparative Analysis of Legume Small GTPases
Even though the complete genome sequence of two model legume plants, Medicago truncatula and Lotus japonicus, is not available yet, large amount of sequence data including ESTs has accumulated.17 These two model organisms have been at utmost importance for providing insights about the symbiotic associations between legumes and Rhizobium spp. Once the full genome sequence become available, the comparative analysis between leguminous and non-leguminous genome sequences would be of great value for winnowing the genes evolved to perform Fabaceae-specific biological processes such as nodulation.18 Since small GTPases are the main switch molecules involving in the regulation of myriad of biological processes such as early and late protein trafficking pathway,19 high salinity tolerance20 and disease resistance,4,21 it is reasonable to assume that these genes could have subfunctionalized in a legume-specific manner to regulate processes related to nodulation. Indeed, we, conducted a comparative phyletic from several leguminous species including Medicago truncatula and Lotus japonicus, and of small GTPases with the homologs from several Metazoa and Plantae species including Arabidopsis thaliana and Homo sapiens with the purpose of finding out whether a functional, phyletical or expressional divergence has occurred in Fabaceae-lineage of Embryophyta.22 As a result of the study, we identified a total of 10 small GTPases that could have gained nodule-specific spatiotemporal expressional characteristics, implying a possible subfunctionalization. Medicago is estimated to have about 120 small GTP-binding genes,22 which is about same number of genes with Populus trichocarpa, 117 estimated from a recently published genome sequence22,23 and much higher than Arabidopsis, 93;10 which may suggest that these genes could have expanded at differential rates in two main lineages of eudicots, eurosid I and eurosid II. RABs have been the fastest growing subfamily of small GTPases in Fabaceae since the divergence from the most recent common ancestor with Arabidopsis. This level of expansion of these genes could also mean that they are likely to gain more lineage-specific functions; e.g., nodule-specific. Even though for the most clades in comparative phyletic tree of Fabacea RABs are heterogenic; that is, containing orthologs from both legumes and Arabidopsis, there are occasional variations in duplication rates and level of sequential divergence between phyletic groups.22 Furthermore, there are RAB cladistic groups that are homogenous for the legume sequences, hinting a likely legume-specific expansion and subfunctionalization. Interestingly, some Arabidopsis RAB homologs such as RABH1a, RABH1b, RABH1c, RABH1d and RABH1e is not associated with any of the leguminous homologs, but are phyletically grouped together with a yeast gene, YPT6, and the homologs from poplar, grape and Chlamydomonas reinhardtii, and Physcomitrella patens RABs (Yuksel et al. unpublished data). This could mean that this group of genes was present in the early common ancestors of both Metazoa and Plantae and later deleted in Fabaceae lineage of Embryophyta. Thus, even though the majority of RABs are conserved across whole Eucaryotae due to their basic roles such as vesicle-mediated trafficking, some of which could have been evolving much faster with regard to both of their functional and sequential characteristics. Even though ROPs (Rho of Plants) are phyletically much closer to each other relative to their Metazoa/Fungi equivalents, RACs, the interfamilial divergence is likely to have been evolving at a higher rate when compared to other three Embryophyta small GTPase subfamilies; ARF, RAB and ROP.22,24 For instance, Arabidopsis ROP6, did not cluster with any of the genes from other Embryophyta including Vitis vinifera,25 Populus thricocarpa,23 Glycine max, Medicago truncatula and Lotus japonicus. (Yuksel et al. unpublished data). Despite their much recent origin, their regulatory role in shaping the morphology of important plant organelles such as polarized-growth of roots and pollen tubes,26,27 and their involvement in plant-pathogen interaction pathways5,28 could be some of the factors driving the lineage-specific diversification in these genes. That is because, both of these biological processes; organelle growth and plant-pathogen interactions, are significant for adaptation of plants to new environments. Therefore, the fast pace evolution of ROPs leading to interlineal diversification would be a natural outcome of their functional identities. As for the other major group of small GTPases, ARFs, some phyletic groups such as Arf1 and Sar1 are universally conserved in all Eukaryotes with respect to both their functional and sequential characteristics. Nevertheless, there are some clades with the homogenous phyletic characteristics, hinting a possible differential deletion or expansion of some genes following the main speciation events. Thus, more comprehensive analysis of the evolutionary and functional characteristics of small GTPases will be beneficial for providing further insights about the role of these genes in the evolution of Embryophta. It is quite reasonable to assume that with the increase of the complexity of organisms; more complicated signaling systems will be needed to commensurate with the increased complexity of intra- and intercellular trafficking.
Role of Fabaceae Small GTPases in Nodular Establishment and Maintenance
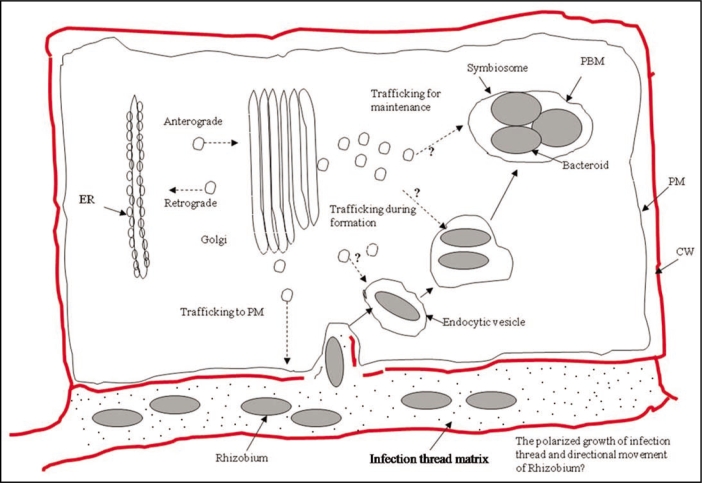
Nodulation is one of the best known models of symbiotic associations, which evolved over millions of years in 10 different families of Embryophyta, including Fabaceae species such as Medicago truncatula and Lotus japonicus.16 These two model legume plants, with an extensive amount of publicly available sequence data, have been the most widely studied organisms for gaining better insights into the molecular components of symbiosis between plants and bacteria. The establishment and maintenance of this highly-specialized, nitrogen-fixing symbiosis organelles (i.e., nodules) involves biologically complex interplay of events between host and symbiont from initial recognition stage and induction of the process,29,30 to engulfment of bacteria31 and final formation of fully-functioning nodules.32 This intricate teamwork between host, plant and bacteria, Rhizobium spp., necessitates the presence of a mechanism regulating the exchange of materials between these partners (Fig. 1). The small GTP-binding proteins are very well-known to be main switch molecules organizing vesicle-mediated trafficking of materials inside cell. Thus, it is logical to think that the main genes playing a major role in the regulation of material transport would likely to be from fabaceous small GTPase homologs. By holding that this statement is true, it could be easily postulated that some members of small GTPases of legumes could be subfunctionalized in the root nodules of legumes as regulatory agents. Furthermore, most of the subfunctionalized genes would be also specialized in terms of their spatiotemporal expressional characteristics. In other words, Fabacea small GTPases evolved to perform nodule-specific functions are likely to be mainly or solely expressed in nodules. Thus, Yuksel et al.22 attempted to identify small GTPase homologs that are uniquely or preponderantly specific to nodules in terms of their spatiotemporal expressional characteristics from two model legume species, M. truncatula and L. japonicus by comparative analysis of EST sequences obtained from nodular and non-nodular tissues, as a result, 10 small GTPase homologs fished out as possible candidates directing the shuttling of materials between symbiotic partners. Majority of these homologs belonged to RAB subfamily, exactly 7 out of 10. The results obtained from experiments conducted to determine the functional identities of these genes in several plant species is consistent with the hypothesis about the likely switch role of these genes in the regulation of trafficking between symbiotic partners. That is because; some of them are implicated to play role in vesical-mediated cargoes to vacuoles.33,34 Hence, it is quite reasonable to assume that some members of this subfamily of small GTPases could have evolved to perform nodule-specific functions. Besides, RAB proteins are also essential part of in the polarized growth of several plant tissues, e.g., pollen tubes,35 tip growth of root hair cells.36 Therefore, it would not be too farfetched to suggest that some of Fabaceae RAB homologs, identified by us, could be playing a regulatory role in the polarized movement of cell wall components during the development of specialized symbiosis structures (i.e., infection thread). However, it needs to be made clear that this argument could be easily tested experimentally in one of two model legume species, Medicago and Lotus, to gain further insights about the involvement of these genes. Furthermore, several previous studies suggested a possible involvement of RABs in the establishment and maintenance of nodular structures; e.g., RAB1p and RAB7 in the formation of peribacteroid membrane and rhizobial endocytosis.34,35 In conclusion, we believe that RABs are likely to be one of the key switch molecules regulating the formation and maintenance of symbiosomes.
Figure 1.
A schematic drawing depicting possible steps where fabaceous small GTPases couldplay role during the development and maintenance of nodules. The dashed arrows indicate the possible addresses of the vesicle-coated cargo and the questions marks limn the locations where small GTPases, identified by us could involve in the regulation of the nodule-specific trafficking. The abbreviations: PM, Plasma Membrane; PBM, Peribacteroid Membrane; CW, Cell Wall; and ER, Endoplasmic Reticulum.
Other than 7 legume RAB small GTPases, Yuksel et al.22 argued that a ROP and two ARFs that could be unique to nodules in terms of their spatiotemporal expressional properties. It is rather easy hypothesize about the putative role of ROP protein; that is, this protein could be a significant component of the molecular mechanisms directing polarized growth of infection thread that requires a complete reorganization of cytoskeleton37 and the pivotal role of ROPs in biological processes requiring an extensive cytoskeletal rearrangement such as pollen tube growth is a well-established fact.38–40 As for ARFs, the similar arguments made for RABs with respect to their role in nodules would be true as well. That is because, ARFs from several plant species are implicated in similar roles to RABs such as vacuolar trafficking.41 In other words, functional overlaps of these two subfamilies of small GTPases in Embryophyta, RABs and ARFs have been observed. Hence, we believe that there could be some members of legume ARFs that could be evolved to perform nodule-specific function.
Conclusions
We suggest that Fabaceae small GTPase homologs could have gained nodule-specific functions including the regulation of cargo-trafficking of materials between symbionts, legume and Rhizobium, and molecular switches coordinating intricate interplay of events that occur during the establishment and maintenance of symbiomes together with tens of already characterized nodular genes. It also needs to be stated that the experimental description of the details of molecular mechanism through which small GTPases take part in the regulation of nodule development and maintenance will greatly contribute to our knowledge about symbiosis biology. Furthermore, better elucidation of the functional and the evolutionary characteristics of small GTPases in whole plant kingdom would be of a great value for furthering our knowledge about how the intracellular trafficking evolved with the increase of organism complexity aftermath of the main divergence points; e.g., the transition from water to land and from nonvascular to vascular systems.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7868
References
- 1.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The Small Gtpase Rab5 Functions as a Regulatory Factor in the Early Endocytic Pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 2.Novick P, Brennwald P. Friends and Family—the Role of the Rab Gtpases in Vesicular Traffic. Cell. 1993;75:597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- 3.Zheng H, Camacho L, Wee E, Batoko H, Legen J, Leaver CJ, Malho R, Hussey PJ, Moore I. A Rab-E GTPase Mutant Acts Downstream of the Rab-D Subclass in Biosynthetic Membrane Traffic to the Plasma Membrane in Tobacco Leaf Epidermis. Plant Cell. 2005;17:2020–2036. doi: 10.1105/tpc.105.031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K. Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA. 2001;98:759–764. doi: 10.1073/pnas.021273498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morino K, Shimamoto K, Umemura K, Iwata M, Kawata M. The rice small GTPase OsRac1, a regulator of the programmed cell death in plant disease resistance, functions during development. Plant Cell Physiol. 2004;45:136. [Google Scholar]
- 6.Wong HL, Sakamoto T, Kawasaki T, Umemura K, Shimamoto K. Downregulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol. 2004;135:1447–1456. doi: 10.1104/pp.103.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira-Leal JB, Hume AN, Seabra MC. Prenylation of Rab GTPases: molecular mechanisms and involvement in genetic disease. FEBS Letts. 2001;498:197–200. doi: 10.1016/s0014-5793(01)02483-8. [DOI] [PubMed] [Google Scholar]
- 8.Kalab P, Pu RT, Dasso M. The Ran GTPase regulates mitotic spindle assembly. Curr Biol. 1999;9:481–484. doi: 10.1016/s0960-9822(99)80213-9. [DOI] [PubMed] [Google Scholar]
- 9.Dasso M. Running on ran: Nuclear transport and the mitotic spindle. Cell. 2001;104:321–324. doi: 10.1016/s0092-8674(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 10.Vernoud V, Horton AC, Yang Z, Nielsen E. Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol. 2003;131:1191–1208. doi: 10.1104/pp.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily—a conserved switch for diverse cell functions. Nature. 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 12.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily—conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 13.Jekely G. Small GTPases and the evolution of the eukaryotic cell. Bioessays. 2003;25:1129–1138. doi: 10.1002/bies.10353. [DOI] [PubMed] [Google Scholar]
- 14.Jiang SY, Ramachandran S. Comparative and evolutionary analysis of genes encoding small GTPases and their activating proteins in eukaryotic genomes. Physiol Genom. 2006;24:235–251. doi: 10.1152/physiolgenomics.00210.2005. [DOI] [PubMed] [Google Scholar]
- 15.Rutherford S, Moore I. The Arabidopsis Rab GTPase family: another enigma variation. Curr Opin Plant Biol. 2002;5:518–528. doi: 10.1016/s1369-5266(02)00307-2. [DOI] [PubMed] [Google Scholar]
- 16.Soltis DE, Soltis PS, Morgan DR, Swensen SM, Mullin BC, Dowd JM, Martin PG. Chloroplast gene sequence data suggest a single origin of the predisposition for symbiotic nitrogen fixation in angiosperms. Proc Natl Acad Sci USA. 1995;92:2647–2651. doi: 10.1073/pnas.92.7.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato S, Nakamura Y, Asamizu E, Isobe S, Tabata S. Genome sequencing and genome resources in model legumes. Plant Physiol. 2007;144:588–593. doi: 10.1104/pp.107.097493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu H, Riely BK, Burns NJ, Ane J-M. Tracing nonlegume orthologs of legume genes required for nodulation and arbuscular mycorrhizal symbioses. Genetics. 2006;172:2491–2499. doi: 10.1534/genetics.105.051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuchi M, Ueda T, Yahara N, Nakano A. Arf1 GTPase plays roles in the protein traffic between the endoplasmic reticulum and the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 2002;31:499–515. doi: 10.1046/j.1365-313x.2002.01372.x. [DOI] [PubMed] [Google Scholar]
- 20.Bolte S, Schiene K, Dietz KJ. Characterization of a small GTP-binding protein of the rab 5 family in Mesembryanthemum crystallinum with increased level of expression during early salt stress. Plant Molec Biol. 2000;42:923–936. doi: 10.1023/a:1006449715236. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal GK, Iwahashi I, Rakwal R. Small GTPase ‘Rop’: molecular switch for plant defense responses. Febs Letts. 2003;546:173–180. doi: 10.1016/s0014-5793(03)00646-x. [DOI] [PubMed] [Google Scholar]
- 22.Yuksel B, Memon AR. Comparative phylogenetic analysis of small GTP-binding genes of model legume plants and assessment of their roles in root nodules. J Exp Bot. 2008;59:3831–3844. doi: 10.1093/jxb/ern223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuskan GA, et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 24.Winge P, Brembu T, Kristensen R, Bones AM. Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics. 2000;156:1959–1971. doi: 10.1093/genetics/156.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The French-Italian Public Consortium for Grapevine Genome Characterization, author. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007 doi: 10.1038/nature06148. advanced online publication. [DOI] [PubMed] [Google Scholar]
- 26.Molendijk AJ, Bischoff F, Rajendrakumar CSV, Friml J, Braun M, Gilroy S, Palme K. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001;20:2779–2788. doi: 10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang JU, Gu Y, Lee YJ, Yang ZB. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell. 2005;16:5385–5399. doi: 10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung YH, Agrawal GK, Rakwal R, Kim JA, Lee MO, Choi PG, Kim YJ, Kim MJ, Shibato J, Kim SH, Iwahashi H, Jwa NS. Functional characterization of OsRacB GTPase—a potentially negative regulator of basal disease resistance in rice. Plant Physiol Biochem. 2006;44:68–77. doi: 10.1016/j.plaphy.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Geurts R, Fedorova E, Bisseling T. Nod factor signaling genes and their function in the early stages of Rhizobium infection. Curr Opin Plant Biol. 2005;8:346–352. doi: 10.1016/j.pbi.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Riely BK, Ané J-M, Penmetsa RV, Cook DR. Genetic and genomic analysis in model legumes bring Nod-factor signaling to center stage. Curr Opin Plant Biol. 2004;7:408–413. doi: 10.1016/j.pbi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Oldroyd GED, Harrison MJ, Udvardi M. Peace talks and trade deals. Keys to long-term harmony in legume-microbe symbioses. Plant Physiol. 2005;137:1205–1210. doi: 10.1104/pp.104.057661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonneke Mulder BH, Bersoult Anne, Cullimore Julie V. Integration of signalling pathways in the establishment of the legume-rhizobia symbiosis. Physiol Plant. 2005;123:207–218. [Google Scholar]
- 33.Sohn EJ, Kim ES, Zhao M, Kim SJ, Kim H, Kim YW, Lee YJ, Hillmer S, Sohn U, Jiang LW, Hwang IW. Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. Plant Cell. 2003;15:1057–1070. doi: 10.1105/tpc.009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heo JB, Rho HS, Kim SW, Hwang SM, Kwon HJ, Nahm MY, Bang WY, Bahk JD. OsGAP1 functions as a positive regulator of OSRab11-mediated TGN to PM or vacuole trafficking. Plant Cell Physiol. 2005;46:2005–2018. doi: 10.1093/pcp/pci215. [DOI] [PubMed] [Google Scholar]
- 35.de Graaf BHJ, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu HM. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell. 2005;17:2564–2579. doi: 10.1105/tpc.105.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preuss ML, Schmitz AJ, Thole JM, Bonner HKS, Otegui MS, Nielsen E. A role for the RabA4b effector protein PI-4K beta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol. 2006;172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokota K, Fukai E, Madsen LH, Jurkiewicz A, Rueda P, Radutoiu S, Held M, Hossain MS, Szczyglowski K, Morieri G, Oldroyd GED, Downie JA, Nielsen MW, Rusek AM, Sato S, Tabata S, James EK, Oyaizu H, Sandal N, Stougaard J. Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell. 2009 doi: 10.1105/tpc.108.063693. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin YK, Yang ZB. Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell. 1997;9:1647–1659. doi: 10.1105/tpc.9.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones MA, Shen JJ, Fu Y, Li H, Yang ZB, Grierson CS. The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell. 2002;14:763–766. doi: 10.1105/tpc.010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu Y, Vernoud V, Fu Y, Yang ZB. ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J Exp Bot. 2003;54:93–101. doi: 10.1093/jxb/erg035. [DOI] [PubMed] [Google Scholar]
- 41.Pimpl P, Hanton SL, Taylor JP, Pinto-daSilva LL, Denecke J. The GTPase ARF1p controls the sequence-specific vacuolar sorting route to the lytic vacuole. Plant Cell. 2003;15:1242–1256. doi: 10.1105/tpc.010140. [DOI] [PMC free article] [PubMed] [Google Scholar]