Abstract
Polyamines are implicated in the regulation of many processes in the plant cell, including functioning of ion channels, DNA replication, gene transcription, mRNA translation, cell proliferation and programmed cell death. Plant polyamines occur either in free form, covalently bound to proteins, or conjugated to hydroxycinnamic acids forming phenol amides. Ustilago maydis is a dimorphic and biotrophic pathogenic fungus responsible for common smut or “huitlacoche” in maize; and it is considered an excellent model for the study of plant-pathogen interactions. Recently, we reported alterations in polyamine metabolism of maize tumors induced on leaf blades by Ustilago maydis infection. Our data revealed a striking increase in maize polyamine biosynthesis, mainly free and conjugated putrescine in the tumors and in the green plant tissue surrounding the tumor. In this addendum, we describe that changes in polyamine metabolism take place even in earlier stages of maize plant infection with Ustilago maydis.
Key words: early stages, polyamines, Ustilago maydis, Zea mays
The diamine putrescine (Put), whose synthesis from amino acids (ornithine and arginine) can be catalyzed by ornithine decarboxylase (ODC, EC 4.1.1.17) or arginine decarboxylase (ADC, EC 4.1.1.19), constitutes an essential precursor for the synthesis of spermidine (Spd) and spermine (Spm). Events occurring through the successive activities of spermidine synthase (SPDS, EC 2.5.1.16) and spermine synthase (SPMS, EC 2.5.1.22) by the addition of aminopropyl groups. The aminopropyl moiety is derived from methionine, which is first converted into S-adenosylmethionine (SAM) and then decarboxylated via S-adenosylmethionine decarboxylase (SAMDC, EC 4.1.1.50). Besides the important functions of polyamines (PAs) in plant growth and development, they are also implicated in biotic stress responses.1–4,10
Conjugated PAs have been associated to reproduction and floral induction processes in plants.5,6 These conjugates participate in the regulation of PA concentration inside the cell,7 and contribute to the formation of a phenolic barrier made by ester-ether linkages, making cell walls more resistant to enzymatic hydrolysis.8 Also, due to their anti-fungal properties,9 a role in the protection against pathogens has been suggested.3,6
Accumulation of Free and Conjugated Putrescine in Ustilago maydis-Induced Chlorotic Lesions
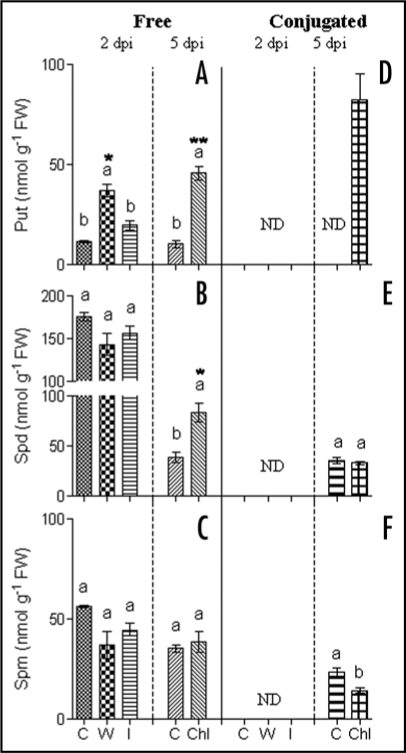
Besides the analysis in maize plant tumors,10 in the present Addendum we describe alterations in PA metabolism at earlier stages of maize plant infection with U. maydis. Specifically, those occurring two days post-inoculation in maize leaves (dpi; 8 d-old seedlings) and during the induction of chlorotic lesions (5 dpi; 11 d-old). In agreement with our previous data,10 the most significant alterations were observed in the levels of free and conjugated Put (Fig. 1). At 2 dpi, an increase (1.7-fold) in free Put content takes place in the infected tissues (I) in comparison to the control non-infected ones; notably the higher accumulation was observed in the wounded (W or mock)-plants (3-fold; Fig. 1A). This result agrees with the observation that a transient accumulation of Put occurs as a characteristic event in response to mechanical wounding in plants.11 In the case of Spd and Spm, a slight reduction in both infected (I) and wounded plants (W) is shown (Fig. 1B and C; 2 dpi). Later on, the chlorotic lesions (Chl) showed a pronounced increase (5-fold) in free Put levels (Fig. 1A; 5 dpi). Chlorosis is one of the early and characteristic symptoms in maize plants infected with U. maydis.12 In these lesions, free Spd was also increased (2-fold; Fig. 1B; 5 dpi). However, Spm levels did not show any significant change (Fig. 1C).
Figure 1.
Determination of free and conjugated polyamine contents in 8 and 11 d-old maize plants infected (2 and 5 dpi) or not with U. maydis. (A and D) Put; (B and E) Spd; and (C and F) Spm. C, control plant; W, wounded plant; Chl, chlorosis; I, inoculated plant. Data are mean ± SE (n = 6) from two repeated experiments. ND, no detected. Different letters indicate significant differences between samples according to Tukey's multiple comparison tests at p < 0.05. Asterisks (* and **) indicate values that differ at p < 0.01 and p < 0.001 respecting control plants.
Notably, free PA levels were found to be higher in younger maize plants than in the older ones. This is observed comparing the controls of the different time points monitored (8 and 11) and 15 d-old maize seedlings (tumor stage),10 suggesting that at early stages of plant growth a higher pool of PAs is required. Regarding soluble conjugated PAs (phenol amides formed by conjugation with hydroxycinnamic acids), a specific accumulation was found in the chlorotic lesions while at 2 dpi they were undetectable (Fig. 1D–F). Put conjugates were the most predominant ones, suggesting that it biosynthesis might be relevant in the chlorotic and tumor stage.10 Recently, it was reported that the total content of hydroxycinnamic acids derivatives as well as the transcription and activity of the phenylalanine-ammonium lyase (PAL) are induced by U. maydis infection.13 These changes are associated to increased cell wall synthesis and reinforcement during tumor development.13
The Zmsamdc Genes are Upregulated in the Chlorotic Lesions Induced by Ustilago maydis
Transcriptional profiling of Adc, Samdc (isoforms 1, 2, 3), Zmspds1 and Zmspms genes of maize was performed by RT-PCR using specific oligonucleotides for each gene.10,14 At 2 dpi, no alterations in the expression levels of these gene transcripts were recorded (data not shown). Conversely, in the chlorotic lesions the Zmsamdc2 and Zmsamdc3 transcripts were upregulated (Fig. 2C and D, lane 2). The Adc transcript was slightly affected (Fig. 2A, lane 2). In the case of the aminopropyltransferase genes, it was observed that Zmspds1 expression suffered no alterations at this time point of the infection, whereas Zmspms transcript levels showed a reduction in the chlorotic areas (Fig. 2E and F, lane 2). In a similar fashion as in tumors,10 an important regulation at the transcriptional level occurs at early stages of infection.
Figure 2.
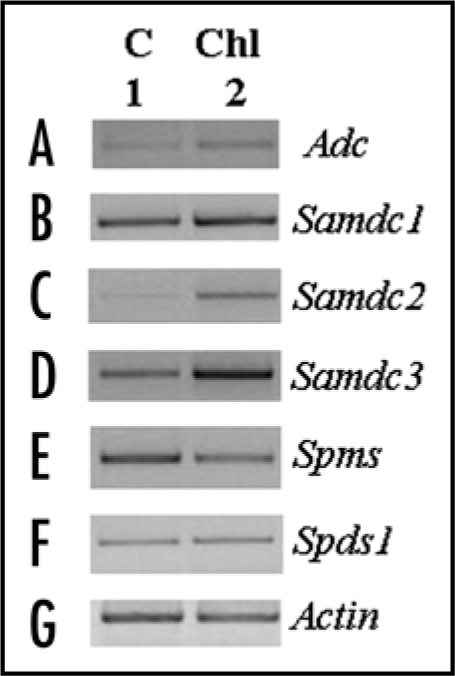
Expression of Adc, Samdc (isoforms 1–3), Zmspds1 and Zmspms genes in leaf blades of 11 d-old maize plants infected (5 dpi) or not with U. maydis. Total RNA was isolated from leaf blades of C and Chl samples as in Figure 1.
The overall changes in PA synthesis, might have several implications in the infection process. First, we suggest that soluble Put conjugates (hydroxycinnamic acids derivatives) might contribute to cell wall synthesis and reinforcement as a consequence of infection and tumor growth, as postulated by others.13 On the other hand, we speculate that free Put might contribute to the enhanced cell division processes as well as pro-survival (anti-programmed cell death) role in tumor development.15
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8089
References
- 1.Takahashi Y, Berberich T, Yamashita K, Uehara Y, Miyazaki A, Kusano T. Identification of tobacco HINI and two closely related genes as spermine-responsive genes and their functional expression during the Tobacco mosaic virus-induced hypersensitive response and during leaf- and flower-senescence. Plant Mol Biol. 2004;54:613–622. doi: 10.1023/B:PLAN.0000038276.95539.39. [DOI] [PubMed] [Google Scholar]
- 2.Uehara Y, Takahashi Y, Berberich T, Miyazaki A, Takahashi H, Matsui K, et al. Tobacco ZFT1, a transcripcional repressor with Cys2/His2 type zinc finger motif that functions in spermine-signaling pathway. Plant Mol Biol. 2005;59:435–448. doi: 10.1007/s11103-005-0272-0. [DOI] [PubMed] [Google Scholar]
- 3.Walters DR. Polyamines and plant disease. Phytochem. 2003a;64:97–107. doi: 10.1016/s0031-9422(03)00329-7. [DOI] [PubMed] [Google Scholar]
- 4.Walters DR. Resistance to plant pathogens: possible roles for free polyamines and polyamine catabolism. New Phytol. 2003;159:109–115. doi: 10.1046/j.1469-8137.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 5.Kakkar RK, Rai VK. Plant polyamines in flowering and fruit ripening. Phytochem. 1993;33:1281–1288. [Google Scholar]
- 6.Martin-Tanguy J. Metabolism and function of polyamines in plants: recent development (new approaches) J Plant Growth Regul. 2001;34:135–148. [Google Scholar]
- 7.Bagni N, Tassoni A. Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids. 2001;20:301–317. doi: 10.1007/s007260170046. [DOI] [PubMed] [Google Scholar]
- 8.Clarke DD. The accumulation of cinnamic acid amides in the cell walls of potato tissue as an early response to fungal attack. In: Wood RKS, editor. Active Defense Mechanisms in Plants. Plenum Press: London; 1982. p. 321. [Google Scholar]
- 9.Walters DR, Meurer-Grimes B, Rovira I. Antifungal activity of three spermidine conjugates. FEMS Microbiol Lett. 2001;10030:1–4. doi: 10.1111/j.1574-6968.2001.tb10765.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez Kessler M, Ruiz OA, Maiale S, Ruiz-Herrera J, Jiménez Bremont JF. Polyamine metabolism in maize tumors induced by Ustilago maydis. Plant Physiol Biochem. 2008;46:805–814. doi: 10.1016/j.plaphy.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Perez Amador MA, Leon J, Green PJ, Carbonell J. Induction of arginine decarboxylase ADC2 genes provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol. 2002;130:1454–1463. doi: 10.1104/pp.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banuett F, Herskowitz I. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development. 1996;122:2965–2976. doi: 10.1242/dev.122.10.2965. [DOI] [PubMed] [Google Scholar]
- 13.Doehlemann G, Wahl R, Horst RJ, Voll LM, Usadel B, Poree F, et al. Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J. 2008;56:181–195. doi: 10.1111/j.1365-313X.2008.03590.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Kessler M, Jímenez-Bremont JF. Zmspds2 maize gene: Coding a spermine synthase? Plant Signal Behav. 2008;3:551–553. doi: 10.4161/psb.3.8.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadakis AK, Roubelakis-Angelakis KA. Polyamines inhibit NADPH oxidase-mediated superoxide generation and putrescine prevents programmed cell death induced by polyamine oxidase-generated hydrogen peroxide. Planta. 2005;220:826–837. doi: 10.1007/s00425-004-1400-9. [DOI] [PubMed] [Google Scholar]