Abstract
In recent years the biology of resistance to aphids and other sap-sucking insects has been studied in detail, whereas the genetic basis underlying this resistance is still poorly understood. Genetic resistance to Acyrthosiphon kondoi Shinji (bluegreen aphid; BGA) has been identified in Medicago truncatula Gaertn and backcrossed into susceptible cultivars. One of these M. truncatula cultivars named Jester also has good resistance to an Australian biotype of pea aphid (PA; A. pisum Harris). Until now it has been unclear whether resistance to each aphid species of the genus Acyrthosiphon is conferred by distinct genes or the same gene termed AKR for A. kondoi resistance. Infestation of the progenitors of the cultivar Jester with both aphid species revealed that resistance to BGA came from a different donor than resistance to PA, demonstrating that resistance to these aphid species is mediated by different resistance genes. However, an interaction between these genes for resistance to Acyrthosiphon species remains a possibility, given that PA resistance was not one of the parameters selected for in the creation of Jester. The identification of resistance to the model aphid, PA, and a closely related aphid BGA in the same genetic background of the model legume M. truncatula makes this system an attractive model for the study of plant-aphid interactions, as well as R gene specificity and evolution.
Key words: aphids, legumes, plant resistance genes, plant-insect interactions, pea aphid
Introduction
Aphids are phloem-feeding insects of the Hemiptera suborder Sternorrhyncha and cause significant damage in agriculture worldwide through direct phloem sap ingestion and transmission of pathogenic viruses.1 The understanding of the genetic and molecular mechanisms underlying plant defense against phloem feeding insects such as aphids is thus of great importance. Medicago truncatula, a model plant species for plant biology studies,2,3 has also been shown to be an excellent model plant for studying plant-aphid interactions.4–8 The pea aphid (PA, Acyrthosiphon pisum) was selected by an international consortium as the model aphid species for genetics and genomics studies and the PA genome sequencing project has just been completed (www.hgsc.bcm.tmc.edu/projects/aphid/). With the wealth of genetic and molecular resources becoming available, M. truncatula and PA provide a powerful system to identify key factors involved in aphid resistance as well as aphid host manipulation factors.
To date, our research focus has been on the biological, genetic and molecular characterization of aphid resistance in M. truncatula cultivars, which are host to a range of legume pests, including blue-green aphid (BGA; A. kondoi), pea aphid and spotted alfalfa aphid (SAA; Therioaphis trifolii). The M. truncatula cv Jester is resistant to BGA, PA and SAA and resistance to all three aphid species in Jester has been characterised in detail and involves antibiosis and antixenosis, and in all three cases is phloem based.6–8 Resistance to BGA in Jester is conferred by a dominant gene called AKR (Acyrthosiphon kondoi resistance) located on the short arm of chromosome three in a region rich in CC-NBS-LRR genes.7 Similarly, SAA resistance in Jester is conferred by a dominant gene termed TTR (Therioaphis trifolii resistance) and is also located on chromosome three approximately 22.8 cM distal of AKR.8 Ten F3 families descended from a cross A17 × Jester, with recombination events tightly linked to the AKR locus controlling BGA resistance were assayed for their PA resistance and the segregation ratios for PA resistance were correlating with the F2 genotypes for the molecular markers linked to AKR.6 This data led to the hypothesis that the same gene might be involved in the resistance to both BGA and PA. However, the transcriptional analysis of genes representing various defense signalling pathways showed that genes involved in the octadecanoid pathway were highly and exclusively induced following BGA infestation in the resistant Jester plants but not in the susceptible A17 plants which are near-isogenic to Jester.4 In contrast, these genes were not induced in either Jester or A17 following PA infestation.6 Here, we provide evidence that resistance to PA is controlled by an independent gene from AKR.
M. truncatula cv Jester's Origin, Breeding and Aphid Resistance Characteristics
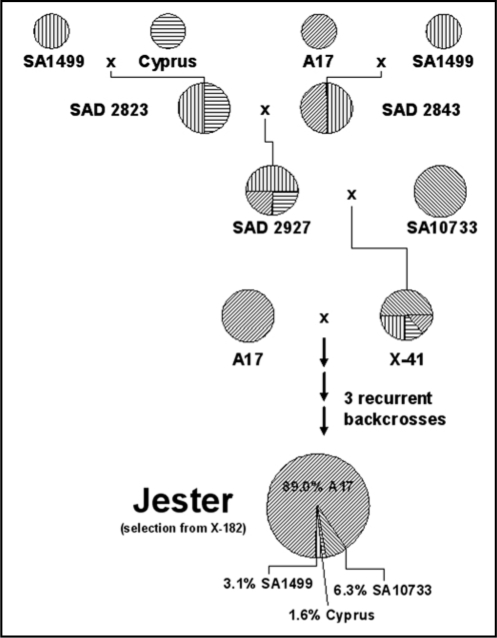
To determine whether BGA and PA resistance came from the same M. truncatula donor, we first investigated the origin of Jester. Jester has a total of four progenitors, being A17 (Jemalong), Cyprus, SA1499 and SA10733 and was generated to introduce BGA and SAA resistance to the elite M. truncatula cultivar, Jemalong (Fig. 1).9 Jester constitutes approximately 1.6% Cyprus, 3.1% SA1499 and 6.3% SA10733 and is thus 89.0% near isogenic to A17. The breeders reported that Cyprus and SA10733 were the sources of SAA resistance, whereas SA1499 was the source of BGA resistance.9 Our research has shown that resistance to PA was also introgressed into Jester, apparently in the absence of selection against this species, but the donor of this resistance was unclear.
Figure 1.
Origin and breeding of the M. truncatula cultivar Jester.
PA Resistance comes from a Different Progenitor than BGA Resistance
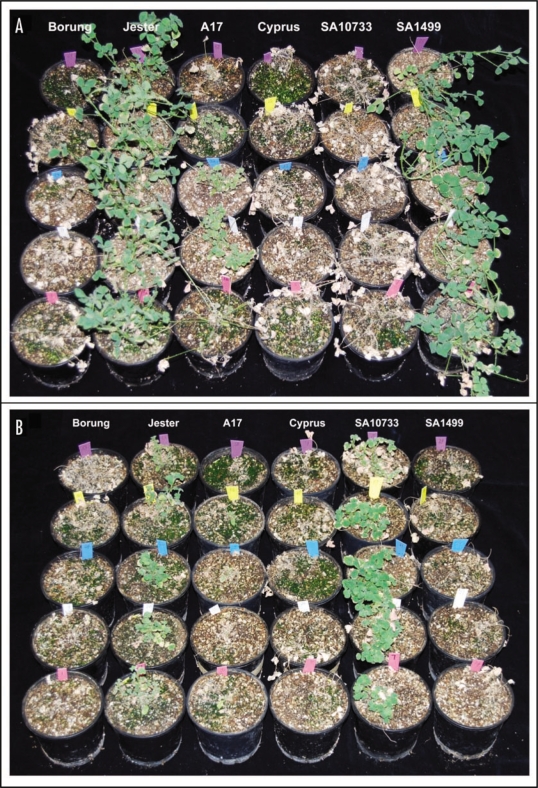
To identify the donor of PA resistance and to confirm the source of resistance for BGA in Jester, the plant tolerance of Jester and its progenitors against BGA and PA was monitored for 28 days following infestation with two apterous adults of BGA or PA on two-week-old plants. The M. truncatula cv Borung has previously been shown to be one of the most susceptible accessions to both BGA and PA, and was therefore included as a susceptible control.5 Twenty-eight days after infestation with BGA, Jester and SA1499 plants were relatively healthy, whereas Cyprus, SA10733 and the susceptible control Borung died (Fig. 2A). The accession A17 showed an intermediate BGA resistance phenotype, with a significantly different level of plant damage compared to its near isogenic line Jester.
Figure 2.
Phenotypes of M. truncatula accessions 28 days post infestation with either (A) bluegreen aphid or (B) pea aphid. Five replicates of two-week-old plants of each accession were infested with two apterous aphids of BGA or PA and monitored up to 28 days post infestation.
In the case of PA, Jester and SA10733 survived 28 days following PA infestation, in stark contrast to the susceptible control Borung, A17, Cyprus and SA1499 (Fig. 2B). The accession A17 was somewhat more resistant than Borung, Cyprus and SA1499 in the early stages of infestation, but by 28 days the majority of the plants died (Fig. 2B). These results clearly demonstrate that AKR in Jester was derived from the accession SA1499, whereas PA resistance was derived from SA10733. It is obvious that AKR is not the main locus conferring resistance to PA, and that PA resistance is controlled by a different gene in Jester, the Acyrthosiphon pisum resistance gene (APR).
Discussion
The value of M. truncatula as a model system to study plant aphid interactions has been demonstrated and three single-gene resistances against three major legume aphids in M. truncatula have been characterised in detail. For BGA and SAA these resistance loci have been fine mapped.7,8 It will therefore be of importance to pursue the fine-mapping of the PA resistance gene, APR. APR could be tightly linked to AKR or APR could have an additive effect on AKR and/or TTR. Either one of these hypotheses could explain why PA resistance was retained in Jester, given that PA resistance was not one of the parameters selected for by the breeders. The presence of an independent resistance gene APR is supported by the fact that different defense related pathways were induced by BGA infestation compared to PA infestation.4 This is also consistent with, the reciprocal pre-infestation experiments with BGA and PA on Jester which suggested that different downstream defense mechanisms act against these two closely related aphid species.6
With the PA genome sequence completed and various PA genomics and proteomics resources available (www.aphidbase.com/aphidbase/), the M. truncatula/PA system has become an even more attractive model to study plant-aphid interactions. It will allow us to study the evolution of R genes that confer resistance to closely related species (and biotypes) as well as the differences in the salivary gland secretome,10,11 the suite of effector proteins which collectively manipulate host plant defence signaling and responses to allow successful feeding. Overall these studies should help shed light on the intimate relationship between plants and phloem-feeding insects.
Acknowledgements
We would like to thank Jenny Reidy-Croft and Elaine Smith for technical assistance on the project. Sumin Guo is supported by a CSIRO/China Scholarship Council fellowship. Lars Kamphuis is the recipient of a CSIRO/OCE Post-Doctoral Fellowship.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8190
References
- 1.Ng JCK, Perry KL. Transmission of plant viruses by aphid vectors. Molec Plant Pathogl. 2004;5:505–511. doi: 10.1111/j.1364-3703.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 2.Küster H, Becker A, Firnhaber C, Hohnjec N, Manthey K, Perlick AM, et al. Development of bioinformatic tools to support EST-sequencing, in silico- and microarray-based transcriptome profiling in mycorrhizal symbioses. Phytochemistry. 2007;68:19–32. doi: 10.1016/j.phytochem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Town CD. Annotating the genome of Medicago truncatula. Curr Opin Plant Biol. 2006;9:122–127. doi: 10.1016/j.pbi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Gao L, Anderson JP, Klingler JP, Nair RM, Edwards OR, Singh KB. Involvement of the octadecanoid pathway in bluegreen aphid resistance in Medicago truncatula. Molec Plant Microb Interact. 2007;20:82–93. doi: 10.1094/MPMI-20-0082. [DOI] [PubMed] [Google Scholar]
- 5.Gao LL, Horbury R, Nair RM, Edwards OR, Singh KB. Characterization of resistance to multiple aphid species (Hemiptera: Aphididae) in Medicago truncatula. Bull Entomol Res. 2007;97:41–48. doi: 10.1017/S0007485307004786. [DOI] [PubMed] [Google Scholar]
- 6.Gao LL, Klingler JP, Anderson JP, Edwards OR, Singh KB. Characterization of pea aphid resistance in Medicago truncatula. Plant Physiol. 2008;146:996–1009. doi: 10.1104/pp.107.111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klingler J, Creasy R, Gao L, Nair RM, Calix AS, Spafford Jacob H, et al. Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol. 2005;137:1445–1455. doi: 10.1104/pp.104.051243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klingler JP, Edwards OR, Singh KB. Independent action and contrasting phenotypes of resistance genes against spotted alfalfa aphid and bluegreen aphid in Medicago truncatula. New Phytol. 2007;173:630–640. doi: 10.1111/j.1469-8137.2006.01939.x. [DOI] [PubMed] [Google Scholar]
- 9.Hill JR. Jester. Plant Varieties Journal. 2000;13:40. [Google Scholar]
- 10.Mutti NS, Louis J, Pappan LK, Pappan K, Begum K, Chen M-S, et al. A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc Natl Acad Sci USA. 2008;105:9965–9969. doi: 10.1073/pnas.0708958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carolan JC, Fitzroy CIJ, Ashton PD, Douglas AE, Wilkinson TL. The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics. 2009 doi: 10.1002/pmic.200800692. In press. [DOI] [PubMed] [Google Scholar]