Abstract
The present study aims at comparing the phloem composition of the tolerant XBJ90.03-16-1-1-1 and the susceptible Bachaar genotypes and the impact of the faba bean genotype on the levels of the major solutes and invertase activities in the parasite Orobanche foetida. In comparison to Bachaar, the XBJ90.03-161-1-1 genotype limited the growth of orobanche tubercles under in vitro conditions. The limited growth was due to low soluble invertase activity, low osmotic potential of the infected roots and the organic nitrogen deficiency of the host phloem sap. The faba bean genotype did not affect the osmoregulation process of O. foetida. Among the organic solutes, stachyose, hexoses, starch and free amino acids, mainly asparagine and aspartate were highly accumulated in orobanche. However, asparagine/aspartate, glutamine/glutamate, alanine, serine, gamma amino butyric acid, stachyose, sucrose were identified as the main organic components in the host phloem exudates. The key role of the enzymes α-galactosidase, asparagine synthetase and aspartate oxaloglutarate aminotransferase in the utilization of the host solutes is proposed in O. foetida parasitizing faba bean.
Key words: asparagine, broomrape, invertase, sucrose, stachyose, amino acid, osmolarity, Vicia faba
Broomrapes (Orobanche spp.) are obligate parasites that infect roots of many economically important crops, causing serious losses in yields.1 In Tunisia, Orobanche foetida is an important agricultural parasite of faba bean in the region of Beja with yield losses varying between 66–90%.2,3 Various strategies were tried to control this plant parasite but without enough success.4,5 Recently, in field experiments, some faba bean genotypes like XBJ90.03-16-1-1-1 were identified as resistant to O. foetida in Tunisia.3 Their resistance was characterized by a decrease in Orobanche attachment and reduction in parasite emergence, nevertheless without observing parasite necrosis on the host roots. In comparison to a susceptible genotype, this genotype showed low stimulatory activity for Orobanche seed germination, and showed a delay of parasite formation and a limited growth of Orobanche tubercles under in vitro conditions.6,7
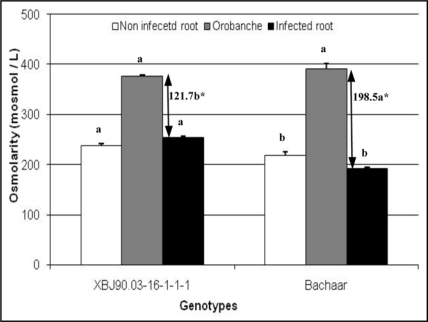
Knowing that tubercle growth is strongly related to uptake of water and nutrient from host, osmotic relationships between host and parasite were investigated by measuring the difference in osmolarity between faba bean roots and tubercles. Orobanche tubercles were taken from Petri dishes at stage 4, stage during which nutriment transfer from the plant host to parasite should be optimal.8 In both tolerant and susceptible genotypes, tubercle osmolarity was significantly higher than root osmolarity, then facilitating water and nutrient fluxes from host to parasite.9,10 The XBJ90.0316-1-1-1 genotype showed higher root osmolarity values than the susceptible cv. Bachaar, then lowering the magnitude in osmolarity (Fig. 1). This should trigger difference in kinetic of tubercle development as previously indicated on these genotypes. In the same way, the cultivar Giza402 was considered tolerant to O. crenata due to its higher osmotic values compared to susceptible cultivars.10,11
Figure 1.
Osmolarity (mosmol/L) of O. foetida tubercles (stage 4) and non infected and infected roots of the two faba bean genotypes. Data are means ± SE (n = 5, p = 0.05). * Arrows represent amplitude of osmolarity difference between infected root and tubercle. Values for the same parameter sharing the same letters did not differ significantly at 0.05 (Duncan test).
These results suggest the presence of a perturbation in the osmoregulation process in the parasite. Knowing that the broomrape depends primarily on phloem sap of the parasitized plant, we carried out the analysis of the composition in soluble sugars, organic acid and free amino acids of orobanche and the phloem exudates from adult leaves of faba bean (at pod setting stage). Our results showed that the phloem exudates of the tolerant line were highly deficient in nitrogen when compared to that of the susceptible line,12 and this may induce the tolerance of the XBJ90.03-16-1-1-1 plants to O. foetida. When attached to roots of the tolerant line, the parasite displayed limited activities of soluble invertases in tubercles, and especially in shoots, suggesting that the low performance of the broomrapes attached to the tolerant line resulted from a reduced capacity to utilize the host-derived carbohydrates.12
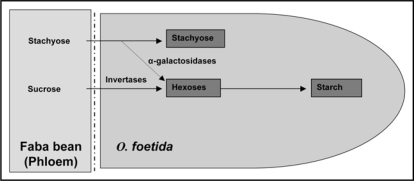
The major organic components potentially transferred from both faba bean genotypes to the parasite were identified as sucrose, stachyose, citrate, malate, asparagine, aspartate, glutamine, glutamate, serine, alanine and gamma amino butyric acid (GABA). However, the parasite accumulated preferentially hexoses, starch, malate and soluble amino acids, especially asparagine and aspartate.12 This difference in the nutrient composition between host and parasite can be explained by the implication of different enzymes (Figs. 2 and 3).
Figure 2.
Schematic representation of the conversion of the host-derived sugar in O. foetida (stage 4).
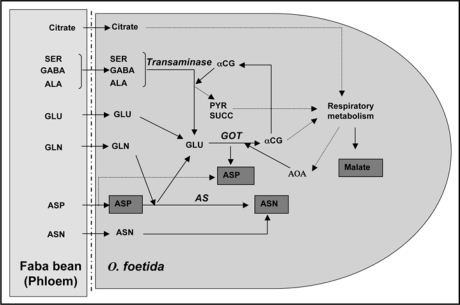
Figure 3.
Schematic representation of the conversion of the host-derived amino acids in O. foetida (stage 4). The hypothesis of the implication of GDH activities was not taken into consideration in this model. ASP, Aspartate; ASN, Asparagine; GLU, Glutamate; GLN, Glutamine; ALA, Alanine; SER, Serine; GABA, Gamma Amino N butyrique acid; αCG, α-cétoglutarate; PYR, Pyruvate; SUCC, Succinate; AOA, Oxaloacetic acid; AS, Asapargine synthetase; GOT, Glutamate oxaloacetate aminotransferase.
The accumulation of hexoses was also observed in O. crenata,11,13 O. aegyptiaca,14 O. hederae15 and O. ramosa.8 This accumulation might be related to the presence of invertases which degrades sucrose. Stachyose can help to accumulate hexoses in presence of α-galactosidase or can be accumulated directly in Orobanche, which makes up evidence of the existence of phloem symplasmic continuum at the interface host-parasite (Fig. 2). However, the starch presents a form of storage of the surplus of hexose.14
The use of the major amino acids present in the phloem exudates by the broomrape leads primarily to an incorporation of nitrogen in the pool aspartate/asparagine. This proves the existence of a particular nitrogen metabolism in O. foetida mainly centred on the couple aspartate/asparagine, which was also described in another parasitic plant, Striga hermonthica.16 This suggests an important role for a glutamine-dependent asparagine synthetase (EC 6.3.5.4) in the N metabolism of the parasite. In addition, as generally assumed in Orobanche,17,18 the glutamine synthetase (GS) activity (EC 6.3.1.2) is probably low in O. foetida, contributing to maintaining the glutamine pool at low level. Besides, the assumed utilization of the host-derived serine, GABA and alanine requires aminotransferase activities resulting in glutamate production. Nevertheless, like glutamine, glutamate is not accumulated in O. foetida indicating a rapid turnover of this component by a glutamate deshydrogenase (GDH) and/or glutamate oxaloacetate aminotransferase (GOT). However, a study using O. minor does not show a GDH activity but a GOT activity was detected,19 thus the implication of a GOT activity was more probable in the case of O. feotida (Fig. 3). The enzyme GOT produces aspartate and α-cetoglutarate which is necessary to transaminase activities and can contribute to respiratory metabolism. On the other hand, the citrate coming from the host phloem, contributes to the accumulation of malate in O. foetida via the respiratory metabolism (Fig. 3). Further studies dealing with the identification and the characterization of those enzymes involved in the N and C metabolism of O. foetida are recommended.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8192
References
- 1.Parker C, Riches CR. Parasitic Weeds of the World: Biology and Control. Wallingford UK: CAB International; 1993. [Google Scholar]
- 2.Kharrat M. Orobanche research activities on faba bean in Tunisia. In: Cubero JI, Moreno MT, Rubiales D, Sillero JC, editors. Resistance to Broomrape, the State of the Art. Sevilla, Spain: Junta de Andalucia; 1999. pp. 77–81. [Google Scholar]
- 3.Abbes Z, Kharrat M, Delavault P, Simier P, Chaïbi W. Field evaluation of the resistance of some faba bean (Vicia faba L.) genotypes to the parasitic weed Orobanche foetida Poiret. Crop Prot. 2007;26:1777–1784. [Google Scholar]
- 4.Kharrat M, Halila MH. Orobanche species on faba bean (Vicia faba L.) in Tunisia: problem and management. In: Pieterse AH, Verkleij JAC, ter Borg SJ, editors. Proceedings of the third International Workshop on Orobanche and Related Striga Research; Biology and Management of Orobanche, Amsterdam, The Netherlands. 1994. pp. 639–643. [Google Scholar]
- 5.Kharrat M, Halila MH. Control of Orobanche foetida on Vicia faba: comparison between different control measures—advances in parasitic plant research. In: Moreno MT, Cubero JI, Berner D, Joel DM, Musselman LJ, Parker C, editors. Proceedings of the sixth International Symposium on Parasitic Weeds; Cordoba. 1996. pp. 734–737. [Google Scholar]
- 6.Abbes Z, Kharrat M, Chaïbi W. Study of the interaction between Orobanche foetida and faba bean at root level. Tun J Plant Prot. 2006;1:55–64. [Google Scholar]
- 7.Abbes Z. Université de Nantes (France): Université Tunis El Manar (Tunisie); 2007. Estimation de la sensibilité et de la tolérance de différents génotypes de féverole (Vicia faba L.) à la plante parasite Orobanche foetida Poiret. Impact du génotype hôte sur les particularités physiologiques et métaboliques du parasite. PhD thesis. [Google Scholar]
- 8.Delavault P, Simier P, Thoiron S, Véronési C, Fer A, Thalouarn P. Isolation of mannose 6-phosphate reductase cDNA, changes in enzyme activity and mannitol content in broom-rape (Orobanche ramosa) parasitic on tomato roots. Plant Physiol. 2002;115:48–55. doi: 10.1034/j.1399-3054.2002.1150105.x. [DOI] [PubMed] [Google Scholar]
- 9.Withney PJ. The carbohydrate and water balance of beans (Vicia faba) attacked by broomrape (Orobanche crenata) Ann App Biol. 1972;70:59–66. [Google Scholar]
- 10.Wegmann K, Von Elert E, Harloff HJ, Stadler M. Tolerance and resistance to Orobanche. In: Wegmann K, Musselman LJ, editors. Progress in Orobanche Research, Proceedings of the International Workshop on Orobanche Research; Obermarchtal. 1991. pp. 318–321. [Google Scholar]
- 11.Wegmann K. Biochemistry of osmoregulation and possible biochemical reasons of resistance against Orobanche. In: ter Borg SJ, editor. Proceedings of a Workshop in Biology and Control of Orobanche; LH/VPO, Wageningen. 1986. pp. 107–117. [Google Scholar]
- 12.Abbes Z, Kharrat M, Delavault P, Chaïbi W, Simier P. Nitrogen and carbon relationships between the parasitic weed Orobanche foetida and susceptible and tolerant faba bean lines. Plant Physiol Biochem. 2009;47:153–159. doi: 10.1016/j.plaphy.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Harloff HJ, Wegmann K. Mannitol pathwayin Orobanche. In: Weber HC, Forstreuter W, editors. Parasitic Flowering Plants; Proceedings of the 4th ISPFP; 1987. pp. 295–309. [Google Scholar]
- 14.Singh M, Singh DV, Misra PC, Tewari KK, Krishnan PS. Biochemical aspects of parasitism by angiosperm parasites: starch accumulation. Physiol Plant. 1968;21:525–538. [Google Scholar]
- 15.Simier P, Fer A, Renaudin S. Identification of the main osmotically active solutes in the unstressed and water-stressed root-hemiparasitic angiosperm Thesium humile and its host Triticum vulgare. Aust J Plant Physiol. 1994;20:223–230. [Google Scholar]
- 16.Pageau K, Simier P, Le Bizec B, Robins RJ, Fer A. Characterization of nitrogen relationships between Sorghum bicolor and the root hemiparasitic angiosperm Striga hermonthica (Del.) Benth. using K15NO3 as isotopic tracer. J Exp Bot. 2003;54:789–799. doi: 10.1093/jxb/erg081. [DOI] [PubMed] [Google Scholar]
- 17.Press MC. In: Carbon and nitrogen relations. Press MC, Graves JD, editors. London: Parasitic Plants, Chapman and Hall; 1995. pp. 103–124. [Google Scholar]
- 18.Igbinnosa I, Thalouarn P. Nitrogen assimilation enzyme activities in witchweed (Striga) in host presence or absence. Weed Sci. 1996;44:224–232. [Google Scholar]
- 19.Rey L, Thalouarn P, Fer A, Renaudin S. About the capacity of achlorophyllous parasitic flowering plants to assimilate inorganic forms of carbon and nitrogen. Beitr Biol Pflanzen. 1990;65:429–441. [Google Scholar]