Abstract
Major regulators of carotenoid biosynthesis have remained rather elusive even though the flux through the branch in the carotenoid pathway can affect plant development in response to environmental stimuli, such as light. Our recent investigations demonstrated that the production of the most abundant carotenoid in plants, lutein, is regulated by carotenoid isomerase (CRTISO) activity at a rate-limiting step of this branch point in carotenoid biosynthesis. CRTISO is required to isomerase cis-carotenes, such as tetra-cis-lycopene to all-trans-lycopene. In order to maintain permissive transcriptional regulation of CRTISO, active marks of histone lysine methylation are targeted to the promoter region by the SET DOMAIN GROUP8 (SDG8) methyltransferase. Mutants of SDG8 (ccr1) and CRTISO (ccr2) show an increase in shoot branching, which may be partly explained by limiting synthesis of the carotenoid-derived branching hormone, strigolactone. In this addendum, we demonstrate new functions for SDG8 in mediating branching in Arabidopsis roots. The roles that carotenoids and SDG8 play in root and shoot development begins to open new doors for investigating the regulation of carotenoid composition in response to epigenetic events.
Key words: carotenoid, epigenetic, shoot branching, chromatin, lutein, arabidopsis, root development, SDG8, histone, methylation, transcription, mRNA, regulation
Essential Functions for Carotenoids during Plant Development
Carotenoids have many functions in plant development processes and are a major class of pigments that provide precursors for the biosynthesis of phytohormones such as abscisic acid and strigolactones (Fig. 1).1–5 Carotenoids play crucial roles in photosynthetic organisms, including photosystem assembly, light-harvesting and photoprotection.6,7 Lutein is the most abundant carotenoid in plants wherein it contributes to light harvesting and photoprotection.8 Carotenoid biosynthesis appears to be tightly regulated throughout the life cycle with dynamic changes in composition matched to developmental requirements including germination, photomorphogenesis and fruit development.9–11
Figure 1.
Schematic diagram of the carotenoid biosynthetic pathway showing key regulatory steps. The bottleneck in the pathway is the first committed step in the synthesis phytoene from geranylgeranyl pyrophosphate and is catalyzed by the light responsive enzyme, phytoene synthase (PSY). The branch point in the pathway involves the isomerisation of tetra-cis-lycopene to all-trans-lycopene by the carotenoid isomerase (CRTISO), which is regulated by a histone methyltransferase, SET DOMAIN GROUP8 (SDG8). Lycopene undergoes further modifications by εLCY (epsilon cyclase) and β-LCY (beta-cyclase) to produce α- and β-carotene, respectively, which serve as substrates for the production of lutein, phytohormones (abscisic acid and strigolactones) and an unknown signaling compound mediated by BYPASS (BPS). ccr: carotenoid and chloroplast regulation, lut2: lutein deficient mutant, CCD: CAROTENOID CLEAVAGE DIOXYGENASE.
The molecular nature of regulatory mechanisms and processes by which carotenoid composition is controlled has been a major challenge in understanding carotenoid metabolism.12 Carotenoid pigments and their corresponding enzymes are located in the plastid, although all known carotenoid biosynthetic genes are nuclear encoded. Recent evidence has shown that plastids are important structures capable of storing, sequestering and retaining carotenoids where they function as lipophilic antioxidants and protect membranes of the cell against oxidative damage.13,14 The control of plastid biogenesis and morphology is an important mechanism by which carotenoid biosynthesis and accumulation is regulated in plants.15 There are a growing number of reports describing carotenoid gene expression changes during developmental processes such as flowering, fruit ripening and seed maturation,2,16,17 that point towards the need for more sophisticated regulatory mechanisms for governing carotenoid biosynthesis. Also, recent discoveries have revealed that epigenetic processes are an integral part of ABA-regulated processes.18
Regulation of the Branch Point of Carotenoid Biosynthesis and Shoot Branching
There are two steps in the primary pathway that can be considered major targets for regulations of carotenoid composition. The first step can be described as the bottleneck in carotenoid biosynthesis and a number of recent studies have focused on the transcriptional regulation of the key enzyme, phytoene synthase (PSY), which converts geranylgeranyl diphosphate to phytoene (Fig. 1).19,20 The second major step in the pathway can be considered the branch point where rate limiting enzymes such as the carotenoid isomerase and epsilon cyclase (εLCY) modulate the synthesis of lutein and β-carotene from lycopene (Fig. 1).21
Our recent investigations demonstrated that a chromatin modifying gene with histone methyltransferase activity, SET DOMAIN GROUP 8 (SDG8), was required to maintain permissive gene expression of CRTISO.22 Mutations in SDG8 (ccr1; carotenoid and chloroplast regulation) resulted in a significant reduction of tri-methylation of histone 3 lysine 4 (H3K4) in chromatin surrounding the CRTISO translation start site and concomitantly transcription of CRTISO was reduced by >90%, presumably by a more closed configuration of chromatin. This reduction in CRTISO mRNA explains the reduced lutein content and demonstrates a novel elegant mechanism for regulating the branching point of carotenoid biosynthesis.22 ccr1 mutants displayed other developmental phenotypes such as a pale green leaf colour, reduced fertility, increased cauline node branching, early flowering in short days and an increase in shoot branching. The early flowering phenotype has been well characterized and results from a decrease in the trimethylation of histone 3 lysine 36 of chromatin surrounding FLOWERING LOCUS C (FLC), which leads to the repression of FLC transcription and stimulates an early flowering habit.23,24 The shoot branching phenotype can be partially explained by the reduction in CRTISO expression, which might perturb biosynthesis of the carotenoid substrates required for synthesis of strigolactones. This was the first report linking regulation of the branch point of the carotenoid pathway to key developmental processes such as shoot branching and began to raise new questions regarding to the affects that carotenoid composition may have on the behavior and the production of root derived signaling compounds.
Carotenoids are Important for Root Development and Signal Transduction
Carotenoid derived signaling compounds appear to play novel functions in mediating Arabidopsis root development. The strigolactone class of metabolites are graft transmissible and inhibit shoot branching in Arabidopsis, pea, petunia and rice while also functioning to stimulate a symbiotic relationship with fungi in the rhizosphere.3,4,25 A novel graft transmissible β-carotene-derived signaling compound was also shown to be required for normal root and shoot development, and is neither abscisic acid, nor a strigolactone as the signal does not require the activity of any single CAROTENOID CLEAVAGE DIOXYGENASE (CCD) (Fig. 1).26 Measurement of carotenoid composition in colourless root tissues has not been investigated in detail, although trace levels of neoxanthin and violaxanthin were reported for Arabidopsis, pea and tobacco (Fig. 1).27
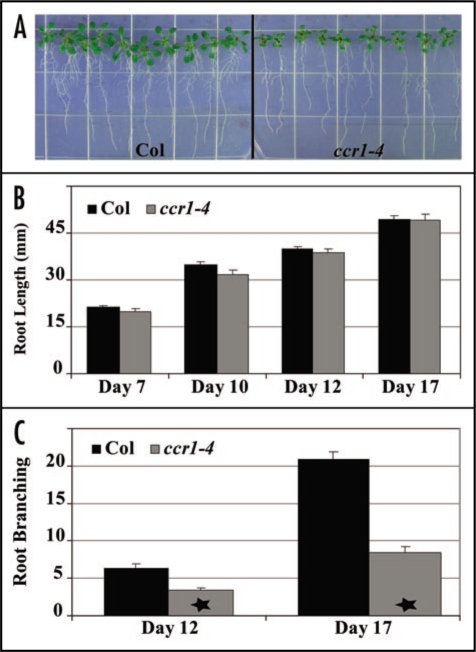
Given the importance of root-derived carotenoid signal compounds in plant development, we investigated root growth and branching habit of ccr1-4 plants. Length of the ccr1-4 primary root was similar when compared to wild type plants over a 17 day period (Fig. 2A and B). Interestingly, there was a significant (p < 0.005) reduction in the total number of secondary roots developed along the older regions of the primary root at both days 12 and 17 (Fig. 2A and C). Our previous investigations demonstrated that ccr1-4 root tissues have a lower abundance of CRTISO transcript in the absence of SDG8 methyltransferase activity.22 It is therefore conceivable that, in the absence of SDG8, the reduction in CRTISO mRNA abundance may perturb the flux of carotenoid derived substrates required for production of signaling compounds involved in secondary root branching. It is clear that carotenoid biosynthesis is required for the production of graft transmissible signaling compounds and regulation at the branch point of the biosynthetic pathway may represent such an elegant mechanism for controlling root and shoot branching.
Figure 2.
Root growth and branching in the carotenoid and chloroplast regulatory mutant ccr1. Seed was sterilized, plated onto Murashige and Skoog media (4.4 g/L MS salts, 1x MS salts, 0.5 g/L sucrose, 0.8% agar) and incubated in the dark for two days at 4°C before transferring plates to a growth chamber maintained at 21°C and illuminated by 150 µE of light for 16 hours. (A) Images of 17 day old plantlets from wild type Columbia (Col) and ccr1-4. (B) Average root length after 7, 10, 12 and 17 days of growth. (C) The average number of secondary root branches along the primary root after 12 and 17 days of growth. Standard error of 18 to 24 individual seedlings are displayed. Statistically significant data are indicated by a star (TTEST, 2 tailed, unpaired, p < 0.005).
Carotenoid Regulation and Epigenetic Insights?
The role that SDG8 plays in controlling carotenoid composition and the behavior of root and shoot branching is paramount to understanding how chromatin modifications are targeted to specific genes during plant development. Further investigations will examine the regulatory function of SDG8 in maintaining permissive transcriptional regulation of CRTISO and therefore controlling carotenoid flux during development. The function of root derived plastids, such as leucoplasts, in the synthesis and storage of carotenoid derived micronutrients is ripe for discovery. The branch point of carotenoid biosynthesis has emerged as a key regulatory step in the pathway controlling many plant developmental processes, however the molecular mechanisms regulating SDG8 and nature for targeting CRTISO remain to be discovered. The fact that SDG8 regulates so many key developmental processes and mediates the vernalization response, begins to reveal novel links between carotenoid biosynthesis, composition and epigenetic regulation.
Acknowledgements
We were supported by the Australian Research Council Centre of Excellence in Plant Energy Biology (CE0561495).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8193
References
- 1.DellaPenna D, Pogson BJ. Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol. 2006;57:711–738. doi: 10.1146/annurev.arplant.56.032604.144301. [DOI] [PubMed] [Google Scholar]
- 2.Howitt CA, Pogson BJ. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ. 2006;29:435–445. doi: 10.1111/j.1365-3040.2005.01492.x. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 4.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 5.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 6.Pogson BJ, Rissler HM, Frank HA. The roles of carotenoids in photosystem II of higher plants. In: Wydrzynski T, Satoh K, editors. Photosystem II: The water/plastoquinone oxidoreductase in photosynthesis. Dordrecht: Springer; 2006. [Google Scholar]
- 7.Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- 8.Pogson BJ, Niyogi KK, Bjorkman O, DellaPenna D. Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc Natl Acad Sci USA. 1998;95:13324–13329. doi: 10.1073/pnas.95.22.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser PD, Enfissi EM, Bramley PM. Genetic engineering of carotenoid formation in tomato fruit and the potential application of systems and synthetic biology approaches. Arch Biochem Biophys. 2008 doi: 10.1016/j.abb.2008.10.009. In press. [DOI] [PubMed] [Google Scholar]
- 10.Hirschberg J. Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol. 2001;4:210–218. doi: 10.1016/s1369-5266(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 11.Welsch R, Beyer P, Hugueney P, Kleinig H, von Lintig J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta. 2000;211:846–854. doi: 10.1007/s004250000352. [DOI] [PubMed] [Google Scholar]
- 12.Lu S, Li L. Carotenoid metabolism: biosynthesis, regulation and beyond. J Integr Plant Biol. 2008;50:778–785. doi: 10.1111/j.1744-7909.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- 13.Cookson PJ, Kiano JW, Shipton CA, Fraser PD, Römer S, Schuch W, et al. Increases in cell elongation, plastid compartment size and phytoene synthase activity underlie the phenotype of the high pigment-1 mutant of tomato. Planta. 2003;217:896–903. doi: 10.1007/s00425-003-1065-9. [DOI] [PubMed] [Google Scholar]
- 14.Lu S, Van Eck J, Zhou X, Lopez A, O'Halloran D, Cosman K, et al. The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high-levels of beta-carotene accumulation. Plant Cell. 2006;18:3594–3605. doi: 10.1105/tpc.106.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuliano G, Diretto G. Of chromoplasts and chaperones. Trends Plant Scie. 2007;12:529–531. doi: 10.1016/j.tplants.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Isaacson T, Ronen G, Zamir D, Hirschberg J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell. 2002;14:333–342. doi: 10.1105/tpc.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alquezar B, Rodrigo MJ, Zacarias L. Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara Cara. Phytochemistry. 2008;69:1997–2007. doi: 10.1016/j.phytochem.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Chinnusamy V, Gong Z, Zhu JK. Abscisic acid-mediated epigenetic processes in plant development and stress responses. J Integr Plant Biol. 2008;50:1187–1195. doi: 10.1111/j.1744-7909.2008.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsch R, Maass D, Voegel T, Dellapenna D, Beyer P. Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiol. 2007;145:1073–1085. doi: 10.1104/pp.107.104828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsch R, Wust F, Bar C, Al-Babili S, Beyer P. A third phytoene synthase is devoted to abiotic stress-induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes. Plant Physiol. 2008;147:367–380. doi: 10.1104/pp.108.117028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuttriss AJ, Chubb A, Alawady A, Grimm B, Pogson B. Regulation of lutein biosynthesis and prolamellar body formation in Arabidopsis. Funct Plant Biol. 2007;34:663–672. doi: 10.1071/FP07034. [DOI] [PubMed] [Google Scholar]
- 22.Cazzonelli C, Cuttriss A, Cossetto S, Pye W, Crisp P, Whelan J, et al. Regulation of carotenoid composition and shoot branching in Arabidopsis by a chromatin modifying histone methyltransferase, SDG8. Plant Cell. 2009 doi: 10.1105/tpc.108.063131. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SY, He Y, Jacob Y, Noh YS, Michaels S, Amasino R. Establishment of the vernalization-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase. Plant Cell. 2005;17:3301–3310. doi: 10.1105/tpc.105.034645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z, Yu Y, Meyer D, Wu C, Shen WH. Prevention of early flowering by expression of FLOWERING LOCUS C requires methylation of histone H3 K36. Nat Cell Biol. 2005;7:1256–1260. doi: 10.1038/ncb1329. [DOI] [PubMed] [Google Scholar]
- 25.Pichersky E. Raging hormones in plants. Nat Chem Biol. 2008;4:584–586. doi: 10.1038/nchembio1008-584. [DOI] [PubMed] [Google Scholar]
- 26.Van Norman JM, Sieburth LE. Dissecting the biosynthetic pathway for the bypass1 root-derived signal. Plant J. 2007;49:619–628. doi: 10.1111/j.1365-313X.2006.02982.x. [DOI] [PubMed] [Google Scholar]
- 27.Parry AD, Horgan R. Abscisic acid biosynthesis in roots 1. The identification of potential abscisic acid precursors, and other carotenoids. Planta. 1992;187:185–191. doi: 10.1007/BF00201936. [DOI] [PubMed] [Google Scholar]