Abstract
Two xanthophyll cycles have been described in higher plants: the ubiquitous violaxanthin (V) cycle and the taxonomically restricted lutein epoxide (Lx) cycle. Both involve the light induced de-epoxidation of an epoxidated xanthophyll (V or Lx) and the epoxidation back in the dark. Evolutionary trends and function of the Lx cycle are still not clear. Up to nowadays, significant amounts of Lx have been found in several unrelated taxa, but it is a character almost exclusive from woody plants (except in the case of the parasitic plant Cuscuta reflexa). We have found an exception to this pattern in Cucumis sativus L., which showed high concentrations of Lx. Since Lx cycle was operative in leaves and cotyledons of this species and Lx concentration were much higher in cotyledons than in leaves, we speculate a role for the early stages of development. To date, this species is the first herbaceous non-parasitic species with operative Lx cycle. Since this species can be much more easily and rapidly grown and investigated than woody plants, these data can open new horizons and new lines of investigation for Lx cycle.
Key words: cotyledon, cucurbitaceae, cucumber, lutein epoxide, violaxanthin, xanthophyll cycles
The photosynthetic apparatus of higher plants must be highly adaptable to quick and large changes in quantum flux1 and plants have to solve a challenging problem: they need to optimize their energy gain for carbon fixation and they need to protect the light harvesting system from photodamage. The excess of light energy can produce an overexcitation of the photosynthetic apparatus, which may lead to the formation of reactive oxygen species, causing photodynamic bleaching and perturbation of cellular metabolism.7 Plants have evolved photoprotective strategies for getting rid of the excess of light energy, which represent a trade-off between photosynthetic efficiency and photoprotection. One of these strategies is the modulation of the rate of thermal energy dissipation through the violaxanthin (V) cycle.4 The cycle implies inter-conversions between three carotenoids in the thylakoid membrane: violaxanthin (V), antheraxanthin (A) and zeaxanthin (Z). Violaxanthin de-epoxidase (VDE) catalyzes the light-induced de-epoxidation of V to Z via A and zeaxanthin epoxidase (ZE) catalyzes the epoxidation of Z back to V, upon weak light. The combined activity of both enzymes generates a light-dependent daily cycle. The V cycle is ubiquitous, occurring not only in land plants but also in all eukaryotic algae expect of Cryptophyta, Glaucophyta and Rhodophyta.11 In parallel to V cycle, lutein epoxide (Lx) cycle operation has been described, but it is restricted to some families of higher plants.10
The Lx cycle consists on a light-driven de-epoxidation of Lx into lutein (L) catalysed also by VDE. The complete operation of the Lx cycle requires the epoxidation back of L to Lx. However, in this cycle this step still remains unclear. Lutein has one β-ring, and it could be in principle a substrate for ZE. However, plant species and tissues seem to differ markedly in the extent to which L serves as a substrate for ZE.2 Indeed, ZE seems to be the key enzyme to complete the Lx cycle. So far, two different responses have been described with respect to Lx cycling. In some species, such as Cuscuta reflexa,3 Amyema miquelii12 and Virola elongata15 a complete Lx cycle operates, with full recovery of the initial Lx pool in the dark in a short-term. In others, a truncated Lx cycle takes place, with no overnight recovery of initial Lx pool. Indeed, L epoxidation seems to be irreversible in a short-term. This truncated cycle seems to operate in Quercus rubra,8 Inga sapindoides,13 L. nobilis5 and in photosynthetic tissues of enclosed buds of some woody plants where an irreversible conversion of L following bud-burst also takes place.9
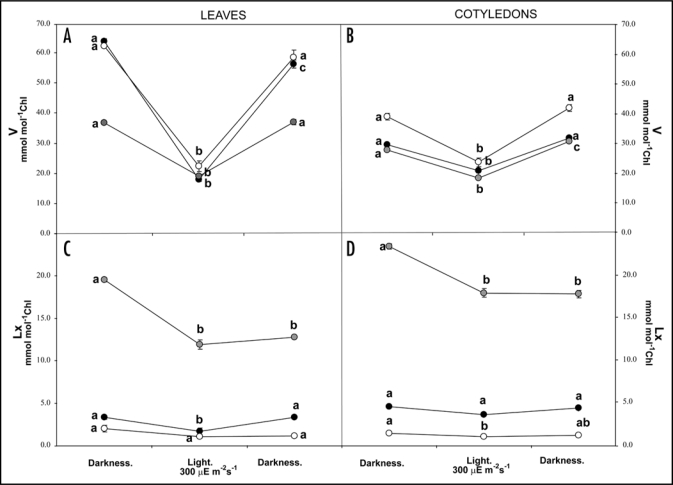
Our recent article shows that Lx cycle, contrasting with V cycle, is not an ancient character in plants since Lx is absent in more primitive groups. Besides, its concentration is not phylogenetically determined, rather, it would be determined by ecological constraints. In fact, we have reported that high Lx concentration (>10 mmol mol−1 chl) is related to woody habit and perennial leaves. The only exception to this trend was found in cucumber (Cucumis sativus L.) (Cucurbitaceae),6 apart from the parasitic Cuscuta reflexa.3 These are the only herbaceous plants described up to now with high contents of Lx. The presence of significant amounts of Lx in the family Cucurbitaceae has also been recently confirmed by Matsubara et al.15 (2009). However, presence of high Lx levels does not necessarily ensure the functionality of the Lx cycle. Hence, in this addendum, we investigate whether Lx is present in leaves and cotyledons of some species (C. sativus, Cucurbita pepo L. and Cucurbita maxima Duch.) of the Cucurbitaceae family and whether the Lx cycle is fully operative in theses species. Dark adapted leaf discs of each species were illuminated to determine the short-term performance of both xanthophyll cycles and followed by an overnight dark period. We observed the typical V cycle performance characterised by a decrease in V pool under light, followed by a recovery in both leaves and cotyledons of all species. However, Lx pool under light only decreased to significant extent (≈8 mmol mol−1 Chl) in leaves and cotyledons of C. sativus and Lx pool did not recover after the dark period. So, this species presents a truncated cycle in which VDE converts Lx into L, but regeneration of Lx by ZE is extremely slow o null (Fig. 1). Interestingly, V pool was similar between both Cucurbita species and higher than in C. sativus. Conversely, Lx pool was 5-fold higher in the cucumber. As a result total xanthophyll pool (V + Lx) was remarkably similar in the three species indicating that Lx and V may be exchangeable in their binding sites. A similar pattern was observed in cotyledons but this tissue presents higher Lx content than leaves.
Figure 1.
Induction of V and Lx cycles in leaves and cotyledons of C. sativus (grey symbols), C. maxima (black symbols) and Cucurbita pepo (white symbols) in dark adapted samples, after illumination of 30 minutes at 300 µE m−2s−1 and following by a dark recovery for 16 hours. Each value represents the mean of 3 replicates ± S.E. Analyses of Variance (ANOVAs) was performed, considering type of treatment (darkness, light treatment and darkeness) as fixed factors in each species. Different letters denote statistically significant differences at p < 0.05 among treatments in each species after Student-Newman-Keul test.
The significance and function of Lx cycle still remain a challenge since the simultaneous operation of both cycles make difficult to distinguish the role of each xanthophyll cycle. All the observations point out a dual role for Lx in photosynthetic performance: light harvesting under limiting light14 and photoprotection mechanism under sudden irradiance changes.10 Both functions may have an adaptive value in seedlings, representing a mechanism to maximize light harvesting efficiency and at the same time a reservoir of photoprotective L to protect against sudden changes in light environment, such as a formation of gaps in the forest canopy. Taking all this into consideration, the operation and significance of Lx cycle in non-woody species should not be underestimated. Therefore, the function of Lx cycle in C. sativus may resemble the function of Lx cycle in seedlings of woody plants growing in forest environment. For C. sativus, a species with epigenous germination, photosynthetic cotyledons need to grow fast, maximizing light harvesting efficiency, which implies highest Lx pool. When this pool converts to L, it could play a photoprotective role in leaves growing in a more irradiated environment.
Since the Lx cycle was discovered in C. reflexa3 and considered as an accessory cycle, an important effort has been made to understand the function and role of this cycle. Nowadays, we have a clear overview of the Lx cycle but experimental approaches have always encountered the additional difficulty and limitation of having to work with long-lived woody plants. From this perspective, the presence of an operative Lx cycle in the model species C. sativus described in this addendum opens new horizons in the investigation of Lx cycle.
Acknowledgements
R.E. received a fellowship from the Basque Government. This research was supported by research projects from the Ministry of Education and Science of Spain (BFU 2007-62637) and from University of the Basque Country/Basque Government (UPV/EHU-GV IT-299-07).
Abbreviations
- A
anteraxanthin
- L
lutein
- Lx
lutein epoxide
- V
violaxanthin
- VDE
violaxanthin de-epoxidase
- Z
zeaxanthin
- ZE
zeaxanthin epoxidase
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8197
References
- 1.Björkman O. Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Physiological Plant Ecology I. Responses to the Physical Environment. Berlin: Springer; 1981. pp. 57–107. [Google Scholar]
- 2.Bouvier F, d'Harlingue A, Hugueney P, Marin E, Marion-Poll A, Camara B. Xanthophyll biosynthesis. J Biol Chem. 1996;271:28861–28867. doi: 10.1074/jbc.271.46.28861. [DOI] [PubMed] [Google Scholar]
- 3.Bungard RA, Ruban AV, Hibberd JM, Press MC, Horton P, Scholes JD. Unusual carotenoid composition and a new type of xanthophyll cycle in plants. Proc Natl Acad Sci USA. 1999;96:1135–1139. doi: 10.1073/pnas.96.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demmig-Adams B, Adams WW. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- 5.Esteban R, Jiménez MS, Morales D, Jiménez ET, Hormaetxe K, Becerril JM, et al. Short- and long-term modulation of the lutein epoxide and violaxanthin cycles in two species of the Lauraceae: sweet bay laurel (Laurus nobilis L.) and avocado (Persea Americana Mill.) Plant Biol. 2008;10:288–297. doi: 10.1111/j.1438-8677.2008.00036.x. [DOI] [PubMed] [Google Scholar]
- 6.Esteban R, Olano JM, Castresana J, Fernández-Marín B, Hernández A, Becerril JM, García-Plazaola JI. Distribution and evolutionary trends of photoprotective isoprenoids (xanthophylls and tocopherols) within the plant kingdom. Physiol Plant. 2009 doi: 10.1111/j.1399-3054.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- 7.Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- 8.García-Plazaola JI, Hernández A, Olano JM, Becerril JM. The operation of the lutein epoxide cycle correlates with energy dissipation. Funct Plant Biol. 2003;30:319–324. doi: 10.1071/FP02224. [DOI] [PubMed] [Google Scholar]
- 9.García-Plazaola JI, Hormaetxe K, Hernández A, Olano JM, Becerril JM. The lutein epoxide cycle in vegetative buds of woody plants. Funct Plant Biol. 2004;31:815–823. doi: 10.1071/FP04054. [DOI] [PubMed] [Google Scholar]
- 10.García-Plazaola JI, Matsubara S, Osmond B. The lutein epoxide cycle in higher plants: its relationships to other xanthophyll cycles and possible functions. Funct Plant Biol. 2007;34:759–773. doi: 10.1071/FP07095. [DOI] [PubMed] [Google Scholar]
- 11.Larkum A. Light-harvesting systems in algae. In: Larkum A, Douglas SE, Raven JA, editors. Photosynthesis in Algae. Dordrecht: The Netherlands: Springer; 2003. pp. 277–304. [Google Scholar]
- 12.Matsubara S, Gilmore AM, Osmond CB. Diurnal and acclimatory responses of violaxanthin and lutein epoxide in the Australian mistletoe Amyema miquelii. Aust J Plant Physiol. 2001;28:793–800. [Google Scholar]
- 13.Matsubara S, Naumann M, Martin R, Nichol C, Rascher U, Morosinotto T, et al. Slowly reversible de-epoxidation of lutein-epoxide in deep shade leaves of a tropical tree legume may ”lock in“ lutein-based photoprotection during acclimation to strong light. J Exp Bot. 2005;56:461–468. doi: 10.1093/jxb/eri012. [DOI] [PubMed] [Google Scholar]
- 14.Matsubara S, Morosinotto T, Krause H, Seltmann M, Winter K, Osmond B, et al. Achieving better light harvesting in the shade: Accumulation of lutein epoxide increases light-harvesting efficiency in shade leaves of Inga species. Photosynth Res. 2007;91:250–251. [Google Scholar]
- 15.Matsubara S, Krause GH, Aranda J, Virgo A, Beisel KG, Jahns P, Winter K. Sun-shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Funct Plant Biol. 2009;36:20–36. doi: 10.1071/FP08214. [DOI] [PubMed] [Google Scholar]