Abstract
Transmission of electrical charge between a lobe and the midrib causes closure of the trap and induces an electrical signal propagating between a lobe and a midrib. The Venus flytrap can accumulate small subthreshold charges, and when the threshold value is reached, the trap closes. The cumulative character of electrical stimuli points to the existence of short-term electrical memory in the Venus flytrap. We investigated the electrical properties of the upper leaf of the Venus flytrap and proposed the equivalent electrical circuit in agreement with the experimental data.
Key words: plant memory, electrophysiology, electrical circuits, venus flytrap, Dionaea muscipula ellis
Signaling and memory play fundamental roles in plant responses.1–9 The existence of different forms of plant memory is well known.7 Depending on the duration of memory retention, there are three types of memory in plants: sensory memory, short term memory and long term memory.2,7,10 Learning and memory, usually associated with brain or neuronal activity, have been observed recently in the amoeba Physarum polycephalum, a unicellular organism.11 It is well-established that cells receive, interpret and adjust to environment.4,10,12,13 Plants are intelligent organisms and capable of functions such as learning, plasticity and memory.10 Some plants exhibit clues of an electrical memory as well.1,2,7
We found that Venus flytrap has a short term electrical memory.1,2,7 Rapid closure of the carnivorous plant Dionaea muscipula Ellis (Venus flytrap) has been attracting the attention of researchers and as a result its mechanism has been widely investigated. When an insect touches the trigger hairs, these mechanosensors generate an electrical signal that acts as an action potential, which activates the trap closing.
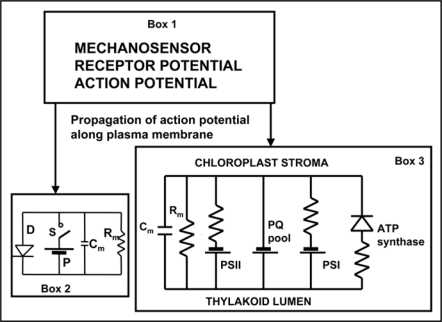
The reason why plants have developed pathways for electrical signal transmission most probably lies in the necessity to respond rapidly to environmental stress factors. Different environmental stimuli evoke specific responses in living cells, which have the capacity to transmit a signal to the responding region. In contrast to chemical signals such as hormones, electrical signals are able to rapidly transmit information over long distances with speed up to 270 m/s.14 Electrical potentials have been measured at the tissue and whole plant levels.4 In terms of electrophysiology, Venus flytrap responses can be considered in three stages: (i) stimulus perception, (ii) signal transmission and (iii) induction of response (Fig. 1).
Figure 1.
Biologically closed electrical circuits in Venus flytrap. Abbreviations: Cm, membrane capacitance; Rm, membrane resistance; PS, photosystem; PQ pool, plastoquinone pool; D, diode as a model of a voltage gated ion channel; S, electrical charge or voltage sensor; P, ATP dependent H+ pump.
Recently, we investigated the biologically closed electrical circuits in the upper leaf of the Venus flytrap and proposed the equivalent electrical circuit of the trap (Fig. 1, Box 2).1 It is often convenient to represent the real electrical and electrochemical properties of biointerfaces with idealized equivalent electrical circuit models consisting of discrete electrical components.
Using the charge injection method,2 it was evident that the application of an electrical stimulus between the midrib (positive potential) and a lobe (negative potential) causes Venus flytrap to close the trap without any mechanical stimulation. Application of a single 14 µC electrical charge from a capacitor causes trap closure and induces an electrical signal propagating between the lobes and the midrib. The Venus flytrap can accumulate and sum small charges, and when the threshold value is reached, the trap closes. A summation of stimuli is demonstrated through the repetitive application of smaller charges. The capacitor discharges exponentially with time, so although a 14 µC charge was applied, not all of this charge was accumulated to assist in closing the trap. As soon as 9.01 µC charge is transmitted between a lobe and midrib from the capacitor, the trap begins to close at room temperature. It was shown that voltage-gated ion channels have a time-dependent “molecular” memory phenomenon with profound implication on the biophysical properties of voltage-gated ion channels.15
Biologically closed electrical circuits operate over large distances in biological tissues. The activation of such circuit can lead to various physiological and biophysical responses. Action potentials play an important role in triggering photosynthetic responses in plants.16–20 The equivalent electrical circuit of photosynthesis is shown on Figure 1 (Box 3).
It is common knowledge that the leaves of the Venus flytrap actively employ turgor pressure and hydrodynamic flow for fast movement and catching insects. In these processes the upper and lower surfaces of the leaf behave quite differently. During the trap closing, the loss of turgor by parenchyma lying beneath the upper epidermis, accompanied by the active expansion of the tissues of the lower layers of parenchyma near the under epidermis, closes the trap. The cells on the inner face of the trap jettison their cargo of water, shrink and allow the trap lobe to fold over. The cells of the lower epidermis expand rapidly, folding the trap lobe over. These anatomical features constitute the basis of the new hydroelastic curvature model.21
There are many different biologically closed electrical circuits in plants (Fig. 1): photosynthetic electrical circuits (Box 3), plasma membrane electrical circuits responsible for receptor, action and resting potentials (Box 1), the electrical starter of a hydroelastic trap closing in Dionaea muscipula Ellis (Box 2), and others.
Abbreviations
- Cm
membrane capacitance
- Rm
membrane resistance
- PS
photosystem
- PQ pool
plastoquinone pool
- D
diode as a model of a voltage gated ion channel
- S
electrical charge or voltage sensor
- P
ATP dependent H+ pump
Addendum to: Volkov AG, Carrell H, Markin VS. Biologically closed electrical circuits in Venus flytrap. Plant Physiol. 2009 doi: 10.1104/pp.108.134536. In press.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8219
References
- 1.Volkov AG, Carrell H, Markin VS. Biologically closed electrical circuits in Venus flytrap. Plant Physiol. 2009 doi: 10.1104/pp.108.134536. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol. 2008;146:694–702. doi: 10.1104/pp.107.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkov AG, Adesina T, Jovanov E. Closing of Venus flytrap by electrical stimulation of motor cells. Plant Signal Behavior. 2007;2:139–145. doi: 10.4161/psb.2.3.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkov AG, editor. Plant electrophysiology—theory & methods. Berlin: Springer; 2006. [Google Scholar]
- 5.Volkov AG. Green plants: Electrochemical interfaces. J Electroanal Chem. 2000;483:150–156. [Google Scholar]
- 6.Plant Electrophysiology. In: Volkov AG, editor; Bard AJ, Inzelt G, Scholz F, editors. Electrochemical dictionary. Berlin: Springer; 2008. pp. 503–5044. [Google Scholar]
- 7.Volkov AG, Carrell H, Adesina T, Markin VS, Jovanov E. Plant electrical memory. Plant Signal Behav. 2008;3:490–492. doi: 10.4161/psb.3.7.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkov AG, Coopwood KJ, Markin VS. Inhibition of the Dionaea muscipula Ellis trap closure by ion and water channels blockers and uncouplers. Plant Sci. 2008;175:642–649. [Google Scholar]
- 9.Volkov AG, Brown CL. Nanodevices in Nature. In: Kumar CSSR, editor. Nanodevices for life sciences. Weinheim: Wiley-VCH; 2006. pp. 440–463. [Google Scholar]
- 10.Barlow PW. Reflections on “plant neurobiology”. Biosystems. 2008;92:132–147. doi: 10.1016/j.biosystems.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Saigusa T, Tero A, Nakagaki T, Kuramoto Y. Amoebae anticipate periodic events. Phys Rev Lett. 2008;100:1–4. doi: 10.1103/PhysRevLett.100.018101. [DOI] [PubMed] [Google Scholar]
- 12.Baluška F, Mancuso S, Volkman D, editors. Neuronal Aspects of Plant Life. Berlin: Springer; 2006. Communication in Plants. [Google Scholar]
- 13.Trewavas A. Aspects of plant intelligence. Annals Botany. 2003;92:1–20. doi: 10.1093/aob/mcg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra NS, Mallick BN, Soropy SK. Electrical signal from root to shoot in Sorghum bicolor: induction of leaf opening and evidence for fast extracellular propagation. Plant Sci. 2001;160:237–245. doi: 10.1016/s0168-9452(00)00378-2. [DOI] [PubMed] [Google Scholar]
- 15.Nayak TK, Sikdar SK. Time-dependent molecular memory in single voltage-gated sodium channel. J Membr Biol. 2007;219:19–36. doi: 10.1007/s00232-007-9058-4. [DOI] [PubMed] [Google Scholar]
- 16.Bulychev AA, Kamzolkina NA. Effect of action potential on photosynthesis and spatially distributed H+ fluxes in cells and chloroplasts of Chara coralline. Russ J Plant Physiol. 2006;53:5–14. [Google Scholar]
- 17.Pikulenko MM, Bulychev AA. Light-triggered action potentials and changes in quantum efficiency of photosystem II in Anthoceros cells. Russ J Plant Physiol. 2005;52:660–666. [Google Scholar]
- 18.Bulychev AA, Krupinina NA. Action potential opens access for the charged cofactor to the chloroplasts of Chara coralina cells. Russ J Plant Physiol. 2008;55:192–201. [Google Scholar]
- 19.Bulychev AA, Niyazova MM, Turovetsky V. Electroinduced changes of chlorophyll fluorescence in individual intact chloroplasts. Biochim Biophys Acta. 1986;850:218–225. [Google Scholar]
- 20.Koziolek C, Grams TEE, Schreiber U, Matysek R, Fromm J. Transient knockout of photosynthesis mediated by electrical signals. New Phytol. 2003;161:715–722. doi: 10.1111/j.1469-8137.2004.00985.x. [DOI] [PubMed] [Google Scholar]
- 21.Markin VS, Volkov AG, Jovanov E. Active movements in plants: mechanism of fly catching by Venus flytrap. Plant Signal Behav. 2008;3:778–783. doi: 10.4161/psb.3.10.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]