Abstract
Blood pressure is a heritable trait, but no common genetic variants contributing to blood pressure in humans have been definitively established. Natriuretic peptides (NP) have blood pressure-lowering properties. Genotyping SNPs at the NPPA/NPPB locus in 14,743 individuals of European ancestry identified associations of plasma atrial natriuretic peptide with rs5068 (P=8×10−70), rs198358 (P=8×10−30), and rs632793 (P=2×10−10), and of plasma B-type natriuretic peptide with rs5068 (P=3×10−12), rs198358 (P=1×10−25), and rs632793 (P=2×10−68). In 29,717 individuals, the alleles of rs5068 and rs198358 related to increased circulating NP concentrations were associated with lower systolic (P=2×10−6 and 6×10−5, respectively) and diastolic blood pressure (P=1×10−6 and 5×10−5), and reduced odds of hypertension (odds ratio 0.85, 95% confidence interval, 0.79–0.92, P=4×10−5; odds ratio 0.90, 95% confidence interval, 0.85–0.95, P=2×10−4, respectively). Common genetic variants related to circulating NP concentrations contribute to inter-individual variation in blood pressure and hypertension.
Hypertension affects a billion individuals worldwide, and represents a potent risk factor for cardiovascular disease.1 Because the relationship between blood pressure and cardiovascular risk is continuous, even small increments in blood pressure confer excess hazard.2,3 However, the underlying determinants of inter-individual variation in blood pressure are poorly defined. Epidemiologic studies have documented substantial heritability of blood pressure, suggesting a role for genetic factors.4 Although rare genetic variants have been described for monogenic forms of hypertension5 and more recently for blood pressure in the general population,6 no common variants for blood pressure have been convincingly demonstrated. While many pathways, including the renin-angiotensin-aldosterone system and the adrenergic system, have been found to modulate blood pressure in experimental models, studies have failed to show that genetic variation in these pathways contributes to inter-individual differences in blood pressure.
Since the discovery that the heart secretes a family of vasodilatory and natriuretic hormones in response to increased wall stress,7 it has been speculated that these molecules, known as the natriuretic peptides, might be involved in blood pressure regulation in humans. In mice, knockout of one copy of the atrial natriuretic peptide (ANP) gene, but not the B-type natriuretic peptide (BNP) gene, is associated with salt-sensitive hypertension.8. Overexpression of ANP through gene therapy in hypertensive mice lowers systolic blood pressure.9 To date, however, clinical studies have failed to establish a causal link between the natriuretic peptide axis and blood pressure variation in humans.
Natriuretic peptide production is stimulated by increased systolic blood pressure, via increased cardiac afterload. The competing influences of a potential blood-pressure lowering effect of natriuretic peptides and a natriuretic peptide-raising effect of elevated blood pressure make cross-sectional studies difficult to interpret, and suggest a role for genetic studies, in which directionality is unambiguous. We sought to demonstrate that cis-acting genetic variants influence natriuretic peptide levels and, in turn, that genetically-determined variation in natriuretic peptide concentrations contributes to inter-individual variation in blood pressure and hypertension (Supplementary Figure 1).
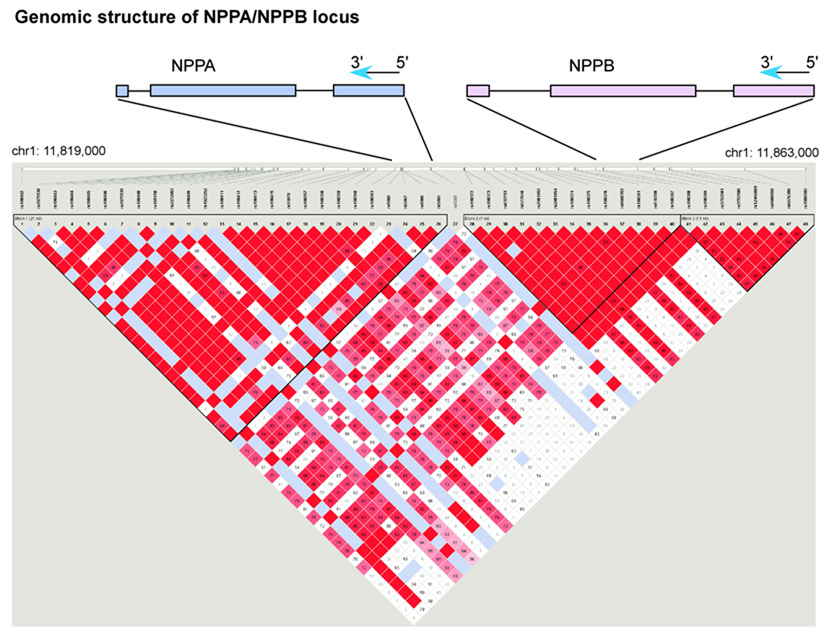
The natriuretic peptide precursor A (NPPA) and natriuretic peptide precursor B (NPPB) genes lie in tandem 9.7 kb apart on chromosome 1. We genotyped a set of 13 SNPs that captured the majority of common variation at the NPPA/NPPB locus (Figure 1, Supplementary Methods, Supplementary Table 1). Clinical characteristics of the study samples and covariate relationships to ANP and BNP are presented in Table 1 and Supplementary Table 2. Of the 13 SNPs tested in stage 1 in 1,705 unrelated Framingham Heart Study participants, multiple SNPs were nominally associated with ANP concentration (Supplementary Table 3), with the strongest association involving a missense SNP in NPPA (rs5063, V32M, P=7×10−55). Several SNPs showed nominal association with BNP concentration, with the strongest statistical support for rs632793 (P=2×10−8). Because many of the stage 1 SNPs were correlated, we performed additional analyses adjusting for the SNP with the lowest p-value (rs5063 for ANP, and rs632793 for BNP), in order to determine the statistical support for the remaining SNPs separately (Supplementary Table 4). Based on these analyses and on the linkage disequilibrium patterns, we selected 3 SNPs associated with ANP and 1 SNP associated with BNP for genotyping in stage 2.
Figure 1. Linkage disequilibrium map across NPPA, NPPB locus in CEPH reference sample.
The exon structures of NPPA and NPPB are shown schematically relative to the SNPs genotyped in the reference samples. The two genes fall on the negative strand of the human genome reference sequence and thus are in 3’ to 5’ order as shown. All SNP alleles in the text are shown according to the coding (negative) strand sequence. The pairwise linkage disequilibrium (LD) relationships among the 48 polymorphic (minor allele frequency ≥5%) SNPs passing quality control in the CEPH reference sample are shown. The linkage disequilibrium between all pairs of SNPs tested is represented at the bottom of the figure: red square indicates significant LD, white indicates weak LD, and light blue reflects inadequate power to determine LD. Three blocks of strong linkage disequilibrium are shown. The NPPA gene falls within the first block and a small region of LD breakdown between the first and second blocks. The NPPB gene lies within the second block of strong LD. Figure prepared using HaploView v2.03, defining blocks using the ‘spine of LD’ method. 35 The genomic position on chromosome 1 is indicated with reference to the hg17 assembly of the human reference sequence (May 2004, NCBI build 35). The SNP numbering 1–48 corresponds to the SNPs as ordered in Supplementary Table 1.
Table 1.
Characteristics of study samples
| Framingham Men (N=1,165) |
Framingham Women (N=1,291) |
Malmö Men (N=2,127) |
Malmö Women (N=3,069) |
Finrisk97 Men (N=3,711) |
Finrisk97 Women (N=3,838) |
Malmö Preventive Project Men (N=9,716) |
Malmö Preventive Project Women (N=4,800) |
|
|---|---|---|---|---|---|---|---|---|
| Samples 1 and 2 | Sample 3 | Sample 4 | Sample 5 | |||||
| Age, years | 58 ±10 | 58 ±10 | 58 ±6 | 58 ±6 | 49 ±13 | 47 ±13 | 43 ±6 | 50 ±7 |
| Body mass index, kg/m2 | 28.7 ±4.4 | 27.4 ±5.8 | 26.2 ±3.5 | 25.5 ±4.2 | 27.0 ±3.9 | 26.3 ±5.0 | 24.5 ±3.0 | 24.2 ±3.9 |
| Systolic blood pressure, mm Hg | 130 ±17 | 127 ±20 | 143 ±19 | 140 ±19 | 140 ±19 | 132 ±20 | 128 ±13 | 126 ±16 |
| Diastolic blood pressure, mm Hg | 78 ±9 | 74 ±9 | 89 ±10 | 86 ±9 | 85 ±11 | 80 ±11 | 86 ±9 | 83 ±9 |
| Anti-hypertensive therapy, % | 30 | 25 | 18 | 16 | 15 | 11 | 3 | 8 |
| Diabetes mellitus, % | 13 | 8 | 12 | 6 | 6 | 5 | 3 | 3 |
| N-terminal proANP, pmol/L | 356 ±265 | 395 ±222 | -- | -- | -- | -- | -- | -- |
| Mid-regional N-terminal proANP, pmol/L* | -- | -- | 69 ±39 | 76 ±32 | 49 ±38 | 52 ±27 | -- | -- |
| N-terminal proBNP, pg/mL | -- | -- | 94 ±261 | 106 ±143 | -- | -- | -- | -- |
| BNP, pg/mL | 14 ±21 | 16 ±20 | -- | -- | 22 ±63 | 25 ±31 | -- | -- |
Shown are the clinical characteristics for the Framingham Heart Study (Unrelated, sample 1, and Related, sample 2, combined), the Malmö Diet and Cancer-Cardiovascular Arm (MDC-CVA, sample 3), the Finrisk97 cohort (sample 4) and the Malmö Preventive Project (sample 5). Values are reported as mean ± SD for continuous traits, and percents for dichotomous traits.
Mid-regional N-terminal proANP refers to the assay for N-terminal pro-ANP that uses an antibody against an epitope in the mid-region of the molecule, as opposed to the N-terminal epitopes used in the Framingham assays. Assays used to measure ANP and BNP are described in the Supplementary Methods. -- = not available
In stage 2, we attempted to validate the association of 4 SNPs with natriuretic peptide concentrations, using 4 study samples totaling 14,743 individuals (Framingham Unrelated and Related, Malmö Diet and Cancer, Finrisk97). SNP rs5063 showed a highly significant association with ANP in the Framingham Related sample (P=2×10−8), but no association in Malmö Diet and Cancer (P=0.24) or Finrisk97 (P=0.07), despite the >6-fold higher sample sizes in the latter two studies. Because rs5063 is a missense SNP encoding an amino acid change within the binding site of the N-terminal pro-ANP assay used in Framingham but not in Malmö or Finrisk97,10 we hypothesized that the association in Framingham was artifactual (Supplementary Methods). Mature ANP was measured in Framingham samples using an assay that does not bind to the region containing rs5063 and failed to show association with rs5063 genotype (P=0.97, Supplementary Note). Missense SNPs have received particular attention because of their greater likelihood of being functional compared with non-coding SNPs. This example illustrates a potential pitfall of relating missense variants in a gene with the concentration of its protein product.
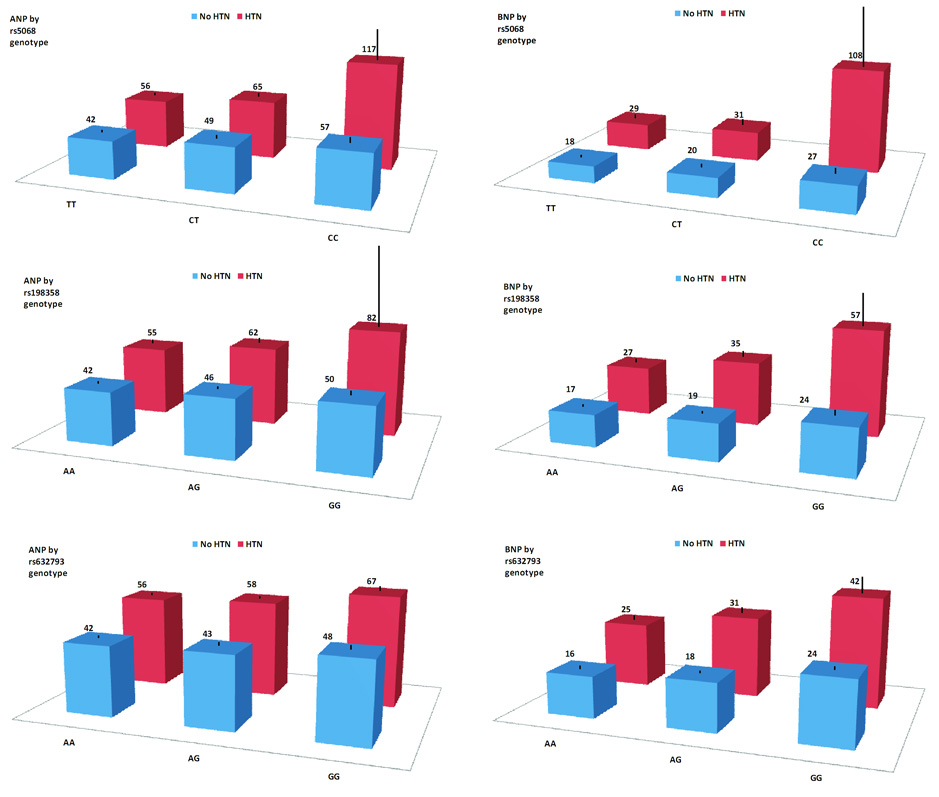
Stage 2 results for the remaining 3 SNPs are summarized in Table 3; cohort-specific results are shown in Supplementary Table 5. Associations with higher ANP concentration were observed for the minor alleles of rs5068 (P=8×10−70), rs198358 (P=8×10−30), and rs632793 (P=2×10−10). Associations with higher BNP were observed for the minor alleles of rs5068 (P=3×10−12), rs198358 (P=9×10−25), and rs632973 (P=2×10−68). All SNPs were associated with comparable effects on BNP (+0.17 to 0.21 SD) but rs5068 was associated with strong (+0.42 SD), rs198358 intermediate (+0.20 SD), and rs632793 weak (+0.08 SD) effects on ANP (Table 2). ANP and BNP were highly correlated (r=0.71) but pairwise r2 values among rs5068, rs198358, and rs632793 were all <0.3. When both rs5068 and rs198358 were entered into a single regression model predicting ANP, the association of each was attenuated but statistically significant in Framingham Unrelated (rs5068 P=0.005, rs198358 P=0.02) and Finrisk97 samples (rs5068 P=2×10−18, rs198358 P=0.003). rs5068 and rs198358 thus appear independent due to their divergent effects on ANP relative to BNP and their significance in a single regression model. A graded relationship of increasing ANP and BNP concentrations with increasing copies of the minor alleles of rs5068, rs198358, and rs632793 was observed in both hypertensive and non-hypertensive groups (Supplementary Table 6, Figure 2).
Table 3.
Association of confirmed natriuretic peptide variants with blood pressure (stages 3 and 4).
| Stage 3 Blood pressure Samples 1–4 N=15,201 |
Stage 4 Blood pressure Sample 5 N=14,516 |
Stage 3 + Stage 4 Blood pressure Samples 1–5 N=29,717 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP ID | SBP | DBP | SBP | DBP | SBP | DBP | ||||||
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | |
| rs5068 | −0.09 | 1×10−4 | −0.07 | 0.002 | −0.07 | 0.005 | −0.09 | 1×10−4 | −0.08 | 2×10−6 | −0.08 | 1×10−6 |
| (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | |||||||
| Adjusted for ANP | 1×10−8 | 0.001 | -- | -- | -- | -- | ||||||
| rs198358 | −0.07 | 5×10−5 | −0.06 | 4×10−4 | −0.03 | 0.10 | −0.04 | 0.03 | −0.05 | 6×10−5 | −0.05 | 5×10−5 |
| (0.02) | (0.02) | (0.02) | (0.02) | (0.01) | (0.01) | |||||||
| Adjusted for ANP | 7×10−8 | 4×10−4 | -- | -- | -- | -- | ||||||
| rs632973 | −0.02 | 0.16 | −0.02 | 0.12 | -- | -- | -- | -- | ||||
| (0.01) | (0.01) | |||||||||||
Association results for systolic and diastolic blood pressure (SBP, DBP) adjusted for age, sex and BMI, are shown for rs5068, rs198358 and rs632793 in stage 3 (samples 1–4), and for rs5068 and rs198358 in an independent sample in stage 4 (sample 5,n=14,516), as well as summary meta-analysis results in the combined stage 3 and stage 4 samples (samples 1–5). In stage 3 samples, analyses were repeated with additional adjustment for ANP. Blood pressure associations (stages 3 and 4) were only tested under a single genetic model, corresponding to the one most supported by the natriuretic peptide concentration association results (Table 2).For rs5068 and rs198358 a dominant model was used, and for rs632793 an additive model was used. β = effect estimate from linear regression, SE = standard error , -- = not available.
Table 2.
Meta-analysis of natriuretic peptide concentration associations (stage 2).
| Stage 2 Samples 1–4 N=14,473 |
||||
|---|---|---|---|---|
| SNP ID | ANP | BNP | ||
| β (SE) | p-value | β (SE) | p-value | |
| rs5068 | +0.42 | 8×10−70 | +0.17 | 3×10−12 |
| MAF 0.06 | (0.02) | (0.02) | ||
| rs198358 | +0.20 | 8×10−30 | +0.18 | 9×10−25 |
| MAF 0.19 | (0.02) | (0.02) | ||
| rs632973 | +0.08 | 2×10−10 | +0.21 | 2×10−68 |
| MAF 0.38 | (0.01) | (0.01) | ||
In stage 2, results are shown from meta-analysis of association results with atrial and B-type natriuretic peptides (ANP, BNP) for rs5068, rs198358, and rs632793 in samples 1–4. Effect sizes (β) are shown on the standard deviation scale. Meta-analysis was performed using inverse variance weights. Association results are shown for the genetic model (dominant, additive, recessive) that yielded the lowest p-value for association. Results for all 3 genetic models in each individual sample are shown in Supplementary Table 5. β = effect estimate from linear regression, SE = standard error , MAF = minor allele frequency.
Figure 2. ANP and BNP concentration in 7,091 individuals from Finrisk97 by genotype and by hypertension status.
Genotype classes are shown from major homozygotes (left) to minor homozygotes (right). Mean plasma concentration of natriuretic peptide is shown by genotype in individuals with (red) and without (blue) hypertension. Hypertension is defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or use of antihypertensive medication. ANP units are pmol/L and BNP units are pg/mL. Standard errors of the mean are shown as black lines.
Although the location of rs5068 in the 3’ untranslated region of ANP raises the possibility that it could alter transcript stability, this would not explain its association with BNP. The fact that all 3 SNPs were associated with both peptides suggests coordinate regulation of the 2 peptides at the genetic level, perhaps through shared enhancer elements. If a genetic variant directly influenced either ANP or BNP concentration alone, one would expect compensatory changes in concentration of the other peptide (in the opposite direction), as observed in knockout mice.8 In contrast, we found the same directionality of genetic association for each peptide. Although a genetic influence on natriuretic peptide clearance is also possible, associations were noted with both N-terminal pro-peptides and mature peptides, which have different clearance mechanisms. Additional work will be required to define the precise mechanism of causal genetic variation at the NPPA/NPPB locus.
The 3 SNPs with genome-wide significant associations with natriuretic peptide concentrations in stage 2 (rs5068, rs198358, rs632793) were carried into stage 3, in which we tested for association with blood pressure in the 4 samples, and combined results using meta-analysis (overall n=15,201). Overall meta-analysis of stage 3 association results are shown in Table 3, with cohort-specific BP means by genotype in Supplementary Table 6 and cohort-specific association results in Supplementary Table 7. Carriers of the rs5068 minor allele had lower systolic blood pressure (P=1×10−4) and lower diastolic blood pressure (P=0.002), compared with major allele homozygotes. Carriers of the rs198358 minor allele had lower systolic blood pressure (P=5×10−5) and lower diastolic blood pressure (P=4×10−4), compared with major allele homozygotes. SNP rs632793 was not significantly associated with either systolic or diastolic blood pressure.
The effects of SNPs on blood pressure roughly paralleled their effects on ANP, suggesting that ANP could have a stronger influence on blood pressure than BNP. This is consistent with the experimental observation that hypertension results from knockout of ANP but not BNP in mice.8 An alternative explanation is that rs632973 is associated with an altered form of BNP with less biological activity.
Because counter-regulatory effects of blood pressure on natriuretic peptide production could mask the primary effect of genetic variants on blood pressure, we also repeated the blood pressure association analyses after adjustment for concurrent ANP concentrations. In models adjusted for ANP, rs5068 and rs198358 showed larger effects on blood pressure, particularly for systolic blood pressure (rs5068 P=1×10−8, rs198358 P=7×10−7, Table 3). These models adjusted for the counter-regulatory changes in natriuretic peptide concentrations that result from a genetically-determined decrease in blood pressure.
We sought additional validation by genotyping rs5068 and rs198358 in 14,516 subjects in the Malmö Preventive Project (stage 4). The minor alleles of rs5068 and rs198358 were associated with lower systolic and diastolic blood pressures in this cohort (Table 3), with clear replication of the rs5068 association (cohort-specific P=0.005 for systolic blood pressure, P=1×10−4 for diastolic blood pressure). Incorporation of the results into the overall meta-analysis led to strengthened associations for rs5068 (systolic, P=2×10−6; diastolic, P=1×10−6). The minor alleles of rs5068 and rs198358 were associated with reductions of 0.9–1.5 mm Hg for systolic blood pressure and 0.3–0.8 mm Hg for diastolic blood pressure.
We examined the association of rs5068 and rs198358 with prevalent hypertension, defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive therapy. The alleles associated with higher natriuretic peptide concentrations were associated with reduced risk of hypertension (odds ratios, 0.85 [P=4×10−5] for rs5068, 0.90 [P=2×10−4] for rs198358) (Table 4).
Table 4.
Association of confirmed natriuretic peptide variants with hypertension.
| Hypertension FHS, MDC, Finrisk97 N=15,201 |
Hypertension FHS, MDC, Finrisk97, MPP N=29,717 |
|||||
|---|---|---|---|---|---|---|
| SNP | OR | 95% CI | p-value | OR | 95% CI | p-value |
| rs5068 | 0.84 | 0.76–0.93 | 7×10−4 | 0.85 | 0.79–0.92 | 4×10−5 |
| With adjustment for ANP | 2×10−5 | |||||
| rs198358 | 0.87 | 0.80–0.94 | 2×10−4 | 0.90 | 0.85–0.95 | 2×10−4 |
| With adjustment for ANP | 4×10−5 | |||||
Shown are results of association of SNPs with dichotomous hypertension, defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or use of antihypertensive therapy. Hypertension was adjusted in logistic regression models for age, sex and body mass index, with or without adjustment for ANP. ANP was not available in the Malmö Preventive Project, so results including this sample are shown separately.
In summary, we report the first common genetic variants for blood pressure and hypertension in the general population. Our findings were consistent across multiple, large cohorts, and supported by the demonstration that these variants (or variants in close association) are functionally active, influencing circulating concentrations of molecules known to modulate vascular tone and sodium excretion.
It has been difficult to establish that common genetic variants influence blood pressure,11–13 which may reflect the complex nature of blood pressure. In contrast to prior studies, we used an intermediate trait, natriuretic peptide concentrations, to select a limited number of SNPs a priori for carrying forward into blood pressure analyses. Each positive association satisfied the hypothesis that variants leading to higher natriuretic peptide concentrations would be associated with lower blood pressure.
Although it is known that disruption of the natriuretic peptide axis in genetically-engineered animals or infusion of large doses of these molecules in experimental protocols alters blood pressure, relevance to normal human physiology has not been previously established. Indeed, natriuretic peptide concentrations in healthy individuals are orders of magnitude lower than those observed in either experimental models or heart failure patients,14 and the effects of small variations in natriuretic peptide concentrations at this low end of the range are unknown.
The present study uses the natural experiment of the random Mendelian assortment of alleles to show that genetically-determined alterations in natriuretic peptide concentrations are associated with changes in blood pressure. Compared with other studies based on the concept of Mendelian randomization,15 our investigation is unique in that the biomarker (ANP) and the clinical trait (blood pressure) lie in a feedback loop, such that natriuretic peptides exert a negative influence on blood pressure, but blood pressure has a positive effect on natriuretic peptide release. Use of genetic variants, clearly upstream of both natriuretic peptide concentrations and blood pressure, eliminates the risk of confounding or reverse causality from this feedback loop.
Most studies relating natriuretic peptide genes to human blood pressure variation have been small and inconclusive. Recently, two larger studies reported associations between missense variants in NPPA and incident hypertension16 and response to diuretic therapy,17 but statistical significance was modest and the studies lacked replication. Dries et al. reported that a missense variant in corin (which cleaves pro-ANP and pro-BNP) was associated with blood pressure in African-American samples.18
Although the reductions in blood pressure associated with the minor alleles of rs5068 and rs198358 may appear modest, even a 1 mm Hg decrement in systolic blood pressure is associated with an 8% lower risk of death from stroke or ischemic heart disease in observational studies.3 Furthermore, potentially lifelong exposure to changes in blood pressure, as can occur due to differences in genotype, could magnify these effects.
Therapeutic agents that chronically activate the natriuretic peptide system are under active development. Our finding that genetically-determined natriuretic peptide concentrations are associated with blood pressure and hypertension suggests that these agents could be useful for the treatment of hypertension. However, further clinical and mechanistic studies with in-depth phenotyping are warranted to fully understand the role of these genetic variants in blood pressure regulation and cardiovascular physiology.
METHODS
Study design
The Framingham Heart Study is an epidemiologic study that includes an offspring cohort enrolled in 1971.19 The Framingham participants are predominantly white of European ancestry. DNA from 1809 unrelated participants attending a routine examination of the offspring cohort (1995–98) was available for genetic analyses in the first stage (sample 1, “Framingham Unrelated”). Selected SNPs with nominal evidence of association with natriuretic peptide levels in the first stage underwent genotyping in a second stage, comprising 3 independent study samples: a family-based sample from Framingham (sample 2, “Framingham Related”),20 unrelated participants in the Malmö Diet and Cancer-Cardiovascular Arm (sample 3),21 and unrelated participants from the Finrisk97 cohort (sample 4).22 All stage 2 Framingham individuals were unrelated to stage 1 individuals. For stage 3, three SNPs were carried over from stage 2 to examine the association with blood pressure in all four samples. In stage 4, an additional set of individuals from the Malmö Preventive Project (sample 5) were added to samples 1–4 to test the relationship of two SNPs with blood pressure and hypertension.23 The Malmö Preventive Project baseline examination (1974–1992) was used in continuous blood pressure analyses because of the lower rates of treatment (4%), and the follow-up examination (2002–2006) was used in hypertension analyses because of the higher prevalence of hypertension.
Subjects in stages 1 and 2 were excluded if they had prior heart failure, missing natriuretic peptide levels or DNA, or, in Framingham, serum creatinine >2.0 mg/dl. After exclusions, samples sizes were 1,705 (sample 1), 751 (sample 2), 5,196 (sample 3), 7,091 (sample 4), and 14,516 (sample 5). An additional 458 subjects in Finrisk97 with blood pressure but not natriuretic peptide levels were available for stage 3. A study design overview is shown in Supplementary Figure 1. Studies were approved by local institutional review boards. All subjects gave written informed consent.
Clinical evaluation
Natriuretic peptides assays are described in the Supplementary Methods. Risk factor ascertainment in the Framingham Heart Study, Malmö Diet and Cancer, Finrisk97, and Malmö Preventive Project is described elsewhere.22–25 Clinical variables were measured contemporaneously with the blood draws for natriuretic peptides. Blood pressures in Framingham and Finrisk97 were averaged from 2 measures using a mercury column sphygmomanometer in seated participants resting for at least 5 minutes.26 In Malmö Diet and Cancer, blood pressure was measured once using a mercury column sphygmomanometer in supine individuals after 10 minutes of rest.27 In the Malmö Preventive Project, blood pressure was the average of two supine and two standing measures, 10 minutes apart.23
SNP selection and genotyping
We characterized linkage disequilibrium patterns by genotyping SNPs across the NPPA/NPPB locus in 96 independent CEPH chromosomes and selected 13 tag SNPs for genotyping in stage 1 (Supplementary Methods, Figure 1, Supplementary Table 1). All SNPs genotyped in Framingham had call rates >85% (average 96.8%). Genotyping in Framingham and Finrisk97 was performed on the Sequenom platform (San Diego, CA), and in the Malmö cohorts with Taqman using ‘assays by design’. After quality control, genotyping call rates for rs5068, rs198358 and rs632793 were ≥99.8%, 99.9%, and 99.9% in Finrisk97, 96.9%, 96.4%, and 97.6% in Malmö Diet and Cancer, and 93.1% and 98.1% in the Malmö Preventive Project (rs632793 was not genotyped in the Malmö Preventive Project). All SNPs were in Hardy-Weinberg equilibrium.
Statistical analysis
Natriuretic peptide concentrations were log-transformed. Residuals were obtained using sex-specific regression models in which natriuretic peptide levels were adjusted for age, body mass index, diabetes, systolic and diastolic blood pressure, antihypertensive therapy, myocardial infarction, atrial fibrillation (Framingham), and serum creatinine (Framingham).20,28 As previously described,29 Tobit regression was used to generate residuals in Framingham, to account for left-censoring of BNP by the assay detection limit (4 pg/ml). Linear regression was used in Malmö Diet and Cancer and Finrisk97, because N-terminal proBNP distributions had minimal left censoring. Covariate relations are shown in Supplementary Table 2.
The natriuretic peptide residuals were standardized (mean=0, SD=1). Using linear regression, residuals were screened for association in the Framingham Unrelated sample with individual SNPs using a 2 degree-of-freedom test (general model). Four SNPs were chosen for validation in stage 2, based on the strength of association and linkage disequilibrium patterns. Association testing was performed in sample 2 using SAS PROC MIXED to account for familial correlations. In the other samples, which consisted of unrelated subjects, linear regression was used. Data from all four samples were combined using fixed-effects meta-analysis with inverse variance weights. Dominant, additive, and recessive models were tested for rs198358 and rs632793, whereas only a dominant model was examined for rs5068 because of the small number of minor homozygotes.
For stage 3, association testing with blood pressure, we selected the 3 SNPs with convincing association with ANP, BNP, or both, in the first 2 stages. For each SNP, we identified the genetic model (dominant, additive, or recessive) for the blood pressure analysis based on the model that showed the strongest association with natriuretic peptide levels. Blood pressure in treated individuals was imputed using an approach described previously.4 Systolic and diastolic blood pressure residuals from regression on age, sex, and body mass index were standardized, and results from all five samples meta-analyzed using inverse-variance weights. The average weights for each study sample were: 4% (sample 1), 1% (sample 2), 18% (sample 3), 34% (sample 4), and 43% (sample 5).
We used SAS v8.1 (Cary, NC) for analyses in Framingham and Finrisk97, and SPSS v15.0 (Chicago, IL) in Malmö Diet and Cancer and Malmö Preventive Project. Two-sided p-values are shown. Because cumulative meta-analysis is more powerful than a derivation/validation design, results at successive stages include meta-analysis of all samples tested cumulatively.30 In stage 1, we performed 26 tests (13 SNPs, 1 genetic model, 2 natriuretic peptides) and therefore considered P<0.002 (0.05/26) to be significant. In stage 2, we performed 24 tests (4 SNPs, 3 genetic models, 2 natriuretic peptides) and considered P<0.002 (0.05/24) to be significant. In stages 3 and 4, we performed up to 6 tests (3 SNPs, 1 genetic model, 2 blood pressure traits) and considered P<0.008 (0.05/6) to be significant.
Supplementary Material
Acknowledgments
Role of the funding source
Drs. Newton-Cheh, Melander, and Wang had full access to the study data and take responsibility for its content. BRAHMS, AG generated the ANP data in the Malmö Diet and Cancer sample. The funding sources had no role in the design, analysis and interpretation, the writing of the manuscript or the decision to submit for publication.
Contributors
CN-C, MGL, OM, and TJW designed the study. CN-C, KDB, AS, CG, JS, NGM, AB, SB, FK, and OM generated the data. CN-C, MGL, XY, OM were responsible for the analyses. CN-C and TJW drafted the manuscript. All authors contributed to interpretation of the data and critical review of the manuscript and approved the final version.
The Framingham Heart Study of National Heart, Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine work was supported by Contract No. N01-HC-25195 and the CardioGenomics Program for Genomic Applications (HL66582). The ANP measurements in Finrisk97 were performed by the MORGAM Biomarkers Study funded by the Medical Research Council, UK (G0601463: 80983). Dr. Newton-Cheh was supported by NIH K23-HL-080025, a Doris Duke Charitable Foundation Clinical Scientist Development Award, and a Burroughs Wellcome Fund Career Award for Medical Scientists. Dr. Wang was supported by NIH K23-HL-074077, R01-HL-086875, R01-HL-083197, and the American Heart Association. Dr. Vasan was supported by a research career award from the NIH (K24-HL-04334). Dr. Bloch was supported by R01-HL-070896. Dr. Peltonen was supported by the Center of Excellence in Complex Disease Genetics of the Academy of Finland, Biocentrum Helsinki Foundation, Finland and the Nordic Center of Excellence. Dr. Salomaa was supported by the Sigrid Juselius Foundation. Dr. Nilsson was supported by the Ernhold Lundstrom Foundation and the Swedish Heart and Lung Foundation. Dr. Melander was supported by the Swedish Medical Research Council, the Swedish Heart and Lung Foundation, the Medical Faculty of Lund University, Malmö University Hospital, the Albert Påhlsson Research Foundation, the Crafoord foundation, the Ernhold Lundströms Research Foundation, the Region Skane, the Hulda and Conrad Mossfelt Foundation, the King Gustaf V and Queen Victoria Foundation and the Lennart Hanssons Memorial Fund. The authors also wish to thank the following companies for their support of the natriuretic peptide assays: Shionogi, BRAHMS, and Dade-Behring.
Footnotes
Conflict of interest statement
Dr. Bloch has sponsored research agreements with and serves on the scientific advisory board of IKARIA/INOTherapeutics. Dr. Bloch reports no conflicts of interest related to this manuscript. Drs. Struck, Morgenthaler and Bergmann are employees of and Dr. Bergmann holds stock in BRAHMS, AG. BRAHMS, AG holds patent rights on the midregional pro-ANP assay. Dr. Hirschhorn has an equity interest in and receives consulting fees from Correlagen Diagnostics. No other potential conflict of interest relevant to this article was reported.
References
- 1.Chobanian AV, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N.Engl.J.Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 4.Levy D, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension. 2000;36:477–483. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 6.Ji W, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 8.John SW, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 9.Schillinger KJ, et al. Regulatable atrial natriuretic peptide gene therapy for hypertension. Proc.Natl.Acad.Sci.U.S.A. 2005;102:13789–13794. doi: 10.1073/pnas.0506807102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Numata Y, et al. Immunoradiometric assay for the N-terminal fragment of proatrial natriuretic peptide in human plasma. Clin.Chem. 1998;44:1008–1013. [PubMed] [Google Scholar]
- 11.Levy D, et al. Framingham Heart Study 100K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Med.Genet. 2007;(Suppl 1)(8):S3. doi: 10.1186/1471-2350-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato N, et al. High-density association study and nomination of susceptibility genes for hypertension in the Japanese National Project. Hum.Mol.Genet. 2008;17:617–627. doi: 10.1093/hmg/ddm335. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am.J.Cardiol. 2002;90:254–258. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 15.Davey SG, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int.J.Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 16.Conen D, et al. Natriuretic peptide precursor a gene polymorphisms and risk of blood pressure progression and incident hypertension. Hypertension. 2007;50:1114–1119. doi: 10.1161/HYPERTENSIONAHA.107.097634. [DOI] [PubMed] [Google Scholar]
- 17.Lynch AI, et al. Pharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertension. JAMA. 2008;299:296–307. doi: 10.1001/jama.299.3.296. [DOI] [PubMed] [Google Scholar]
- 18.Dries DL, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Feinleib M, McNamara PM. An investigation of coronary heart disease in families: The Framingham Offspring Study. American Journal of Epidemiology. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 20.Wang TJ, et al. Heritability and genetic linkage of plasma natriuretic peptide levels. Circulation. 2003;108:13–16. doi: 10.1161/01.CIR.0000081657.83724.A7. [DOI] [PubMed] [Google Scholar]
- 21.Berglund G, Elmstahl S, Janzon L, Larsson SA. The Malmo Diet and Cancer Study. Design and feasibility. J.Intern.Med. 1993;233:45–51. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 22.Vartiainen E, et al. Cardiovascular risk factor changes in Finland, 1972–1997. Int.J.Epidemiol. 2000;29:49–56. doi: 10.1093/ije/29.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Berglund G, et al. Long-term outcome of the Malmo preventive project: mortality and cardiovascular morbidity. J.Intern.Med. 2000;247:19–29. doi: 10.1046/j.1365-2796.2000.00568.x. [DOI] [PubMed] [Google Scholar]
- 24.Cupples LA, d'Agostino R., Jr . Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Study: 30-year follow-up. Washington, DC: Government Printing Office; 1987. [Google Scholar]
- 25.Nilsson PM, Engstrom G, Hedblad B. The metabolic syndrome and incidence of cardiovascular disease in non-diabetic subjects--a population-based study comparing three different definitions. Diabet.Med. 2007;24:464–472. doi: 10.1111/j.1464-5491.2007.02142.x. [DOI] [PubMed] [Google Scholar]
- 26.Kastarinen MJ, et al. Trends in blood pressure levels and control of hypertension in Finland from 1982 to 1997. Journal of Hypertension. 1998;16:1379–1387. doi: 10.1097/00004872-199816090-00019. [DOI] [PubMed] [Google Scholar]
- 27.von WF, et al. Genetic variance of SGK-1 is associated with blood pressure, blood pressure change over time and strength of the insulin-diastolic blood pressure relationship. Kidney Int. 2005;68:2164–2172. doi: 10.1111/j.1523-1755.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang TJ, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, et al. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–1353. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 30.Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for twostage genome-wide association studies. Nat.Genet. 2006;38:209–213. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.