Abstract
It is widely thought that, after peripheral injury, some low-threshold mechanoreceptive (LTMR) afferents “sprout” into pain-specific laminae (I–II) of the dorsal horn and are responsible for chronic pain states such as mechanical allodynia. Although recent studies have questioned this hypothesis, they fail to account for a series of compelling results from single-fiber analyses showing extensive projections from large-diameter myelinated afferents into nocireceptive layers after nerve injury. Here we show that, in the thoracic spinal cord of naïve adult mouse, all myelinated nociceptors gave rise to terminal projections throughout the superficial dorsal horn laminae (I–II). Most (70%) of these fibers had large-diameter axons with recurving flame-shaped central arbors that projected throughout the dorsal horn laminae I–V. This morphology was reminiscent of that attributed to sprouted LTMRs described in previous studies. After peripheral nerve axotomy, we found that LTMR afferents with narrow, uninflected somal action potentials did not sprout into superficial laminae of the dorsal horn. Only myelinated noiceptive afferents with broad, inflected somal action potentials were found to give rise to recurving collaterals and project into superficial “pain-specific” laminae after axotomy. We conclude that the previously undocumented central morphology of large, myelinated cutaneous nociceptors may very well account for the morphological findings previously thought to require sprouting of LTMRs.
Indexing terms: nociceptor, regeneration, spinal cord, pain, dorsal root ganglion, reinnervation
Numerous studies have established that peripheral nerve injury leads to reorganization of the central terminals of myelinated low-threshold cutaneous afferents within the dorsal horn of the spinal cord (see, e.g., Woolf et al., 1992; Shortland and Woolf, 1993; Koerber et al., 1994, 1999; Kohama et al., 2000; Okamoto et al., 2001). One aspect of this reorganization with important clinical implications is the potential formation of ectopic projections by LTMRs in the outer substantia gelatinosa and marginal zone (laminae IIo and I, respectively), a region normally receiving inputs predominantly from nociceptors (Light et al., 1992; Light and Willcockson, 1999). Such sprouting by LTMRs into “pain-specific” regions is thought to provide an anatomical substrate for nerve injury-induced chronic pain states (e.g., mechanical allodynia) resulting from the inappropriate activation of pain circuitry via low-intensity tactile stimulation.
Much of the evidence for sprouting of myelinated fibers stems from bulk transport of beta-cholera toxin-horseradish peroxidase (B-HRP) conjugates, a technique that has recently been shown to be suspect (Tong et al., 1999; Bao et al., 2002; Santha and Jancso, 2003; Shehab et al., 2003). Nevertheless, more powerful single-fiber analyses have demonstrated a small number of afferents with large-diameter (Aβ) axons projecting throughout laminae I/IIo after injury. These injured afferents exhibit dorsally recurving “flame-shaped” arbors that, projections into more superficial laminae notwithstanding, are strikingly similar to those from LTMRs (Woolf et al., 1992; Shortland and Woolf, 1993; Koerber et al., 1994, 1999). However, these findings have also been recently challenged by Hughes et al. (2003). By using intraaxonal staining following sciatic nerve ligation, they found a lack of evidence for sprouting of putative LTMR projections into the more superficial laminae.
Apart from altered laminar termination patterns, additional phenotypic changes in sensory neurons have also been reported after nerve injury, ranging from neurochemical (Noguchi et al., 1995; Koerber et al., 1999) to anatomical formation of peptidergic pericellular baskets via intraganglionic sprouting (McLachlan and Hu, 1998). Taken together, these findings suggest that sensory neurons exhibit a wide variety of responses to injury and that many, if not all, of these postaxotomy alterations may be correlated.
To address these possibilities and to gain a better understanding of potential mechanisms involved in injury-induced alterations in the laminar termination of cutaneous primary afferents, it is important first to identify those afferent fiber types capable of sprouting into laminae I/IIo. For example, is sprouting restricted to a single class of afferents, or is this a generalized phenomenon across multiple afferent types? Toward this end, we have employed a mouse ex vivo somatosensory system preparation (Woodbury et al., 2001; Koerber and Woodbury, 2002) to allow intrasomal staining of previously axotomized adult cutaneous afferents. Unlike earlier studies using intrafiber injections, this preparation allows a more complete evaluation of peripherally axotomized sensory neurons than central anatomy alone, providing information on somal action potentials, intraganglionic morphology, neuropeptide content, and peripheral anatomy, as well as characterization of the cells’ peripheral response properties. Thus, we can now directly address a number of key questions surrounding sensory neuron plasticity.
MATERIALS AND METHODS
The present experiments were conducted in adult male Swiss-Webster mice (Hilltop Farms, Scottdale, PA). The effects of prior nerve injury on the central projections of primary afferents were examined through both bulk-labeling and single-neuron analyses. All procedures used in the present study were approved by the Animal Care and Use Committee of the University of Pittsburgh and conform to NIH guidelines.
Bulk-labeling studies
Both sciatic and dorsal cutaneous nerves (DCNs) were included in bulk-transport studies carried out in separate animals. Mice were anesthetized with ketamine and xylazine (90 mg/kg and 10 mg/kg, respectively, i.m.). For studies of the sciatic nerve, hair on the thigh was clipped and an incision made in the skin overlying the femur. The sciatic nerve was exposed at midfemur level and either 1) transected with iridectomy scissors or 2) crushed (twice for 3 seconds each) with No. 5 watchmaker’s forceps; in the latter, subsequent examination at the site of nerve crush verified an obvious lesion characterized by a distinctive translucent appearance. For DCN studies, hair over the dorsal midline was clipped and a longitudinal incision made along the dorsal midline. The skin was retracted, and five to nine contiguous DCNs on the right side were either 1) transected with iridectomy scissors 2–3 cm proximal to the skin or 2) crushed in this same region following procedures as described above. Incised skin was then closed with sutures, and the animals were allowed to recover from anesthesia.
Two to four weeks later, animals were reanesthetized with ketamine/xylazine (90 and 10 mg/kg, respectively, i.m.). Previously injured nerves were again exposed and injected with a 1% solution of choleragenoid-horseradish peroxidase (B-HRP) conjugate (w/v in dH2O). Uninjured nerves on the opposite side were also injected with B-HRP; in the case of DCNs, results were also compared with similar transport studies in animals in which no previous surgeries had been performed (Woodbury et al., 2000). Forty-eight hours later, animals were overdosed with the same anesthetic, heparinized, and transcardially perfused with 70 ml warm 0.9% saline followed by 70 –100 ml ice-cold modified Karnovsky’s fixative [1% paraformaldehyde, 1.25% glutaraldehyde, 5% sucrose in 0.1 M phosphate buffer (PB), pH 7.4]. The spinal cord was dissected free and postfixed for 20 minutes in the same fixative. Appropriate segments were blocked, cryoprotected overnight at 4°C in 30% sucrose in PB, and serially frozen sectioned (40 μm) on a sliding microtome. Transganglionically transported HRP was visualized via standard tetra-methylbenzidine histochemical protocols.
Single-neuron analyses
Single-cell analyses were conducted only using DCNs in order to restrict our analyses to cutaneous afferents. Two to seven weeks following nerve injury, animals were prepared for intrasomal recording and staining of DRG neurons in vitro.
Ex vivo preparation
The in vitro preparation used in the present experiments on adult mice is similar to one developed previously in neonatal mice that has been described in detail elsewhere (e.g., Woodbury et al., 2001; Koerber and Woodbury, 2002). Modifications of the neonatal preparation to allow its extension into adults are emphasized here.
Adult mice were anesthetized with a mixture of ketamine and xylazine (100 mg/kg and 20 mg/kg, respectively, i.m.). Hairs over the entire dorsal and dorsolateral surface of the trunk were clipped and then carefully trimmed to a fine stubble using a foil-head electric shaver. The heart was exposed via ventral midline incision, heparin was administered, and the left ventricle was cannulated. The cannula was glued in place using cyanoacrylate adhesive, the right atrium was cut, and the mouse was transcardially perfused with ice-chilled (14 –17°C) oxygenated artificial cerebrospinal fluid (aCSF) in which sucrose was substituted for sodium chloride at equivalent osmolarity (in mM: 253.9 sucrose, 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26 NaHCO3, 10.0 D-glucose).
The perfusion was continued as a longitudinal incision was made in the dorsal skin 2–5 mm to the left of the midline. A laminectomy was quickly performed, and the cord and spinal column were transected at upper cervical and lower sacral levels. The isolated spinal column and entire right trunk were then dissected from the carcass and placed in a circulating bath containing the same chilled, oxygenated, sodium-depleted aCSF. The dura mater and arachnoid membranes were dissected away, and a parasagittal hemisection was made along the left side of the spinal cord. Excess spinal column bone, ribs, muscle, and loose connective tissue were then removed to produce an isolated cord/dorsal root ganglion (DRG)/nerve/skin preparation. This ex vivo somatosensory system was then transferred to a Sylgard-coated recording chamber containing chilled, oxygenated aCSF, now with replenished sodium chloride in place of sucrose (127.0 mM NaCl). The hemisected cord was suspended directly over a continuous inflow and the skin pinned out flat onto a mesh platform at the bath–air interface (see Fig. 2a). An additional flow of oxygenated aCSF was directed beneath the skin to maintain physiological viability. The bath was then slowly warmed to 30 –31°C in preparation for physiological recording.
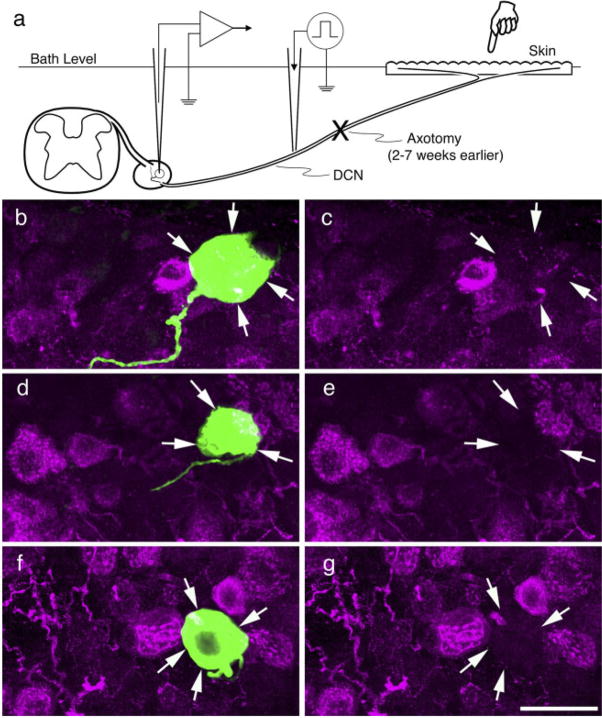
Fig. 2.
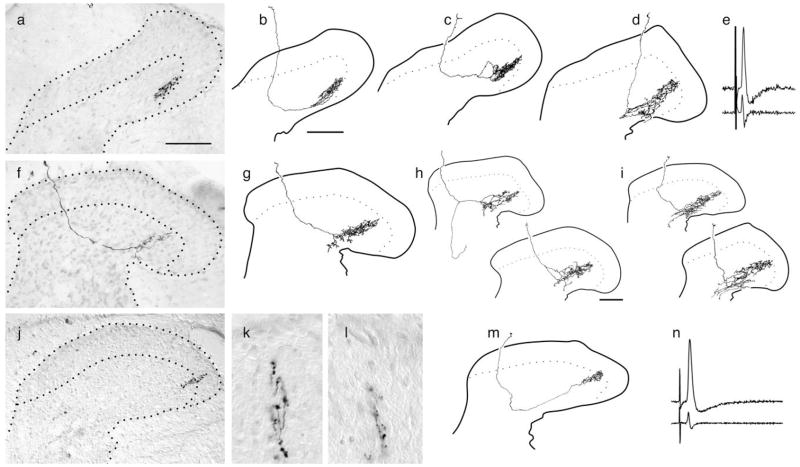
Schematic representation of the adult ex vivo preparation used to stain axotomized cutaneous afferents (a) and intracellularly stained cells double-labeled for CGRP. b–g: Confocal micrographs of labeled somata from previously axotomized myelinated afferents double-labeled for neurobiotin (green) and CGRP immunohistochemistry (red); arrows indicate position of labeled somata in each. b,c: Putative guard hair follicle afferent. d,e: Putative D-hair follicle afferent. e,f: Putative nociceptor with recurving, flame-shaped arbor. All lacked intraganglionic arborizations and were CGRP negative. Scale bar = 20 μm.
Individual sensory neurons were impaled using glass microelectrodes containing 10 –20% Neurobiotin (Vector, Burlingame, CA) in 1 M potassium acetate (70 –100 MΩ). Electrodes were advanced into the DRG with a stepping microdrive while brief electrical stimuli (0.05 msec at 0.5 Hz) at an intensity supramaximal for myelinated fibers were delivered to the DCN through an en passant suction electrode proximal to the site of nerve injury. Once an adequate intracellular penetration was made, the somal spike was recorded and the skin gently searched with a fire-polished glass stylus or the tips of sharpened forceps to locate the peripheral receptive field. For fibers in recorded in naïve mice and those afferents that had successfully reinnervated the skin, the cell’s peripheral mechanical threshold and response properties to graded stimulus intensities were determined using calibrated von Frey filaments. In some cases, a feedback-controlled Peltier device (3 × 5 mm) was centered on the mechanical receptive field to assess thermal sensitivity.
Histochemical and immunohistochemical processing
After physiological characterization, sensory neurons (one per DRG) were iontophoretically injected with Neurobiotin (+10 –20 nA/minute total current). Three to nine hours later, tissues were fixed in 4% paraformaldehyde in PB, embedded in 10% gelatin, postfixed in the same fixative, cryoprotected overnight in 30% sucrose, and serially sectioned (25–50 μm) with a sliding microtome. Spinal cord frozen transverse sections (50 μm) were serially collected in PB; DRG were also cut at 50 μm, whereas the peripheral nerves, including the transection sites, were cut at 25 μm. The somata, central, and peripheral projections of stained neurons were visualized using standard ABC/DAB protocols (Elite kit; Vector) to visualize neurobiotin. Sections were rinsed, mounted on slides, counter-stained with neutral red, dehydrated, cleared, and cover-slipped with Permount (Fisher Scientific, Fair Lawn, NJ).
In some cases, labeled somata were processed for fluorescent double labeling with antibodies against CGRP and analyzed by using confocal microscopy as described previously (Ritter et al., 2000). Neurobiotin (Vector) was visualized by using fluorescein isothiocyanate (FITC)-conjugated avidin (Vector; incubation of 1–2 hours), and CGRP staining in the same sections was performed by using polyclonal rabbit anti-CGRP antiserum (Chemicon, Temecula, CA) overnight at 4°C: The CGRP antiserum (1:2,000; Chemicon; catalog No. AB1971) was developed against the whole rat α-calcitonin gene-related peptide conjugated to bovine serum albumin (BSA). Preabsorption with the full CGRP peptide (10 μM) completely blocked staining in control DRG sections. CGRP was then visualized by using Cy3-conjugated goat anti-rabbit secondary. After final washes, sections were mounted, and fluorescence was viewed with an Olympus confocal microscope under 3100 oil. Tissue was included in the analysis only if the following two criteria were met: only a single, brightly labeled neurobiotin-positive cell was found in the ganglion, and the quality of the CGRP staining was optimal.
Images were digitally stored in Adobe Photoshop (Adobe Systems Inc., San Jose, CA). In a few instances, this software was also used to make minor adjustments in the brightness and contrast levels to improve uneven illumination and to lighten the background.
RESULTS
Bulk transport of cholera toxin conjugates in mice
To evaluate mice as a potential nerve injury model, we first conducted bulk-transport studies using bilateral nerve injections of B-HRP in six mice to determine whether nerve injury in mice produces alterations in central labeling patterns comparable to those in other species (Woolf et al., 1992; LaMotte and Kapadia, 1993). Both crush and complete transection injuries were assessed in mixed (sciatic) and purely cutaneous nerves (DCN). Uninjured contralateral nerves served as controls.
Two weeks after sciatic nerve injury in mice, B-HRP labeling extended throughout the substantia gelatinosa on the injured side, notably in lamina IIo, which remained largely devoid of labeling on the control side (Fig. 1). Thus, nerve injury in mice was effective in bringing about this result previously documented in rats. Additional differences were evident between labeling patterns from the injured and control nerves. The most prominent was that B-HRP uptake and/or transport were markedly enhanced in all injured elements, both sensory and motor, relative to the uninjured control side. This enhanced labeling was also seen following transection injuries of DCNs (Fig. 1c, d). Here, a disruption of the normal labeling pattern, with relatively coarse, punctate labeling distributed throughout lamina IIo, was evident by 2 weeks postinjury.
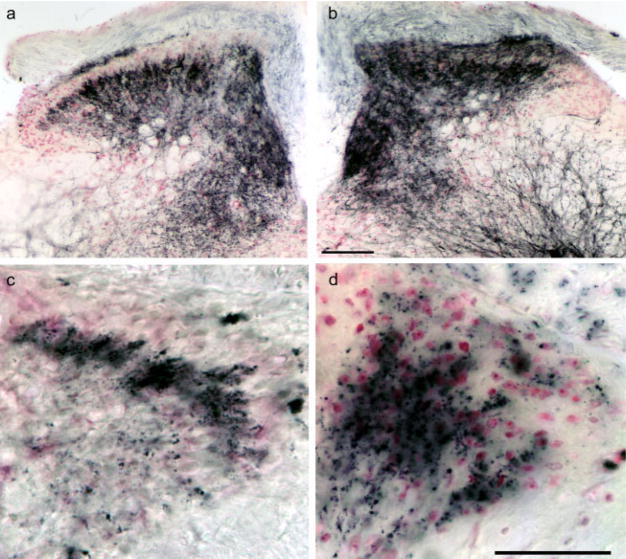
Fig. 1.
Bulk transport of B-HRP in control and previously injured sciatic and dorsal cutaneous nerves (DCNs) of adult mice. a,b: Sciatic. c,d: DCNs. a,c: Uninjured control nerves; b: crush injury, 2 weeks survival; d: transection injury, 2 weeks survival. Enhanced labeling is evident in the dorsal horn of animals with injured nerves. DCN transection resulted in a disruption of normal laminar bulk-labeling patterns. Scale bars = 100 μm in b (applies to a,b); 50 μm in d (applies to c,d).
Single-neuron analyses
Although these findings show that nerve injury produces B-HRP labeling in superficial laminae of mice equivalent tyo that of other mammals (Woolf et al., 1992; LaMotte and Kapadia, 1993; Tong et al., 1999), it is clear that bulk transport provides little if any information on the functional identity of afferents, particularly after injury, when enhanced transport may be seen in all fibers (Tong et al., 1999; Bao et al., 2002; Santha and Jancso, 2003; Shehab et al., 2003). Therefore, we undertook single-neuron analyses in animals that had cutaneous nerves previously axotomized to gain additional insight into the identities of afferents capable of ectopic sprouting. We used an ex vivo somatosensory system preparation (Fig. 2a) wherein afferents are stained from their cell bodies in the DRG (Woodbury et al., 2001; Koerber and Woodbury, 2002) rather than from their fibers in the dorsal columns as in earlier studies (see, e.g., Woolf et al., 1992; Koerber et al., 1994). This somal approach confers a major advantage because of the strong correlation between somal spike shape and afferent identity (Koerber et al., 1988; Ritter and Mendell, 1992; Djouhri et al., 1998), which appears to be quite resilient to nerve injury (Czeh et al., 1977; Gurtu and Smith, 1988; Koerber et al., 1995). In addition, staining of these characterized cells allows for immunohistochemical analysis (Fig. 2b–g).
Myelinated nociceptors project throughout superficial laminae in naïve adults
In this study we injected a total of 14 myelinated nociceptive neurons in 12 naïve adult mice. Fibers with conduction velocities >1.2 m/second and <10 m/second were classified as conducting in the Aδ range and those with conduction velocities ≥10 m/secomd were considered to be conducting in the Aβ range (Koltzenburg et al., 1997; McIlwrath et al., 2007). The fibers’ response properties were analyzed by using von Frey filaments of increasing bending forces (0.07–39 mN) and a contact peltier device. Mechanical thresholds for these fibers ranged from 0.7 to 2.5 mN. In total, 10 well-stained fibers were recovered from these preparations. We found that all cutaneous myelinated nociceptors innervating back skin, regardless of peripheral conduction velocity, projected throughout the superficial dorsal horn in intact adult preparations. In addition, seven of these fibers had collateral axons in the dorsal column that gave rise to dorsally recurving “flame-shaped” arbors that extended throughout “pain-specific” (I and IIo) as well as deeper (IIi–V; Fig. 3a–c) laminae. All 10 neurons had broad, inflected somal spikes (Fig. 3d), conduction velocities in the upper part of the Aδ, and the lower part of the Aβ range, and were capable of encoding stimulus intensity well into the noxious range (i.e., 39 mN; Fig. 3e). Most of these fibers responded only to mechanical stimuli, but three of these 10 recovered fibers were found to respond to both mechanical and heat stimuli (Fig. 3f).
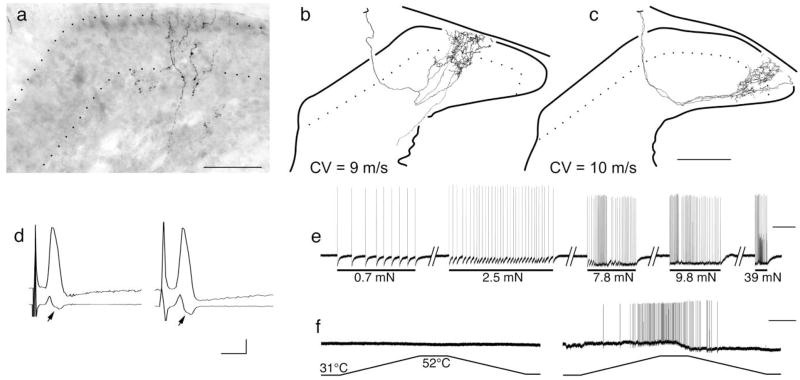
Fig. 3.
Flame-shaped arbors extending throughout dorsal laminae in naïve adult mice (8 weeks old). a–f: Central anatomy and physiology from two different myelinated nociceptors innervating the hairy skin in naïve adult mouse. a: Photomicrograph of arbor reconstructed in b showing extensive albeit diffuse arborization throughout the superficial laminae. c: Reconstructed collateral arbor from a different myelinated nociceptor. Both afferents were similar in many physiological respects, exhibiting receptive fields consisting of a cluster of individual “hot spots” as well as similar conduction velocities (9.0 and 10.0 m/second, respectively), identical mechanical thresholds (0.7 mN), similar adaptation rates and response properties to increasing forces (e shows example from afferent in b), and similar somal spikes [d, top left shows that of b, top right shows that of c, first derivatives below spike traces reveal the break or inflection in the falling phase (arrows)]. Although basically indistinguishable in terms of central anatomy, peripheral receptive field organization, and a wide variety of physiological parameters, the afferent in b showed no response to multiple applications of noxious heat (left trace in f), whereas the afferent in c was exquisitely sensitive (right trace in f). Scale bars = 50 μm in a; 100 μm in c (applies to b,c). Calibration bars = horizontal 2.5 msec (d), 1 sec (e), 5 sec (f); vertical 20 mV (d–f).
Injury effects on DRG neurons
In all, we iontophoretically injected somata of 32 previously axotomized, myelinated cutaneous afferents in 20 mice. Among these, the central projections of 26 afferents were very well labeled, including 18 from afferents exhibiting narrow, uninflected somal action potentials [putative low-threshold mechanoreceptors (LTMRs)] and eight from afferents exhibiting inflected somal spikes [putative high-threshold mechanoreceptors (HTMRs)]. This sample was nearly evenly split between afferents that had successfully reinnervated the skin and those for which no peripheral receptive field could be located; some of the latter were from nerves forming a terminal neuroma after failing to regenerate (see, e.g., Fig. 4c). Thus in effect, they were chronically axotomized as in earlier studies (Woolf et al., 1992). Multiple aspects of labeled afferents were then examined anatomically, including their somata, peripheral axons, and central arbors.
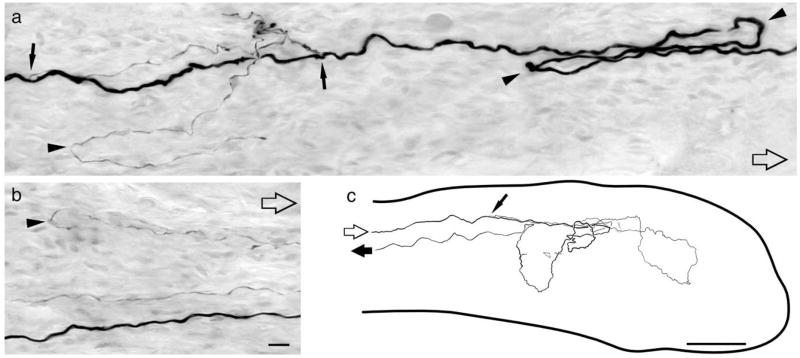
Fig. 4.
Axotomy induces supernumerary peripheral axons differing in size and trajectory. a: Putative D-hair follicle afferent axon in the nerve proximal to the site of axotomy. Open arrows point distally. Solid arrows indicate branch points where fine unmyelinated axons originate. Arrowheads point to hairpin loops. b: Axons from same afferent distal to the site of injury. c: Camera lucida drawing showing the course and branching pattern of a putative guard hair follicle afferent within a terminal neuroma (outline of neuroma indicated by large solid line). Both afferents were axotomized 7 weeks earlier. Scale bars = 10 μm in b (applies to a,b); 100 μm in c.
Recent reports suggest that nerve injury triggers major phenotypic changes within the DRG, including the formation of intraganglionic arbors by large-diameter afferents (McLachlan and Hu, 1998) and de novo synthesis of neuropeptides (Noguchi et al., 1995; Koerber et al., 1999). It was of interest, therefore, to examine the extent to which myelinated cutaneous afferents may be involved in these phenomena. We saw no evidence of either phenotypic alteration. For example, all 32 previously axotomized afferents in our sample exhibited normal ganglionic morphology (Fig. 2b–g), with no more than a single central and peripheral process within the ganglion as assessed via serial reconstructions. We also examined for CGRP content in a subset of these cells (six LTMRs and four HT-MRs), and all were CGRP negative (Fig. 2b–g) whether they successfully reinnervated the skin or not. This finding is more consistent with previous studies showing that somal CGRP immunostaining decreased following peripheral injury (Doughty et al., 1991; Zhang et al., 1993).
Injury effects on peripheral axons
Peripheral axotomy has also been suggested to have differential effects on myelinated and unmyelinated afferent populations. With the outstanding diffusion properties of Neurobiotin, it was often possible to examine the peripheral effects of axotomy on single afferents directly. As anticipated, branching in the vicinity of nerve transection was common. Rather than being restricted to a single site, branching could occur at multiple, widely separated locations along the axon. Branching was often highly asymmetrical, with large myelinated axons giving rise to extremely fine-diameter and presumably unmyelinated sister axons (Fig. 4a,b). The ultimate outcome of these supernumerary axons was highly variable: some remained trapped within the neuroma in a chaotic tangle, others reversed direction abruptly and continued centrally (Fig. 4c), and still others successfully negotiated the injury site and potentially reached the skin alongside the myelinated parent fiber (Fig. 4a,b). Overall, the trajectories followed by regenerating axons were tortuous, forming hairpin loops at multiple, independent locations both distal and caudal to the injury site. As a result of these multiple bifurcation points and tortuous growth, the amount of branching was difficult to quantify. Nevertheless, it is clear from these findings that the effect of axotomy on myelinated and unmyelinated afferent populations would be difficult to assess accurately from standard fiber counts, because a single myelinated afferent may be represented in any given transverse plane by multiple myelinated and unmyelinated profiles.
Injury effects on central arbors
The primary objective of these studies was to determine the identity of myelinated cutaneous afferents that sprout into nocireceptive regions of the dorsal horn after nerve injury. Although multiple classes of afferents were examined, in view of earlier work implicating LTMRs (Woolf et al., 1992; Shortland and Woolf, 1993; Koerber et al., 1994, 1999), our initial efforts were focused primarily on afferents exhibiting narrow, uninflected somal action potentials, a key trait of LTMRs under normal conditions.
Well-labeled central projections from a total of 18 afferents exhibiting narrow, uninflected somal action potentials (LTMRs) were recovered. Many (n = 10) had central anatomy characteristic of guard hair follicle afferents (Fig. 5a–d); that is, they gave rise to dorsally recurving “flame-shaped” arbors, and, more importantly, these arbors were longitudinally oriented to form continuous, mediolaterally flattened sheets of neuropile between collaterals. This identification was further supported physiologically by the rapidly adapting response properties among those that had regenerated (not shown). A few (n = 3) exhibited similarly dorsally recurving arbors, but with far less overlap between adjacent arbors than the above-described group. In addition, these arbors also had a ventrally projecting component along the lateral edge of lamina V, a central morphology that was highly indicative of slowly adapting type I afferents (Fig. 5f–i). Five other LTMRs exhibited conduction velocities and central anatomy indicative of D-hair follicle afferents, with dorsally recurving arbors forming continuous, longitudinally oriented columns of neuropil between collaterals (Fig. 5j, m).
Fig. 5.
Representative examples of central anatomy and somal action potentials from different classes of axotomized afferents; all axotomies were performed at about 5 weeks of age, and afferents were stained 4 –7 weeks later. a–e: Putative guard hair follicle afferents. f–i: Putative slowly adapting type I afferents. j, k: D-hair follicle afferent (uninjured). l–n: Putative D-hair follicle afferent. All were axotomized except for the normal D-hair follicle afferent in adult mice (j, k), shown here for the first time for comparison. Note that all putative low-threshold mechanoreceptors fail to sprout ectopically into nocireceptive laminae (dotted lines in a–d,f–j,m indicate outline of substantia gelatinosa). Scale bars = 100 μm (note different scale in h and i).
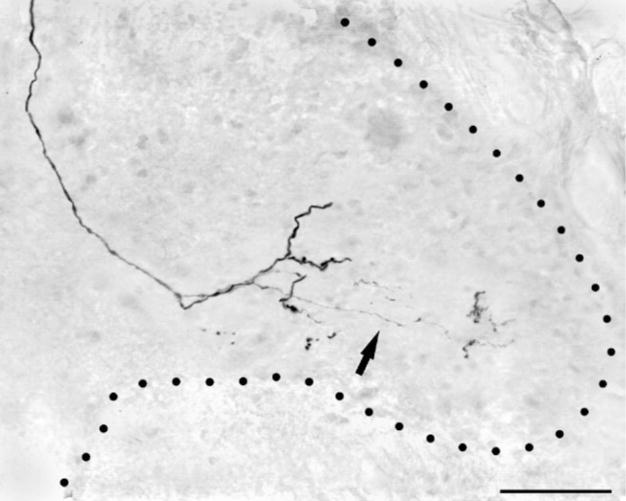
In terms of the laminar locations of these central arbors, the results of these experiments were consistent: no axotomized neuron with a narrow, uninflected spike gave rise to any collateral projecting into the outer half of the substantia gelatinosa or marginal zone. This was the case regardless of survival time (2–7.5 weeks) or successful reinnervation of the skin by the fiber. Although signs of altered central anatomy were seen among some of these axotomized LTMRs, instead of progressive growth (i.e., dorsal sprouting) the changes observed were predominantly regressive (i.e., degenerative) overall. For example, arbors from axotomized LTMRs were outwardly diminished in density and complexity (cf. Fig. 5k, l), their dorsalmost extent was often reduced, and many were simply truncated collaterals ending as unbranched axons either in the dorsal columns or shortly after entering the gray matter. Such truncated collaterals, often capped by large, dilated tips indicative of retraction bulbs, were seen far more frequently in injured than in uninjured afferents (not shown). Not all changes were regressive, however; evidence suggestive of new growth was also seen in a few collaterals. In some, long, thin linear processes arose from much larger diameter collateral branches, occasionally arborizing outside the field of the larger parent arbor in novel mediolateral areas suggestive of somatotopic reorganization. Importantly, however, this putative “novel” growth arborized deep to nocireceptive laminae (Fig. 6).
Fig. 6.
Photomicrograph showing an example of possible central sprouting of a putative hair follicle afferent 6 weeks following injury. This fiber gave rise to very fine collaterals that terminated lateral to the main projection field. Note that this collateral did not extend into the superficial laminae. Scale bar = 25 μm.
Injured myelinated nociceptors project throughout superficial laminae
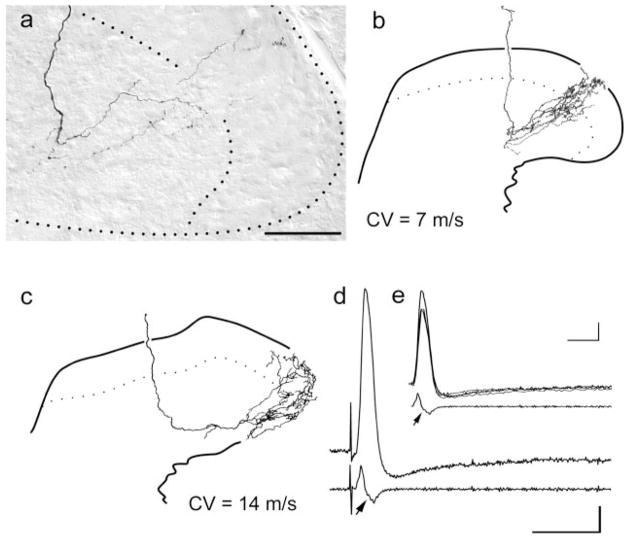
In marked contrast to the failure of LTMRs to sprout into pain-specific regions of the superficial dorsal horn, all afferents exhibiting broad, inflected somal spikes (putative HTMRs) gave rise to extensive projections throughout nocireceptive regions. Among axotomized HTMRs, two collateral morphologies were observed overall. One of these, from HTMRs with the most slowly conducting peripheral axons (4 – 6 m/second), entered the dorsal horn laterally from parent axons traveling in Lissauer’s tract and were similar overall to classical lamina I/IIo-specific myelinated nociceptors (Light and Perl, 1979; Woodbury and Koerber, 2003). The other, from HTMRs with faster conducting axons (7–14 m/second), entered the dorsal horn medially from axons in the dorsal column (Fig. 7a–c); these HTMRs gave rise to dorsally recurving “flame-shaped” arbors that were in all respects similar to those of LTMRs except that they extended throughout the substantia gelatinosa and marginal layer. In transverse camera lucida reconstructions, some of these recurving arbors were very similar to the morphology of putative “sprouted LTMRs” documented in earlier studies (Woolf et al., 1992; Shortland and Woolf, 1993). In single sections, however, the arbors were more diffuse than those of LTMRs (see, e.g., Fig. 5).
Fig. 7.
Representative examples of the central anatomy and somal spikes of previously axotomized putative myelinated nociceptors. a: Photomicrograph of recurving collateral extending through laminae I–IV. b: Camera lucida reconstruction of a collateral from the same fiber. c: Camera lucida reconstruction of a collateral from a different myelinated nociceptor that had a much higher peripheral conduction velocity. d: Somal action potential and first derivative of the somal action potential of the fibers shown in c. e: Superimposition of the somal action potentials and first derivatives of the regenerated cell in d and the two naïve fibers shown in Figure 3d. Note that these spikes were essentially identical in shape. Scale bar = 50 μm.
DISCUSSION
The primary focus in the present studies was to identify adult mouse myelinated cutaneous sensory neurons that maybe capable of sprouting into nocireceptive dorsal horn laminae (I/IIo) following peripheral axotomy and to determine postaxotomy changes in sensory neuron phenotype that might be associated with this ectopic growth. First we demonstrated that bulk labeling using CTB-HRP following sciatic nerve transaction resulted in the same appearance of projections in of the lamina II as seen previously in rat (see, e.g., Woolf et al., 1995). In addition, we also show that bulk labeling of a small cutaneous nerve (DCN) also leads to increased staining in lamina II. One notable difference in the labeling of the projections of these two different injured nerves was that the labeling in lamina II was less pronounced after DCN labeling. This most likely is due to fundamental differences between these two nerves. For example, in addition to cutaneous afferents, the sciatic nerve contains numerous myelinated muscle afferents that have been shown to project to lamina II among others (see, e.g., Hoheisel et al., 1989). In addition, the innvervation density is much higher on the distal extremity than that present for the back. Thus much greater numbers of afferents are projecting to a given location in the dorsal horn.
Next, we used an ex vivo somatosensory system preparation that allowed previously injured cutaneous afferents to be recorded and stained from their somata. Thus, unlike previous studies of injured single fibers, here we have many measures of cell phenotype with which to address the question of fiber type prior to the lesion. For example, because of the tight correlation between somal action potential shape and afferent identity in normal animals (Koerber et al., 1988; Ritter and Mendell, 1992; Djouhri et al., 1998; Woodbury et al., 2001; Koerber and Woodbury, 2002; Woodbury and Koerber, 2003), and the possibility that this correlation remains intact after nerve injury (Czeh et al., 1977; Gurtu and Smith, 1988; Koerber et al., 1995; but also see Stebbing et al., 1999; Liu et al., 2000; Ma et al., 2003), this somal approach provided additional physiological insight into the preaxotomy identity of individually stained afferents.
Central projections of myelinated cutaneous nociceptors in naïve mice
Earlier studies regarding sprouting of large-diameter, myelinated fibers were working under the assumption that all myelinated nociceptive fibers project to laminae I and V. However, in a recent study of myelinated nociceptors in neonatal and juvenile mice (Woodbury and Koerber, 2003), we documented two different termination patterns for myelinated nociceptive fibers in the thoracic spinal cord. One pattern was similar to that described previously by Light and Perl (1979), with the focus of the projection in lamina I, whereas the second pattern from fibers with faster conduction velocities was novel in that the projections extended across all laminae (I–V) in the dorsal horn. This earlier study also showed that this novel morphology persisted well into juvenile life (3 weeks) at a time when B-HRP transport would suggest otherwise (Beggs et al., 2002).
Here we have confirmed the existence of this population of fast conducting, myelinated cutaneous nociceptive fibers that maintains projections across all dorsal horn laminae in the adult mouse. In addition, many of these fibers (70%) had central axons that gave rise to recurving collaterals that produced terminal endings reminiscent of “flame-shaped” arbors. This terminology was first used in the classic studies of A.G. Brown and colleagues (Brown et al., 1977) to describe the characteristic endings of Aβ hair follicle afferents in cats. Subsequently, this morphology, combined with conduction velocity measurements, has been used to identify the central projections of low-threshold, rapidly adapting Aβ fibers following peripheral nerve transection and ligation (see, e.g., Shortland and Woolf, 1993; Hughes et al., 2003). Here we demonstrate that myelinated fibers with response properties characteristic of nociceptors also exhibit similar morphology with the addition of projections penetrating through lamina II into lamina I. It is clear that, without the knowledge of the existence of fibers with this projection pattern, the observation of this morphology following peripheral injury could very well be interpreted to represent collateral sprouting.
This “novel” class of myelinated nociceptors may very well correspond to the “moderate pressure” category identified in pioneering studies of myelinated nociceptors (Burgess and Perl, 1967), many of which conduct in the Aβ range. As seen previously in neonates, these afferents in the back skin of adult mice were relatively sensitive to mechanical stimuli, with many having von Frey hair mechanical thresholds of about 0.7 mN. Multiple “hot spots” could be detected in the receptive field, where low to moderate stimulus intensities elicited a sustained and fairly regular discharge. These afferents respond in an increasingly vigorous fashion to increasing force by faithfully encoding stimulus intensity and projecting to nocireceptive laminae. Thus, these fibers are ideally suited to warn of impending tissue damage. That these fibers are indeed nociceptors is further attested to by the sensitivity to noxious heat in about 30% of the fibers. Interestingly, these heat-sensitive myelinated nociceptors typically respond to noxious heating with a much more vigorous and sustained response than unmyelinated polymodal nociceptors (not shown). Apart from thermal sensitivity, these distinct heat-sensitive and -insensitive subpopulations of myelinated nociceptors may be otherwise indistinguishable, exhibiting similar if not identical somal spikes, peripheral conduction velocities, mechanical thresholds, adaptation rates, and central morphology. The myelinated nociceptive fibers described here in mice have many of the same characteristics as found in other species (for review see Djouhri and Lawson, 2004). Given the prevalence of these fibers across species, the morphology observed in mouse may very well exist in other species. However, confirmation of this fact would obviously require further experiments in different species.
Central projections of injured myelinated cutaneous nociceptors
For putative LTMRs, we found that axotomy-induced changes in central arbors were primarily regressive overall. None gave rise to ectopic projections into nocireceptive laminae after injury, whether or not they reinnervated the skin. By contrast, all previously axotomized myelinated HTMRs gave rise to widespread projections throughout nocireceptive laminae of the dorsal horn. Most of the latter had central axons in the dorsal columns, which gave rise to dorsally recurving, “flame-shaped” arbors extending throughout the entire dorsal horn. This distinctive morphology is very similar to that used to support conclusions of injury-induced sprouting of LTMRs in earlier studies (e.g., Shortland and Woolf, 1993). We show that, rather than representing a novel, injury-induced central morphology, these previously undocumented central arbors of myelinated nociceptors were already present prior to injury.
These findings contradict the perception that LTMRs invade spinal pain circuitry after injury (Woolf et al., 1992; Shortland and Woolf, 1993; Koerber et al., 1994, 1999; Kohama et al., 2000). This work, coupled with findings from recent bulk-labeling studies (Tong et al., 1999; Bao et al., 2002; Santha and Jancso, 2003; Shehab et al., 2003) and single-fiber analysis (Hughes et al., 2003), indicates that the entire concept of injury-induced ectopic central sprouting of cutaneous myelinated afferents requires reevaluation. The only clear example of sprouting that we observed among axotomized afferents was the production of supernumerary peripheral axons near the site of injury. In addition to a lack of central sprouting, we also found no evidence for intraganglionic sprouting of myelinated afferents as reported earlier (McLachlan and Hu, 1998), suggesting that the possible intraganglionic alterations at these time points following axotomy are few and are not correlated with central changes. It has also been suggested that large-diameter sensory neurons may show de novo synthesis of neuropeptides following injury (Noguchi et al., 1995; Koerber et al., 1999). We did not observe any evidence of de novo expression here, but it should be pointed out that the postinjury times examined (2–7.5 weeks) overlap with the time frame in which earlier studies have shown that somal CGRP immunostaining is decreased following peripheral injury (Doughty et al., 1991; Zhang et al., 1993). Therefore, de novo expression may occur at postinjury times longer than those examined here.
With regard to earlier single-fiber evidence used to support the hypothesis of central sprouting, it is important to note that afferents were labeled from their axons in the dorsal columns (Koerber et al., 1989, 1994, 1999; Woolf et al., 1992; Shortland and Woolf, 1993), so diagnostic information on afferent identity from somal spike properties was unavailable. The use of mixed nerves in these earlier studies (Woolf et al., 1992; Shortland and Woolf, 1993; Koerber et al., 1994, 1999) also clouds the issue somewhat, in that noncutaneous afferents projecting to both superficial and deep laminae (Hoheisel et al., 1989) might have been labeled alongside cutaneous nociceptors. Ultrastructural analyses revealed a small number of large, dense-core vesicles, similar to those in intact myelinated nociceptors in the synaptic terminals of “sprouted” afferents (Koerber et al., 1999). This observation, coupled with earlier findings that “sprouted” afferents and intact HTMRs generate similar field potentials (Koerber et al., 1994), was interpreted to suggest that LTMRs not only alter their function by invading and activating novel pain circuitry after injury but also alter their neurochemical phenotype to match the local environment as well.
It is possible that, after injury, some LTMR sensory neurons undergo an all-inclusive phenotypic change (e.g., somal spike shape, acquisition of thermal sensitivity, central sprouting). This cannot be completely ruled out. However, in view of the present findings, a far more parsimonious interpretation would be that “sprouted LTMRs” in previous studies were most likely this morphologically distinct class of myelinated nociceptors (Koerber et al., 1989).
These results also raise the question of why this population of myelinated fibers had remained undiscovered in intact preparations yet appear to become more accessible following peripheral injury. One possible explanation could be central hypertrophy of the injured myelinated nociceptors. Previous anatomical and physiological studies have shown that the central axons of afferent fibers are smaller and conduct more slowly than their peripheral counterparts (Lee et al., 1986; Mirnics and Koerber, 1997). This slowing begins in the dorsal root and continues in the spinal cord, and myelinated nociceptors exhibit a more pronounced slowing than their low-threshold counterparts (Traub and Mendell, 1988). In addition, another recent study has shown a significant increase in the central conduction velocities of myelinated nociceptors following peripheral inflammation (Djouhri and Lawson, 2001). This suggests that the central axons of myelinated nociceptors may hypertrophy, thus increasing the probability of obtaining intraaxonal recordings following peripheral injury.
In conclusion, the results presented here provide definitive proof of the existence of a population of mechanical and polymodal myelinated nociceptors with previously unseen central morphology. Even in the absence of injury-induced ectopic sprouting of cutaneous myelinated afferents, it is entirely conceivable that this “novel” group of afferents may account for the heat hypersensitivity and mechanical allodynia that are often associated with nerve injury. Not only do these fibers have relatively low transduction thresholds in normal animals, but nociceptors in general may also show pronounced sensitization following peripheral reinnervation (Andrew and Greenspan, 1999). Thus, their successful regeneration coupled with injury-induced disinhibition of spinal pain circuitry (Sivilotti and Woolf, 1994; Moore et al., 2002) could lead to a substantial increase in the capacity of these myelinated nociceptors to drive spinal pain networks.
Acknowledgments
We thank Julie Kopsak and Weiwen Wang for excellent technical assistance. We also thank Drs. Brian Davis, Michael Jankowski, and Kristopher Rau for helpful comments on the manuscript.
Grant sponsor: National Institutes of Health; Grant number: NS23725 (to H.R.K.); Grant number: NS44094 (to C.J.W.).
Footnotes
Published online in Wiley InterScience (www.interscience.wiley.com).
LITERATURE CITED
- Andrew D, Greenspan JD. Modality-specific hyper-responsivity of regenerated cat cutaneous nociceptors. J Physiol. 1999;516:897–906. doi: 10.1111/j.1469-7793.1999.0897u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Wang HF, Cai HJ, Tong YG, Jin SX, Lu YJ, Grant G, Hokfelt T, Zhang X. Peripheral axotomy induces only very limited sprouting of coarse myelinated afferents into inner lamina II of rat spinal cord. Eur J Neurosci. 2002;16:175–185. doi: 10.1046/j.1460-9568.2002.02080.x. [DOI] [PubMed] [Google Scholar]
- Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–1258. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- Brown AG, Rose PK, Snow PJ. The morphology of hair follicle afferent fibres collaterals in the spinanl cord of the cat. J Physiol. 1977;272:779–797. doi: 10.1113/jphysiol.1977.sp012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967;190:541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh G, Kudo N, Kuno M. Membrane properties and conduction velocity in sensory neurones following central or peripheral axotomy. J Physiol. 1977;270:165–180. doi: 10.1113/jphysiol.1977.sp011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri J, Lawson SN. Increased conduction velocity of nociceptive primary afferent neurons during unilateral hindlimb inflammation in the anaesthetised guinea-pig. Neuroscience. 2001;102:669– 679. doi: 10.1016/s0306-4522(00)00503-0. [DOI] [PubMed] [Google Scholar]
- Djouhri J, Lawson SN. Aβ-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46:131–145. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. J Physiol. 1998;513:857– 872. doi: 10.1111/j.1469-7793.1998.857ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty SE, Atkinson ME, Shehab SA. A quantitative study of neuropeptide immunoreactive cell bodies of primary afferent sensory neurons following rat sciatic nerve peripheral axotomy. Regul Pept. 1991;35:59–72. doi: 10.1016/0167-0115(91)90254-e. [DOI] [PubMed] [Google Scholar]
- Gurtu S, Smith PA. Electrophysiological characteristics of hamster dorsal root ganglion cells and their response to axotomy. J Neurophysiol. 1988;59:408– 423. doi: 10.1152/jn.1988.59.2.408. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Lehmann-Willenbrock E, Mense S. Termination patterns of identified group II and III afferent fibres from deep tissues in the spinal cord of the cat. Neuroscience. 1989;28:495–507. doi: 10.1016/0306-4522(89)90195-4. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Scott DT, Todd AJ, Riddell JS. Lack of evidence for sprouting of Abeta afferents into the superficial laminas of the spinal cord dorsal horn after nerve section. J Neurosci. 2003;23:9491–9499. doi: 10.1523/JNEUROSCI.23-29-09491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Woodbury CJ. Comprehensive phenotyping of sensory neurons using an ex vivo somatosensory system. Physiol Behav. 2002;77:589–594. doi: 10.1016/s0031-9384(02)00904-6. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Seymour AW, Mendell LM. Mismatches between peripheral receptor type and central projections after peripheral nerve regeneration. Neurosci Lett. 1989;99:67–72. doi: 10.1016/0304-3940(89)90266-8. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Mirnics K, Brown PB, Mendell LM. Central sprouting and functional plasticity of regenerated primary afferents. J Neurosci. 1994;14:3655–3671. doi: 10.1523/JNEUROSCI.14-06-03655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Mirnics K, Mendell LM. Properties of regenerated primary afferents and their functional connections. J Neurophysiol. 1995;73:693–702. doi: 10.1152/jn.1995.73.2.693. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Mirnics K, Kavookjian AM, Light AR. Ultrastructural analysis of ectopic synaptic boutons arising from peripherally regenerated primary afferent fibers. J Neurophysiol. 1999;81:1636–1644. doi: 10.1152/jn.1999.81.4.1636. [DOI] [PubMed] [Google Scholar]
- Kohama I, Ishikawa K, Kocsis JD. Synaptic reorganization in the substantia gelatinosa after peripheral nerve neuroma formation: aberrant innervation of lamina II neurons by Abeta afferents. J Neurosci. 2000;20:1538–1549. doi: 10.1523/JNEUROSCI.20-04-01538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- LaMotte CC, Kapadia SE. Deafferentation-induced terminal field expansion of myelinated saphenous afferents in the adult rat dorsal horn and the nucleus gracilis following pronase injection of the sciatic nerve. J Comp Neurol. 1993;330:83–94. doi: 10.1002/cne.903300107. [DOI] [PubMed] [Google Scholar]
- Lee KH, Chung K, Chung JM, Coggeshall RE. Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol. 1986;243:335–346. doi: 10.1002/cne.902430305. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Light AR, Willcockson HH. Spinal laminae I–II neurons in rat recorded in vivo in whole cell, tight seal configuration: properties and opioid responses. J Neurophysiol. 1999;82:3316–3326. doi: 10.1152/jn.1999.82.6.3316. [DOI] [PubMed] [Google Scholar]
- Light AR, Shults RC, Jones SL. The initial processing of pain and its descending control: spinal and trigeminal systems. Basel: Karger; 1992. [Google Scholar]
- Liu CN, Michaelis M, Amir R, Devor M. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: relation to neuropathic pain. J Neurophys. 2000;84:205–215. doi: 10.1152/jn.2000.84.1.205. [DOI] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophys. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- McIlwrath SL, Lawson JJ, Anderson CE, Albers KM, Koerber HR. Overexpression of neurotrophin-3 enhances the mechanical response properties of slowly adapting type 1 afferents and myelinated nociceptors. Eur J Neurosci. 2007;26:1801–1812. doi: 10.1111/j.1460-9568.2007.05821.x. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Hu P. Axonal sprouts containing calcitonin gene-related peptide and substance P form pericellular baskets around large diameter neurons after sciatic nerve transection in the rat. Neuroscience. 1998;84:961–965. doi: 10.1016/s0306-4522(97)00680-5. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Koerber HR. Properties of individual embryonic primary afferents and their spinal projections in the rat. J Neurophysiol. 1997;78:1590–1600. doi: 10.1152/jn.1997.78.3.1590. [DOI] [PubMed] [Google Scholar]
- Moore KA, Baba H, Woolf CJ. Gabapentin—actions on adult superficial dorsal horn neurons. Neuropharmacology. 2002;43:1077–1081. doi: 10.1016/s0028-3908(02)00226-5. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K. Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J Neurosci. 1995;11:7633–7643. doi: 10.1523/JNEUROSCI.15-11-07633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Baba H, Goldstein PA, Higashi H, Shimoji K, Yoshimura M. Functional reorganization of sensory pathways in the rat spinal dorsal horn following peripheral nerve injury. J Physiol. 2001;532:241–250. doi: 10.1111/j.1469-7793.2001.0241g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Woodbury CJ, Albers K, Davis BM, Koerber HR. Maturation of cutaneous sensory neurons from normal and NGF-overexpressing mice. J Neurophysiol. 2000;83:1722–1732. doi: 10.1152/jn.2000.83.3.1722. [DOI] [PubMed] [Google Scholar]
- Santha P, Jancso G. Transganglionic transport of choleragenoid by capsaicin-sensitive C-fibre afferents to the substantia gelatinosa of the spinal dorsal horn after peripheral nerve section. Neuroscience. 2003;116:621–627. doi: 10.1016/s0306-4522(02)00701-7. [DOI] [PubMed] [Google Scholar]
- Shehab SA, Spike RC, Todd AJ. Evidence against cholera toxin B subunit as a reliable tracer for sprouting of primary afferents following peripheral nerve injury. Brain Res. 2003;964:218–227. doi: 10.1016/s0006-8993(02)04001-5. [DOI] [PubMed] [Google Scholar]
- Shortland P, Woolf CJ. Chronic peripheral nerve section results in a rearrangement of the central axonal arborizations of axotomized A beta primary afferent neurons in the rat spinal cord. J Comp Neurol. 1993;330:65–82. doi: 10.1002/cne.903300106. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- Stebbing MJ, Eschenfelder S, Habler HJ, Acosta MC, Janig W, McLachlan EM. Changes in the action potential in sensory neurons after peripheral axotomy in vivo. Neuroreport. 1999;10:201–206. doi: 10.1097/00001756-199902050-00001. [DOI] [PubMed] [Google Scholar]
- Tong YG, Wang HF, Ju G, Grant G, Hokfelt T, Zhang X. Increased uptake and transport of cholera toxin B-subunit in dorsal root ganglion neurons after peripheral axotomy: possible implications for sensory sprouting. J Comp Neurol. 1999;404:143–158. [PubMed] [Google Scholar]
- Traub RJ, Mendell LM. The spinal projection of individual identified A-delta- and C-fibers. J Neurophysiol. 1988;59:41–55. doi: 10.1152/jn.1988.59.1.41. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Koerber HR. Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. J Neurosci. 2003;23:601– 610. doi: 10.1523/JNEUROSCI.23-02-00601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Ritter AM, Koerber HR. On the problem of lamination in the superficial dorsal horn of mammals: a reappraisal of the substantia gelatinosa in postnatal life. J Comp Neurol. 2000;417:88–102. doi: 10.1002/(sici)1096-9861(20000131)417:1<88::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Ritter AM, Koerber HR. Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. J Comp Neurol. 2001;436:304–323. [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Reynolds M, Ridings J, Doubell T, Coggeshall RE. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J Comp Neurol. 1995;360:121–134. doi: 10.1002/cne.903600109. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ju G, Elde R, Hokfelt T. Effect of peripheral nerve cut on neuropeptides in dorsal root ganglia and the spinal cord of monkey with special reference to galanin. J Neurocytol. 1993;22:342–381. doi: 10.1007/BF01195558. [DOI] [PubMed] [Google Scholar]