Abstract
Fatty acid amides are a new class of signaling lipids that have been implicated in diverse physiological and pathological conditions. Oleamide is a fatty acid amide that induces vasorelaxation. Here, we investigated the mechanisms behind the vasorelaxation effect of oleamide in rat mesenteric resistance arteries. Oleamide-induced concentration dependent (0.01 μM–10μM) vasorelaxation in mesenteric resistance arteries. This relaxation was unaffected by the presence of the fatty acid amide hydrolase (FAAH) inhibitors. The cannabinoid type 1 (CB1) receptor antagonist, AM251 and the non-CB1/CB2 cannabinoid receptor antagonist, O-1918, attenuated the oleamide vasodilatory response, however the cannabinoid CB2 receptor antagonist, AM630, did not affect the vascular response. Moreover, inhibition of the transient receptor potential vanilloid (TRPV) 1 receptor with capsazepine shifted the oleamide-induced vasorelaxation response to the right. In agreement with the vascular functional data, the cannabinoid CB1 and TRPV1 receptor proteins were expressed in mesenteric resistance arteries but cannabinoid CB2 receptors and the FAAH enzyme were not. In endothelium-denuded arteries, the oleamide-mediated vasorelaxation was attenuated and cannabinoid CB1 or non-CB1/CB2 cannabinoid receptor blockade did not further reduce the dilatory response whereas TRPV1 antagonism further decreased the response. These findings indicate that cannabinoid receptors on the endothelium and endothelium-independent TRPV1 receptors contribute to the oleamide vasodilatory response. Taken together, these results demonstrate that the oleamide-induced vasorelaxation is mediated, in part, by cannabinoid CB1 receptors, non-CB1/CB2 cannabinoid receptors, and TRPV1 receptors in rat mesenteric resistance arteries. These mechanisms are overlapping in respect to oleamide-induced mesenteric resistance artery dilation.
Keywords: oleamide, cannabinoid receptors, endothelium, fatty acid amide, mesenteric arteries
1. Introduction
An increasing body of evidence suggests that lipid mediators are involved in a variety of pathophysiological functions and disease development that include participation in intracellular signaling, inflammation and cardiovascular disease. In this regard, fatty acid amides represent a promising class of signaling lipids that have been implicated in a diverse number of physiological and pathological processes. Oleamide is primary fatty acid amide of endogenous lipid mediators, first identified in the cerebrospinal fluid of sleep deprived cats (Cravatt et al., 1995). It has been shown to induce sleep, cause hypomotility and hypothermia, inhibit gap junction-mediated cell-cell communication and increase food intake (Hiley and Hoi, 2007). Leggett et al. (2004) found that oleamide is an endogenous agonist of rat and human cannabinoid (CB) receptors. A previous report demonstrated that oleamide is involved in the regulation of cardiovascular function by inducing vasodilation in rat mesenteric arteries (Hoi and Hiley, 2006). Mesenteric arteries dilation in response to oleamide was demonstrated to be partially dependent on an intact endothelium and activation of the non-CB1/CB2 cannabinoid receptor (Hoi and Hiley 2006). These findings suggest that the vascular actions of oleamide could contribute importantly to cardiovascular function.
Oleamide shares some structural similarity with other fatty acid amides such as anandamide in the form of the amide linkage of fatty acid. Like anandamide, oleamide can be catabolized by fatty acid amide hydrolase (FAAH) (McKinney and Cravatt, 2005). Fatty acid amides have been demonstrated to dilate resistance arteries and there appears to be overlapping mechanisms contributing to the vascular response (Randall et al., 1996; Pratt et al., 1998; Járai et al., 1999; Bátkai et al., 2004; Wang et al., 2005; Watanabe et al., 2005; Hoi and Hiley, 2006). Thus, the contribution of specific mechanisms and their possible overlap motivated us to investigate oleamide-induced vasorelaxation in rat mesenteric resistance arteries. Specifically, we determined the contribution of the endothelium, CB receptors, vanilloid receptor, nitric oxide (NO), potassium (K+) channel and metabolism by FAAH to the mesenteric vascular response evoked by oleamide.
2. Materials and Methods
2.1. Chemicals and reagents
Oleamide (A.G. Scientific Inc, San Diego, CA), 3′-(aminocarbonyl) [1,1′-biphenyl]-3-yl)-cyclohexylcarbamate (URB597), methyl arachidonyl fluorophosphonate (MAFP), 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl–N-1-piperidinyl-1H-pyrazole-3-carboxamide (AM 251), capsazepine and U46619 were purchased from Cayman Chemical, Ann Arbor, MI. 6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl) methanone (AM 630), 1,3-Dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]benzene (O-1918) and abnormal cannabidiol (abn-cbd) were purchased from Tocris Bioscience, Ellisville, MO. Cannabinoid CB1 and CB2 receptor antibodies (Affinity Bioreagents, Golden, CO) and TRPV1 antibody (Santa Cruz Biotechnology Inc. Santa Cruz, CA) were used. FAAH antibody was a gift from Dr. Cecilia J. Hillard. All other chemicals and reagents used were of the highest analytical grade obtained from Sigma Chemicals Co. (St. Louis, MO).
2.2. Isolated mesenteric resistance vessel preparation
The Medical College of Wisconsin Animal Care and Use Committee approved all experimental procedures. Male Sprague-Dawley rats (250–400g) were anesthetized with pentobarbital sodium (50 mg/kg ip). Two mesenteric resistance arteries were obtained from each rat and a different protocol was conducted on each artery. Therefore the n size represents the number of mesenteric resistance arteries and the number of rats used for each protocol. Mesenteric artery segments were obtained from rats and mounted between two cannulae in a pressure myograph system (Danish Myo Technology model 111P). The interior and exterior of the vessel were oxygenated in 95% O2/5% CO2 Krebs physiological salt solution (119.0 mM NaCl, 25.0 mM NaHCO3, 4.6 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.8 mM CaCl2, 11.0 mM glucose) at pH 7.4 and 37°C. Under no flow conditions, the pressure within the vessel was increased in 10 mm Hg increments from 20 to 65 mm Hg. The vessel was then equilibrated at 65 mm Hg for 30 min and remained at that pressure for the duration of the experiment. Lumen diameter measurements were acquired and logged using the MyoView 1.2P user interface (DMT, Aarhus, Denmark). The control lumen diameter was calculated as the mean diameter during the last 1 min of the 30 min equilibration. Vessels were constricted with the thromboxane mimetic U46619, and the diameter of the constricted vessel was calculated as the mean during the last 1 min of 15 min.
Oleamide was dissolved in ethanol and doses (0.01, 0.03, 0.1, 0.3, 1.0, 3.0 and 10 μM) were added to the bathing solution every 5 min, and the response for each dose was measured. Sodium nitroprusside (100 μM) was given at the end of the experimental period to ensure the vascular integrity. To ensure that the vehicle (1–10 μl) into which the drugs were dissolved did not alter vessel reactivity, experiments were conducted using ethanol in the absence of drug. Ethanol did not change vascular reactivity at any of the concentrations used in these experiments (n=6).
To determine the contribution of FAAH enzyme, CB receptors, NO, K+ channel and vanilloid receptors to the mesenteric resistance artery dilation evoked by oleamide, URB597 (1 μM), MAFP (10 μM), AM 251 (1 μM), AM 630 (1 μM), O-1918 (30 μM), capsazepine (10 μM), ruthenium red (1 μM), N-omega-Nitro-L-Arginine methyl ester hydrochloride (L-NAME; 300 μM) and tetraethyl ammonium (TEA, 1 mM) at a volume of 1–10 μl were added to 10 ml of the organ bath. These inhibitors were added to the myograph vessel bath 20 or 30 min prior to constriction of the vessel with U46619. These inhibitors did not alter baseline diameter with the exception of L-NAME that significantly decreased baseline diameter from 293±8 μm to 239±11 μm. In other experiments, the contribution of the endothelium to oleamide-mediated vasorelaxation was determined. Endothelium-denuded vessels were prepared by inserting a human hair into the vessel in a dish of Krebs physiological salt solution. The successful endothelium removal was confirmed by absence of the vasorelaxation effect in acetylcholine (10 μM). If an acetylcholine-induced relaxation was not observed, the vessel was rinsed, and allowed to equilibrate 30 min before constriction with U46619. Different doses of oleamide were added to the vessel chamber as described earlier. At the end of the experimental protocol, sodium nitroprusside (100 μM) was added to the chamber to demonstrate that the endothelium-denuded vessel was capable of relaxation.
2.3. Western blot analysis
Mesenteric resistance artery segments were obtained from male Sprague-Dawley rats. The vessels were homogenized in cell lysis buffer (RIPA lysis buffer, Millipore Corp, MA) with protease inhibitors cocktail (1:200; Sigma Chemicals Co, St. Louis, MO), and the homogenate centrifuged at 10,000g for 10 min at 4°C to sediment any non-homogenized material. The supernatant was removed, and the protein concentration was determined using a bicinchoninic acid protein assay (Pierce Biotechnology, Inc., Rockford, IL). Protein was solubilized in Laemmli sample buffer. The samples were separated by electrophoresis using a 10% stacking Tris-glycine acrylamide gel and then western blotted to PVDF membrane and blocked with 2% bovine serum albumin and 5% dry milk in phosphate-buffered saline containing 0.1% Tween 20 (PBS-T). After blocking, the membranes were washed several times with PBS-T and incubated with primary antibody. The primary antibodies used were rabbit anti-FAAH (1:1000), rabbit anti-cannabinoid CB1 receptor (1:1000), rabbit anti-cannabinoid CB2 receptor (1:1000) and goat anti-TRPV1 receptor (1:1000). Membranes were washed with PBS-T and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase. Goat anti-rabbit (1:5000) was used as a secondary antibody for FAAH, cannabinoid CB1 and CB2 receptors, and donkey anti-goat (1:5000) was used as a secondary antibody for TRPV1 receptors. The membranes were developed and detected proteins using an enhanced chemiluminescence kit (GE Healthcare Limited, Buckinghamshire, UK) and followed by exposure of the membranes to Amersham Hyperfilm ECL (GE Healthcare Limited, Buckinghamshire, UK).
2.4. Statistical analysis
All data were expressed as mean ± S.E.M. (Standard Error Mean). The concentration response curve of oleamide with all inhibitors were expressed as percentage of maximum constriction and analyzed using non-linear regression of sigmoidal curves to calculate the EC50 (GraphPad Prism, San Diego, CA). Statistical significance between two groups was evaluated with Student’s t-test, and a one-way ANOVA was performed to evaluate significance between multiple groups. A P value < 0.05 was considered to reflect a significant difference.
3. RESULTS
3.1. FAAH inhibition on the rat mesenteric resistance artery response to oleamide
To determine vascular actions of oleamide, increasing concentrations of oleamide were administered to isolated pressurized mesenteric resistance arteries and vascular diameter was measured. Oleamide (0.01 μM – 10 μM) elicited a concentration-dependent vasorelaxation in mesenteric resistance arteries (Figure 1). The EC50 (μM) value of oleamide for mesenteric resistance vessels was 0.35 ± 0.26 (n=8). The response of the mesenteric arteries to oleamide in the presence of FAAH inhibitors was also determined. Oleamide-induced vasorelaxation was not influenced by the presence of either URB597 (EC50 0.45 ± 0.20) or MAFP (EC50 0.48 ± 0.35). These results demonstrate that FAAH metabolites of oleamide are not involved in the vasodilatory response. In addition, an oleamide metabolite, oleic acid, did not relax the vessel, but did result in a negligible constriction of the mesenteric resistance vessel.
Figure 1.
Effect of URB597 or MAFP on oleamide mediated dilation of mesenteric resistance arteries. Mesenteric resistance arteries were pressurized and pretreated with vehicle (n=8), URB597 (1μM, n=6) or MAFP (10μM, n=4). The effect of oleic acid (n=5) was also evaluated on intact mesenteric resistance arteries. Starting vessel diameter measured 281 ± 7 μm (n=23) and measured 126 ± 9 μm after treatment with U46619. Values are expressed as mean±S.E.M.
3.2. Effect of cannabinoid receptor antagonists on oleamide-induced vasorelaxation
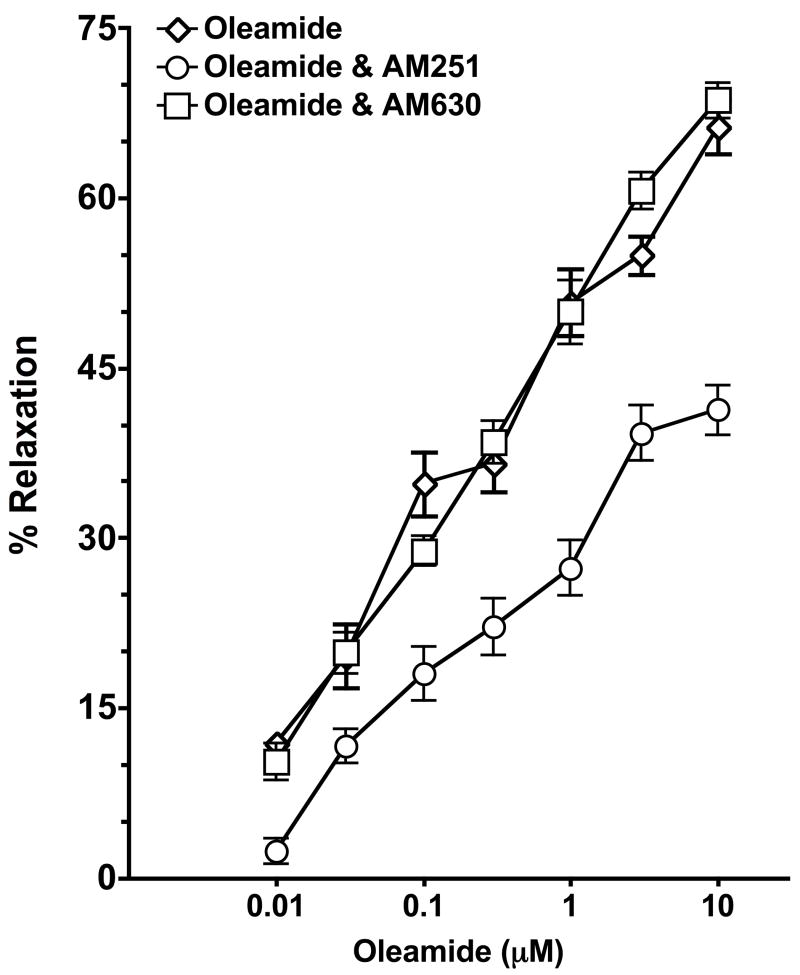
The effectiveness of cannabinoid CB1 receptor antagonist, AM251 and cannabinoid CB2 receptor antagonist, AM630 on oleamide-induced relaxation was determined. In Figure 2, the concentration response curve to oleamide was shifted rightwards in the presence of AM251 (control, EC50 = 0.48 ± 0.19; AM251, EC50 = 0.84 ± 0.71; P<0.05). However, pretreatment with AM630 (EC50 = 0.54 ± 0.48) did not affect the relaxation induced by oleamide. Therefore it appears that the cannabinoid CB1 receptor partially mediates the oleamide-induced mesenteric resistance artery relaxation.
Figure 2.
Effect of the cannabinoid CB1 receptor antagonist AM251 or the CB2 receptor antagonist AM360 on oleamide mediated dilation of mesenteric resistance arteries. Mesenteric resistance arteries were pressurized and pretreated with vehicle (n=4), AM251 (1μM, n=5) or AM630 (1μM, n=6). Starting vessel diameter measured 267 ± 11 μm (n=15) and measured 113 ± 7 μm after treatment with U46619. Values are expressed as mean±S.E.M.
3.3. Role of non-CB1/CB2 cannabinoid receptor in the oleamide-induced vasorelaxation
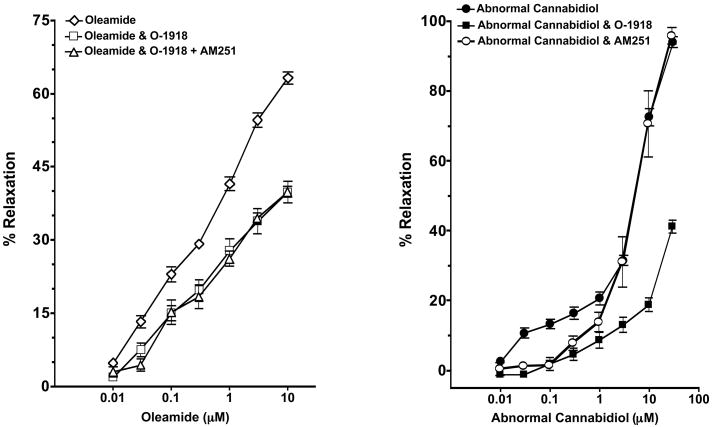
Vascular preparations were preincubated for 30 min with O-1918, an antagonist of the novel non-CB1/CB2 cannabinoid receptor that does not bind to cannabinoid CB1 or CB2 receptor at a concentration of 30 μM (Offertáler et al., 2003). After preconstriction, the concentration curve for oleamide was evaluated (Figure 3, left panel). Oleamide maximally relaxed the mesenteric resistance artery by 63.2 ± 3.6% and this vasorelaxation was significantly attenuated in the presence of the non-CB1/CB2 cannabinoid receptor antagonist O-1918 (39.9 ± 4.4%; P<0.05) or O-1918 and the cannabinoid CB1 receptor antagonist AM251 (39.9 ± 2.4%; P<0.05). There was no difference in the vascular response to oleamide in the mesenteric resistance arteries treated with O-1918 or the combination of O-1918 and AM251. Furthermore, the vasorelaxation effect of abnormal cannabidiol (abn-cbd) was decreased by O-1918, confirming that O-1918 is an antagonist for non-CB1/CB2 cannabinoid receptor (Figure 3, right panel). Hence, the non-CB1/CB2 cannabinoid receptor contributes to oleamide-induced vasorelaxation. The mesenteric resistance artery dilation to abn-cbd was also partially attenuated by AM251 but to a lesser extent than O-1918. This finding suggests that AM251 besides being a selective CB1 receptor antagonist it is also a weak antagonist on the non-CB1/CB2 receptor.
Figure 3.
Effect of the non-CB1/CB2 cannabinoid receptor antagonist O-1918 on oleamide and abnormal cannabidiol (abn-cbd) mediated dilation of mesenteric resistance arteries. Left panel: Mesenteric resistance arteries were pressurized and pretreated with vehicle (n=8), the non-CB1/CB2 cannabinoid receptor antagonist O-1918 (30μM, n=4) or the cannabinoid CB1 receptor antagonist AM251 (1μM) and the non-CB1/CB2 cannabinoid receptor antagonist O-1918 (n=5). Starting vessel diameter measured 283 ± 15 μm (n=17) and measured 122 ± 11 μm after treatment with U46619. Right panel: Mesenteric resistance arteries were pressurized and pretreated with vehicle (n=6), the non-CB1/CB2 cannabinoid receptor antagonist O-1918 (30μM, n=4), or the cannabinoid CB1 receptor antagonist AM251 (1μM, n=4). Starting vessel diameter measured 268 ± 12 μm (n=14) and measured 121 ± 11 μm after treatment with U46619. Values are expressed as mean±S.E.M.
3.4. Effect of endothelial removal on oleamide and cannabinoid receptor vasorelaxation
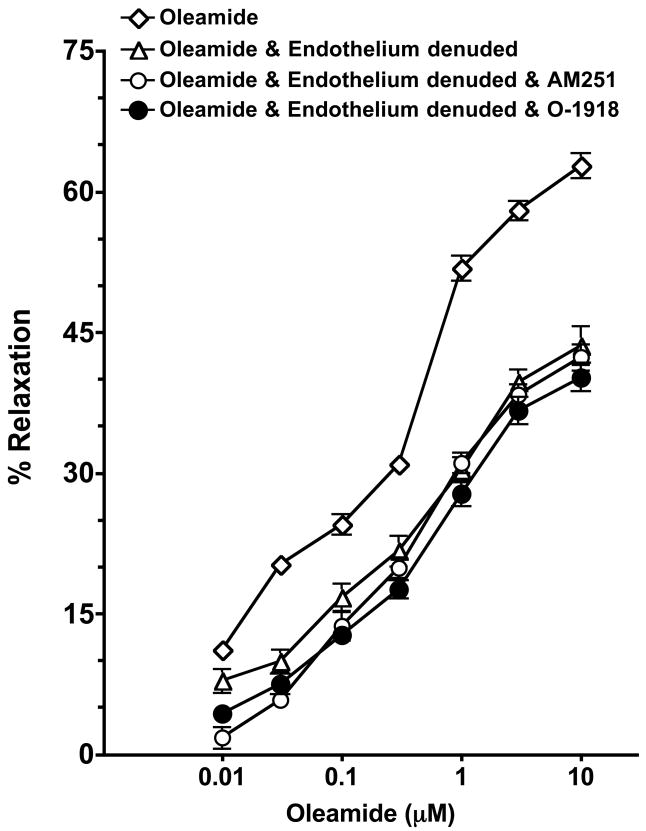
The effectiveness of endothelium removal on oleamide-induced response was evaluated. Removal of the endothelium resulted in a significant reduction in the vasorelaxant potency of oleamide (Figure 4). In endothelium-denuded rings, the concentration-response curve to oleamide was significantly shifted rightward (control, EC50 = 0.30 ± 0.16; endothelium removal, EC50 = 1.28 ± 0.90; P<0.05). It was also found that the additional presence of cannabinoid CB1 receptor antagonist AM251 did not cause any further inhibition of oleamide-induced relaxation (EC50 = 1.89 ± 1.28). Similarly, the presence of non-CB1/CB2 cannabinoid receptor antagonist, O-1918 did not cause any further attenuation of oleamide-induced relaxation (maximal relaxation=41.6 ± 1.2% in endothelium denuded and 40.1 ± 3.0% in endothelium denuded and O-1918). These results indicate that the cannabinoid CB1 and non-CB1/CB2 cannabinoid receptors mediated portion of the oleamide relaxation of the mesenteric resistance arteries appears to be dependent on an intact endothelium.
Figure 4.
Effect of endothelial removal on oleamide and cannabinoid receptor mediated dilation of mesenteric resistance arteries. Mesenteric resistance arteries were pressurized and the response to oleamide determined in endothelial intact (n=7) and endothelium denuded arteries pretreated with vehicle (n=8), the cannabinoid CB1 receptor antagonist AM251 (1μM, n=6) or the non-CB1/CB2 cannabinoid receptor antagonist, O-1918 (30 μM, n=4). Starting vessel diameter measured 273 ± 7 μm (n=25) and measured 133 ± 10 μm after treatment with U46619. Values are expressed as mean±S.E.M.
3.5. Influence of vanilloid receptor antagonist on oleamide-induced vasorelaxation
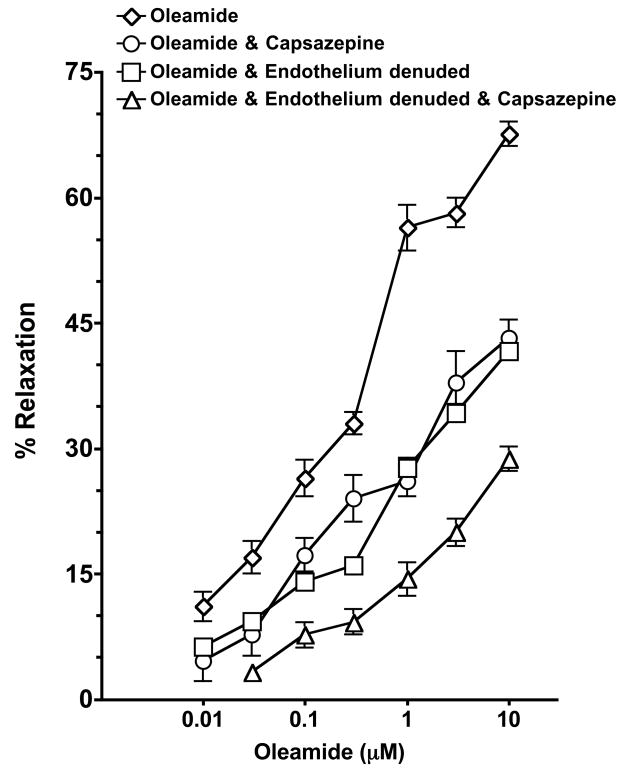
The effect of vanilloid receptor antagonists on oleamide-induced vasorelaxation is shown in Figure 5. Pretreatment with the TRPV1 antagonist, capsazepine resulted in a marked attenuation of the vasorelaxant effect of oleamide (control, EC50 = 0.79 ± 0.31; capsazepine, EC50 =1.56 ± 1.52; P<0.05). In the presence of the cation channel and TRPV inhibitor, ruthenium red, the concentration-response curve to oleamide was also significantly shifted rightwards (EC50 = 1.67 ± 1.64; P<0.05, n=5, data not shown). The mesenteric resistance artery dilation was assessed in the presence of capsazepine and the cannabinoid CB1 receptor antagonist, AM251 (n=5) or capsazepine, AM251 and the non-CB1/CB2 cannabinoid receptor antagonist, O-1918 (n=4). The oleamide-induced vasorelaxation did not further decrease in the presence of the combination of cannabinoid receptor antagonism and capsazepine when compared to capazepine alone (data not shown). Taken together, these results indicate that the vanilloid receptor partially contributes to oleamide-induced vasorelaxation.
Figure 5.
Effect of the TRPV1 receptor antagonist capsazepine on oleamide mediated dilation of mesenteric resistance arteries. Mesenteric resistance arteries were pressurized and pretreated with vehicle (n=7), capsazepine (10μM, n=5). The effect of endothelial removal on oleamide TRPV1 receptor mediated dilation of mesenteric resistance arteries was assessed in endothelium denuded arteries pretreated with vehicle (n=4) or capsazepine (n=4). Starting vessel diameter measured 289 ± 8 μm (n=20) and measured 147 ± 7 μm after treatment with U46619. Values are expressed as mean±S.E.M.
Additional experiments determined if the TRPV1 portion of the oleamide-induced vasorelaxation was dependent on an intact endothelium (Figure 5). As was previously observed, endothelium denuded mesenteric resistance arteries have a blunted vasodilatory response to oleamide (41.6± 2.1%); however, the TRPV1 antagonist, capsazepine further attenuated the vascular response to oleamide in endothelium denuded arteries (28.7 ± 2.9%, P<0.05). These results suggest that a portion of the TRPV1 oleamide-induced vasorelaxation is endothelial independent.
3.5. Effect of L-NAME and TEA on oleamide-induced vasorelaxation
Potassium channel and nitric oxide contributions to the oleamide mediated dilator responses in mesenteric resistance arteries were determined. Figure 6 shows the effect of the NO inhibitor, L-NAME and the non-selective K+ channel inhibitor, TEA on the oleamide-induced vasorelaxation. While oleamide maximally relaxed the mesenteric resistance arteries by 62.6 ± 6.3% this vasorelaxation was significantly decreased in the presence of L-NAME (37.8 ± 5.2%, P<0.05) or in the presence of TEA (38.9 ± 3.9%, P<0.05). Additionally, the combination of L-NAME and TEA did not further decrease the vascular responses to oleamide (37.9 ± 2.8%, data not shown) than to L-NAME or TEA individually. These results indicate that NO and K+ channel are involved in the oleamide-induced mesenteric resistance artery dilation.
Figure 6.
Effect of the NO inhibitor, L-NAME and potassium channel inhibitor, TEA on oleamide mediated dilation of mesenteric resistance arteries. Mesenteric resistance arteries were pressurized and pretreated with vehicle (n=4), the NO inhibitor, L-NAME (300μM, n=4) or the K+ channel inhibitor, TEA (1mM, n=4). Starting vessel diameter measured 272 ± 9 μm (n=12) and measured 142 ± 6 μm after treatment with U46619. Values are expressed as mean±S.E.M.
3.7. Measurement of FAAH, cannabinoid CB1, CB2 and TRPV1 protein expression
To determine whether or not the FAAH enzyme, cannabinoid CB1, CB2 receptors and TRPV1 receptor are expressed in mesenteric resistance vessel, the protein expression was measured by western blotting (Figure 7). FAAH enzyme, cannabinoid CB1, CB2 receptors and TRPV1 receptor were detected in brain samples; however, the FAAH enzyme and cannabinoid CB2 receptors demonstrated much lower levels of expression. Cannabinoid CB1 and TRPV1 receptors were expressed in rat mesenteric resistance arteries. In contrast, protein expression of the FAAH enzyme and cannabinoid CB2 receptor was not detected in mesenteric resistance arteries.
Figure 7.

FAAH enzyme, cannabinoid CB1 receptors, CB2 receptors and TRPV1 receptors protein expression was determined in rat mesenteric resistance arteries. The vessels and brains were homogenized and 10 or 20 μg of protein loaded onto a gel and resolved by electrophoresis.
4. Discussion
Fatty acid amides represent a novel class of signaling lipids that have been suggested to have cardiovascular actions. Many of these actions could be potentially protective to the cardiovascular system. Oleamide is a primary fatty acid amide that has possible protective vascular actions that are not well understood. Thus the aim of the present study was to determine mechanisms involved in oleamide-induced relaxation of rat mesenteric resistance arteries. Our experimental studies demonstrate that oleamide-induced relaxation of rat mesenteric resistance arteries involves cannabinoid CB1 and non-CB1/CB2 cannabinoid receptor activation as well as vanilloid receptor activation.
The initial experiments demonstrate that oleamide causes concentration-dependent relaxation in mesenteric resistance arteries. FAAH is a membrane-associated enzyme that converts oleamide (Cravatt et al., 1996; Deutsch et al., 1997) into oleic acid and ammonia and FAAH metabolism has been implicated in altering the vascular responses to fatty acid amides. An earlier study demonstrated that anandamide-induced vasorelaxation is dependent on its FAAH metabolite, arachidonic acid and subsequent metabolism to vasoactive eicosanoids (Pratt et al., 1998). In the present investigation, we evaluated whether or not the oleamide-induced vasorelaxation is similarly dependent on metabolism by FAAH. The FAAH inhibitors, URB597 or MAFP, did not significantly alter oleamide mediated mesenteric resistance artery dilation. However, the maximal vasodilatory response to oleamide was decreased in the presence of URB597. We do not know the reason for this difference between URB597 and MAFP on the vascular response to oleamide but speculate that it does not relate to FAAH since we failed to detect FAAH enzyme protein expression in isolated mesenteric resistance arteries. Previous studies have demonstrated the presence of FAAH in bovine coronary arteries, kidney endothelial cells and human umbilical vein endothelial cells (Pratt et al., 1998; Deutsch et al., 1997; Maccarrone et al., 2001). On the other hand, there is no direct evidence for the FAAH enzyme in myocardial cells or vascular smooth muscle cells (Hiley and Hoi, 2007). In addition, the fact that the oleamide metabolite, oleic acid did not relax the mesenteric resistance artery further supports the notion that oleamide does not depend on FAAH metabolism to evoke a vascular response.
Previous studies have also provided evidence that anandamide induces arachidonic acid release and eicosanoid production through activation of a cannabinoid receptor and a MAP kinase signaling pathway (Hirasawa et al., 1995; Wartmann et al., 1995). Wartmann et al. (1995) reported that anandamide has been shown to activate cytoplasmic PLA2 and eicosanoid production via activation of MAP kinase signaling pathway in human fetal lung fibroblast cells. In the present study, we utilized MAFP that in addition to inhibiting the FAAH enzyme also acts as an irreversible inhibitor of cytoplasmic PLA2 (Lio et al., 1996). Activation or metabolism by PLA2 does not appear to participate in the vascular response since oleamide-induced vasorelaxation was not altered by MAFP. Our studies are in agreement with a previous report that the cyclooxygenase inhibitor, indomethacin did not significantly effect the oleamide relaxation of rat mesenteric arteries (Hoi and Hiley, 2006). Taken together, the results of our experimental studies support the notion that oleamide vasorelaxation is not dependent on metabolism to vaosactive prostanoid-like metabolites.
Oleamide belongs to the same family of chemical messengers as the endocannabinoid, anandamide; oleamide could thus be expected to act through cannabinoid receptors. We evaluated the contribution of the two main cannabinoid receptors to the oleamide-mediated vasorelaxation. Our results demonstrated that cannabinoid CB1 receptor but not CB2 receptor inhibition attenuated the oleamide-mediated relaxation of the mesenteric resistance arteries. Previous studies by Leggett et al. (2004) found that oleamide is a full cannabinoid CB1 receptor agonist, competitively inhibiting the agonist and antagonist binding to the cannabinoid CB1 receptor, and also inducing the binding of [35S]GTPγS to brain membranes. Evidence has also suggested that the oleamide induced hypnotic action is mediated by cannabinoid CB1 receptors (Mendelson and Basile, 1999). In addition to the vascular functional data, we demonstrated the presence of cannabinoid CB1 receptor protein expression in the mesenteric resistance arteries. The expression of cannabinoid CB2 receptor was not found in rat mesenteric resistance vessels and this finding agrees with several studies demonstrating that the cannabinoid CB2 receptor is predominantly expressed in cells of immune system (Matsuda et al., 1990; Galiègue et al., 1995; Schatz et al., 1997). Overall these data suggest that the cannabinoid CB1 receptor is expressed in the rat mesenteric resistance arteries and contributes to the vasodilatory action of oleamide.
Other experimental studies have provided evidence that oleamide and anandamide vasorelaxation is mediated in part by the non-CB1/CB2 cannabinoid receptor (Járai et al., 1999; Mukhopadhyay et al., 2002; Hoi and Hiley, 2006). Abnormal cannabidiol (abn-cbd), a structural analog of naturally occurring cannabidiol, is a selective agonist (Adams et al., 1977) and O-1918, a selective antagonist (Offertaler et al., 2003) of the non-CB1/CB2 cannabinoid receptor. Abn-cbd has been shown to induce mesenteric vasorelaxation in CB1/CB2 receptor double knockout mice (Jarai et al., 1999), which suggest that novel cannabinoid receptors other than the cannabinoid CB1 and CB2 receptors are present in mesenteric arteries. The present findings indicate that abn-cbn induced vasorelaxation is greatly attenuated by the presence of O-1918 and that O-1918 inhibits the mesenteric vasorelaxant effect of oleamide.
A previous report demonstrated that the cannabinoid CB1 receptor antagonist SR141716A, rimonabant, but not AM251 attenuated the mesenteric resistance artery dilation to oleamide (Hoi and Hiley, 2006). This same study provided initial evidence that rimonabant could have attenuated oleamide-mediated vasorelaxation by acting on the non-CB1/CB2 cannabinoid receptor (Hoi and Hiley, 2006). In contrast, our study demonstrated that the cannabinoid CB1 receptor antagonist AM251 attenuated the mesenteric resistance artery dilation to oleamide. Although AM251 has been touted as a highly selective CB1 receptor antagonist (Hoi and Hiley, 2006; Kruetz et al., 2009), experimental studies have demonstrated that AM251 is a weak antagonist to the abn-cbd mesenteric vasodilation (Ho and Hiley, 2003) and fully antagonizes the vasodilation to the non-CB1/CB2 receptor agonist VSN16 (Hoi et al., 2007). In the present study the vasodilation to abn-cbd was somewhat attenuated by AM251 further supporting the concept that AM251 is a weak antagonist on the yet to be identified non-CB1/CB2 receptor. Moreover, AM251 in combination with non-CB1/CB2 cannabinoid receptor antagonist O-1918 did not further attenuate the vasodilatory response to oleamide. These observations and those of previous reports support the notion that oleamide-induced vasorelaxation is partially mediated through non-CB1/CB2 cannabinoid receptors along with cannabinoid CB1 receptor or that oleamide is acting through a single cannabinoid receptor that is inhibited by AM251 and O-1918 at the doses used in the current experimental setting. Another possibility is that a significant interaction between the CB1 and non-CB1/CB2 receptors are required for the oleamide-induced mesenteric resistance artery dilation.
A recent report has provided evidence that oleamide vasorelaxation is partially endothelium-dependent (Hoi and Hiley, 2006). Our findings in the present study also demonstrate that the endothelium contributes to oleamide-mediated vasodilation. Interestingly, a portion of the oleamide vasodilation remained after endothelial removal. This suggests that oleamide-induced vasorelaxation is partially mediated by means other than the endothelium such as vascular smooth muscle cells and sensory nerves. Interestingly, both endothelium-dependent (Pratt et al., 1998; Chaytor et al., 1999) and endothelium-independent (Zygmunt et al., 1999) actions of the fatty acid amide, anandamide, have been reported in various vascular tissues. Next, we examined whether or not the cannabinoid receptor contribution was endothelium-dependent. The data demonstrate that the cannabinoid CB1 receptor antagonist, AM251, or the non-CB1/CB2 cannabinoid receptor antagonist, O-1918, did not further attenuate the vascular response to oleamide in endothelium-denuded rat mesenteric resistance arteries. This finding that the endothelium-dependent vasodilation in response to oleamide is mediated by cannabinoid receptor activation is in agreement with previous reports that have demonstrated endothelial localization of the cannabinoid CB1 receptor in the rat (Bátkai et al., 2004; Lepicier et al., 2007). Likewise, previous studies in the rat mesenteric arteries and human pulmonary arteries demonstrated endothelial localization of the non-CB1/CB2 cannabinoid receptor (Jarai et al., 1999; Kozlowka et al., 2007). Taken together, these findings demonstrate that the oleamide-induced vasorelaxation is mediated by endothelial cannabinoid receptors.
Another cell signaling mechanism known to be involved in fatty acid amide vascular responses are vanilloid receptors (Zygmunt et al., 1999; Harris et al., 2002). TRPV1 is a ligand-gated ion channel that is activated by multiple stimuli such as capsaicin, proton and heat (Tominaga et al., 1998). In the present study, the competitive antagonist for TRPV1, capsazepine, attenuated responses to oleamide in rat mesenteric resistance arteries. This is consistent with previous reports that TRPV1 receptors contribute to vascular responses evoked by fatty acid amides (Harris et al., 2002). Zygmunt et al. (1999) found that anandamide-induced responses were blocked by TRPV1 receptor and calcitonin gene related peptide (CGRP) antagonists and suggested that when activated by anandamide, TRPV1 receptors induce the release of CGRP and subsequent vasorelaxation. Experimental evidence demonstrates that the CGRP-mediated dilation in rabbit mesenteric arteries, cat cerebral arteries, and guinea pig pulmonary arteries is endothelium independent (Kakuyama et al., 1998; Mejia et al., 1988; Maggi et al., 1990). In agreement with vascular smooth muscle localization of TRPV1 receptors we found that capsazepine further attenuated oleamide-induced dilation in endothelium denuded mesenteric resistance arteries. We also found that the TRPV1 receptor protein is expressed in rat mesenteric resistance arteries and this result agrees with those of Wang et al. (2005). Overall, the present results indicate that TRPV1 receptors participate in an endothelial independent manner in oleamide-mediated dilation of mesenteric resistance arteries.
Next, we investigated whether or not an interaction between cannabinoid CB1 and TRPV1 receptors existed in the mesenteric resistance arteries since the cannabinoid CB1 and TRPV1 receptor antagonists partially attenuated the oleamide-mediated vasorelaxation. The combination of AM251 or AM251 and O-1918 and capsazepine did not further attenuate the vasodilatory response to oleamide. This is in agreement with a previous report that the sequential combination of cannabinoid CB1 and TRPV1 receptor inhibition did not produce additive or incremental hemodynamic effects compared with each drug alone in cirrhotic rats (Moezi et al., 2006). These findings would suggest that cannabinoid and TRPV1 receptors have overlapping functions in regards to vascular responses to oleamide.
Previous investigations have found NO involvement in cannabinoid receptor-mediated vasodilation of rat mesenteric arteries, rat aortic rings and bovine ophthalmic arteries (Hoi et al., 2007; Herradón et al., 2001; Romano and Lograno, 2006). Oleamide has also been shown to induce the Ca2+ activated K+ channel in mesenteric arteries (Hoi and Hiley, 2006). In the present study we evaluated the contribution of NO and K+ channels to oleamide-induced vasorelaxation. Mesenteric resistance artery relaxation in response to oleamide was attenuated by inhibition of NO synthesis as well as K+ channels. A previous report demonstrated that cannabinoid agonists induce the phosphorylation of endothelial nitric oxide synthase (eNOS) through activation of Akt resulting in the NO synthesis (Mukhopadhyay et al., 2002). In addition, anandamide-induced relaxation of rat mesenteric arteries has been shown to be sensitive to K+ channel blockers and high extracellular K+ levels (Randall et al., 1997). Additionally, endothelial generated NO has been shown to directly activate the Ca2+- activated K+ channels on vascular smooth muscle cells (Bolotina et al., 1994). Since the combination of L-NAME and TEA did not have additive effects on the mesenteric artery response to oleamide these findings along with those of previous studies support the notion that NO activation of K+ channels participates in oleamide-mediated vasodilation.
In conclusion, the results of the present study demonstrate that oleamide dose-dependently induces vasorelaxation in mesenteric resistance arteries and that this dilation is partially mediated by endothelial cannabinoid receptors and endothelial independent TRPV1 receptors. These mechanisms appear to overlap in respect to oleamide-induced mesenteric resistance artery dilation. We also provide evidence for a contribution of NO and K+ channels to the oleamide-induced mesenteric resistance artery dilation. Future studies will be required to determine the exact nature of the interactions between these mechanisms with respect to vascular responses evoked by the fatty acid amide, oleamide.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants HL-59699, DK38226 and an American Heart Association Established Investigator Award to J.D. Imig. We thank Dr. Ahmed Elmarakby for his assistance and advice.
References
- Adams MD, Earnhardt JT, Martin BR, Harris LS, Dewey WL, Razdan RK. A cannabinoid with cardiovascular activity but no overt behavioral effects. Experientia. 1977;33:1204–1205. doi: 10.1007/BF01922330. [DOI] [PubMed] [Google Scholar]
- Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, Offertáler L, Mackie K, Rudd MA, Bukoski RD, Kunos G. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Chaytor AT, Martin PE, Evans WH, Randall MD, Griffith TM. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J Physiol. 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Harris D, McCulloch AI, Kendall DA, Randall MD. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J Physiol. 2002;539:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herradón E, Martín MI, López-Miranda V. Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. Br J Pharmacol. 2007;152:699–708. doi: 10.1038/sj.bjp.0707404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CR, Hoi PM. Oleamide: a fatty acid amide signaling molecule in the cardiovascular system? Cardiovasc Drug Rev. 2007;25:46–60. doi: 10.1111/j.1527-3466.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- Hirasawa N, Santini F, Beaven MA. Activation of the mitogen-activated protein kinase/cytosolic phospholipase A2 pathway in a rat mast cell line. Indications of different pathways for release of arachidonic acid and secretory granules. J Immunol. 1995;154:5391–5402. [PubMed] [Google Scholar]
- Ho WSV, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi PM, Visintin C, Okuyama M, Gardiner SM, Kaup SS, Bennett T, Baker D, Selwood DL, Hiley CR. Vascular pharmacology of a novel cannabinoid-like compound, 3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide (VSN16) in the rat. Br J Pharmacol. 2007;152:751–764. doi: 10.1038/sj.bjp.0707470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi PM, Hiley CR. Vasorelaxant effects of oleamide in rat small mesenteric artery indicate action at a novel cannabinoid receptor. Br J Pharmacol. 2006;147:560–568. doi: 10.1038/sj.bjp.0706643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuyama M, Vallance P, Ahluwalia A. Endothelium-dependent sensory NANC vasodilatation: involvement of ATP, CGRP and a possible NO store. Br J Pharmacol. 1998;123:310–316. doi: 10.1038/sj.bjp.0701610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski H, Baranowska M, Schlicker E, Kozlowski M, Laudanski J, Malinowska B. Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J Hypertens. 2007;25:2240–2248. doi: 10.1097/HJH.0b013e3282ef7a0a. [DOI] [PubMed] [Google Scholar]
- Kruetz S, Kocj M, Bottger C, Ghadban C, Korf HW, Dehghani F. 2-Arachidonylglycerol elicits neuroprotective effects on excitotoxically lesioned dentate gyrus cells via abnormal-cannabidiol-sensitive receptors in microglial cells. Glia. 2009;57:286–294. doi: 10.1002/glia.20756. [DOI] [PubMed] [Google Scholar]
- Leggett JD, Aspley S, Beckett SR, D’Antona AM, Kendall DA, Kendall DA. Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br J Pharmacol. 2004;141:253–262. doi: 10.1038/sj.bjp.0705607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lépicier P, Lagneux C, Sirois MG, Lamontagne D. Endothelial CB1-receptors limit infarct size through NO formation in rat isolated hearts. Life Sci. 2007;8:1373–1380. doi: 10.1016/j.lfs.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca(2+)-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta. 1996;1302:55–60. doi: 10.1016/0005-2760(96)00002-1. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Lorenzon T, Bisogno T, Di Marzo V, Finazzi-Agrò A. Anandamide uptake by human endothelial cells and its regulation by nitric oxide. J Biol Chem. 2000;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Patacchini R, Perretti F, Tramontana M, Manzini S, Geppetti P, Santicioli P. Sensory nerves, vascular endothelium and neurogenic relaxation of the guinea-pig isolated pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:78–84. doi: 10.1007/BF00178976. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- Mejia JA, Pernow J, von Holst H, Rudehill A, Lundberg JM. Effects of neuropeptide Y, calcitonin gene-related peptide, substance P, and capsaicin on cerebral arteries in man and animals. J Neurosurg. 1988;69:913–918. doi: 10.3171/jns.1988.69.6.0913. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Basile AS. The hypnotic actions of oleamide are blocked by a cannabinoid receptor antagonist. Neuroreport. 1999;10:3237–3239. doi: 10.1097/00001756-199910190-00021. [DOI] [PubMed] [Google Scholar]
- Moezi L, Gaskari SA, Liu H, Baik SK, Dehpour AR, Lee SS. Anandamide mediates hyperdynamic circulation in cirrhotic rats via CB(1) and VR(1) receptors. Br J Pharmacol. 2006;149:898–908. doi: 10.1038/sj.bjp.0706928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Chapnick BM, Howlett AC. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol Heart Circ Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- Offertáler L, Mo FM, Bátkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Pratt PF, Hillard CJ, Edgemond WS, Campbell WB. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Physiol. 1998;274:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- Randall MD, McCulloch AI, Kendall DA. Comparative pharmacology of endothelium-derived hyperpolarizing factor and anandamide in rat isolated mesentery. Eur J Pharmacol. 1997;333:191–197. doi: 10.1016/s0014-2999(97)01137-0. [DOI] [PubMed] [Google Scholar]
- Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, Kemp PA, McCulloch AI, Kendall DA. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- Romano MR, Lograno MD. Cannabinoid agonists induce relaxation in the bovine ophthalmic artery: evidences for CB1 receptors, nitric oxide and potassium channels. Br J Pharmacol. 2006;147:917–925. doi: 10.1038/sj.bjp.0706687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol. 1997;142:278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kaminski NE, Wang DH. VR1-mediated depressor effects during high-salt intake: role of anandamide. Hypertension. 2005;46:986–991. doi: 10.1161/01.HYP.0000174596.95607.fd. [DOI] [PubMed] [Google Scholar]
- Wartmann M, Campbell D, Subramanian A, Burstein SH, Davis RJ. The MAP kinase signal transduction pathway is activated by the endogenous cannabinoid anandamide. FEBS Lett. 1995;359:133–136. doi: 10.1016/0014-5793(95)00027-7. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]