Abstract
Training exercises can improve perceptual sensitivities. We examined whether progressively training humans and rats to perform a difficult auditory identification task led to larger improvements than extensive training with highly similar sounds (the easy-to-hard effect). Practice improved humans’ ability to distinguish sounds regardless of the training regimen. However, progressively trained participants were more accurate and showed more generalization, despite significantly less training with the stimuli that were the most difficult to distinguish. Rats showed less capacity to improve with practice, but still benefited from progressive training. These findings indicate that transitioning from an easier to a more difficult task during training can facilitate, and in some cases may be essential for, auditory perceptual learning. The results are not predicted by an explanation that assumes interaction of generalized excitation and inhibition, but are consistent with a hierarchical account of perceptual learning in which the representational precision required to distinguish stimuli determines the mechanisms engaged during learning.
Keywords: transfer, discrimination learning, representation, perception
An organism’s ability to distinguish similar stimuli often depends on its experience. For example, pre-exposing an organism to pairs of shapes can facilitate the acquisition of discrimination learning tasks involving those shapes (E. J. Gibson & Walk, 1956). This phenomenon is referred to as perceptual learning (J. J. Gibson, 1959), which reliably improves performance for hours (Karni & Sagi, 1993) or days (Schoups & Orban, 1996). Several mechanisms have been proposed to underlie perceptual learning, including attentional weighting, stimulus imprinting, differentiation, and integration (Goldstone, 1998). Empirical support for these mechanisms comes from investigations of human category learning (Goldstone, 1994), attention (Czerwinski, Lightfoot, & Shiffrin, 1992), and animal discrimination learning (Riley, 1968).
Perceptual Learning
Most research on perceptual learning has focused on the visual system (Poggio, Fahle, & Edelman, 1992). Improvements in acuity after visual learning are often restricted to the particular spatial locations and stimulus features experienced during training (Fahle & Poggio, 2002). As a consequence, most researchers examining visual perceptual learning predict little transfer to new stimuli. Auditory discrimination training enhances the differentiation of sound features in humans and in other animals (Hawkey, Amitay, & Moore, 2004; Recanzone, Schreiner, & Merzenich, 1993). Unlike visual perceptual learning, the effects of auditory perceptual training seem to generalize to a wide variety of untrained sounds (Karmarkar & Buonomano, 2003).
Visual and auditory perceptual learning also differ with respect to the impact that task difficulty has on generalization. In visual perceptual learning, higher task difficulty has been associated with less transfer of training (Ahissar & Hochstein, 2000; Karni & Sagi, 1993). In contrast, generalization of auditory perceptual learning appears to be independent of the difficulty of the training task (Karmarkar & Buonomano, 2003).
The Easy-to-Hard Effect
The easy-to-hard effect refers to the perceptual learning phenomenon that early experience with an easy version of a discrimination task facilitates subsequent learning of a more difficult task involving stimuli that vary along the same dimension; initial training on the easier task usually produces better progress and final results than continuous training on the hard task. Pavlov (1927, p. 121–130) first reported this effect when he observed that progressively trained dogs could learn to distinguish between similar shades (e.g., light grey versus white) that they would fail to learn when trained directly with the harder stimulus contrast. Lawrence (1952) conducted the first systematic investigation of this effect by training rats on a simultaneous brightness discrimination task. Rats trained for forty trials on easier problems before being trained for forty trials on a more difficult discrimination, made fewer errors performing the difficult task than rats that were trained exclusively on the difficult task for eighty trials. The easy-to-hard effect observed by Lawrence differed from the effect reported by Pavlov in that all rats successfully learned to discriminate similar shades of gray, but rats trained first on an easy task required less training trials to reach the same level of performance as rats trained only on the hard task. Since then, variants of the easy-to-hard effect reported by Lawrence have been demonstrated with a wide variety of animals (e.g., rats, pigeons, rabbits, fish, octopuses, honeybees; Walker, Lee, & Bitterman, 1990) and in many sensory domains including: vision, audition, gustation, and magnetic sensitivity (Walker et al., 1990). The breadth of experimental contexts and animals showing the easy-to-hard effect suggests that it reflects a general principle of learning.
A recent report by McLaren and Suret (2000) is one of only a very small number of studies directly examining this phenomenon in humans. Instead of training individuals to discriminate simple stimuli, such as those used in past animal studies, the researchers constructed complex artificial dimensions by morphing between different faces. College students were first trained to perform either an easy or a hard face discrimination task before being tested with the hard contrast. Although both groups showed significant learning of the hard discrimination, the final level of performance was higher for those individuals that first learned the easy discrimination task, demonstrating an easy-to-hard effect. In the auditory domain, the easy-to-hard effect has only been directly demonstrated in humans discriminating the pitch of pure tones (Baker & Osgood, 1954). Direct investigations of the easy-to-hard effect in humans have all used single-session training with a relatively small numbers of trials (e.g., 80 trials in McLaren & Suret, 2000, and 240 trials in Barker & Osgood, 1954).
Despite the small number of studies directly investigating the easy-to-hard effect in humans, the basic principle has been implicitly integrated into various experimental procedures designed to maximize the benefits of training. This protocol is referred to in the literature as fading, which has been commonly used in studies involving training with speech discrimination (Tremblay & Kraus, 2002), non-native phoneme discrimination (McCandliss, Fiez, Protopapas, Conway, & McClelland, 2002) and development of cognitive skills (Anderson, Corbett, Koedinger, & Pelletier, 1995). Unlike the studies that have directly examined the easy-to-hard effect in humans, studies utilizing fading or adaptive training usually involve more than one training session and often spread learning across multiple days.
The underlying mechanisms of perceptual learning with single versus multi-session training might be very different. For example, it has been proposed that within-session fast learning is likely to reflect procedural learning that is task-specific, whereas slow learning across sessions indicates the gradual, possibly structural, modification of perceptual modules (Karni & Sagi, 1993). If that is the case, then it is possible that the easy-to-hard effect may be specific to, or modulated by, the relatively short training sessions used in most animal studies and all human studies that directly examine the phenomenon. Although it generally is assumed that the findings from these limited session training procedures generalize to multi-session training environments, this has not been directly tested in humans, nor has it been fully explored in other animals.
The Current Experiments
The current study was designed to directly examine the effects of progressive versus fixed training on the identification of complex sounds. The series of experiments allows us to compare the two types of training in single and multi-session training situations across two species (humans and rats). If the easy-to-hard effect reflects a basic principle of perceptual learning, and is independent of the amount of specific training, then we would expect to see an easy-to-hard effect in both single and multi-session learning situations across both species. If the effect is specific to, or modifiable by, the degree of training, then we might expect to see differences in the pattern of performance for single versus multi-session training. In either case, the easy-to-hard effect appears to be contrary to the common assumption that learning should transfer best when the training and test involve identical conditions (Baker & Osgood, 1954; Morris, Bransford, & Franks, 1977).
As with other research in perceptual learning, there are many unanswered questions about the easy-to-hard effect in the auditory domain. In view of the inherent complexity of most sounds in the natural environment (Fetterman, 1996), as well as the fact that the processes underlying perception of complex sounds are less well understood than those underlying perception of simple sounds, we chose frequency-modulated (FM) sounds as the stimuli in our identification tasks. Use of these sounds avoids the issue of previous experience and learning that may come with other types of complex sounds like speech. We focused on these sounds to examine whether the easy-to-hard effect applied generally to the perceptual learning of temporally complex auditory stimuli. In Experiment 1, we used these complex sounds and a progressive training regimen to replicate the basic easy-to-hard effect previously demonstrated in humans using photographic stimuli (McLaren & Suret, 2000).
Experiment 1: Single Session Training in Humans
In this experiment, participants were trained to identify FM sounds varying along a complex acoustic dimension. All participants were trained in a single session of 80 trials to distinguish either progressively more similar sounds (the Easy condition) or the most similar pair of sounds (the Hard condition). Participants were then tested on their ability to distinguish all of the sounds used in training across conditions. Given the robustness of the easy-to-hard effect reported in the animal literature, and the findings of McLaren & Suret (2000), we expected participants in the Easy condition to distinguish sounds more accurately.
Method
Participants
Twenty introductory psychology students (male:female = 1.22) from the University at Buffalo, SUNY, participated as partial fulfillment of their course requirements. Ten participants were randomly assigned to the Easy and Hard conditions, respectively. Two participants (one in each training group) were dropped and replaced: one in the Hard condition was dropped for never responding during the training session, and one in the Easy condition for performing significantly below chance on the overall test.
Acoustic stimuli and apparatus
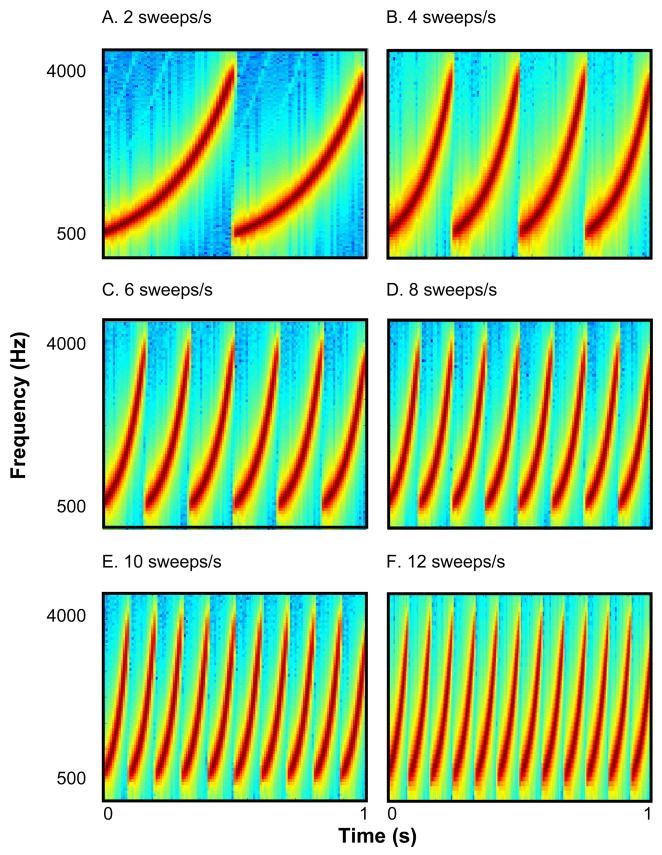
The stimuli were trains of repeated FM sweeps generated with Matlab 6.5 (MathWorks, Natick, MA). Each stimulus was made up of 2 – 12 non-overlapping sweeps, where each sweep was an ascent from 500 to 4000 Hz, or 3 octaves (see Figure 1). Because each stimulus was also 1 s long in duration, the more sweeps making up a stimulus, the shorter and steeper was each individual sweep. Thus, varying sweep rate actually changed multiple acoustic dimensions in parallel. These FM sounds, which can be thought of as the auditory equivalents of moving gratings in the visual domain (Rauschecker, 1998), strongly engage auditory cortical neurons in both owl monkeys (deCharms, Blake, & Merzenich, 1998), and rats (Orduña, Mercado, Gluck, & Merzenich, 2001). All FM sounds described here were also used in Experiment 2. In Experiment 1, only sweep rates of 10, 11, 11.5, and 12 sweeps/s were used.
Figure 1.
Spectrograms of selected sample stimuli used in Experiment 1 and 2.
Sound stimuli were presented, and keyboard button press responses were collected, using DMDX (Forster & Forster, 2003) running on HP Pavillion a300n, IBM compatible desktop computers. Audio-Technica, ATH-M40fs headphones were used to present the stimuli at normal conversational level to the listener.
Design and procedure
The experiment used a single factor between-participant design to compare the two types of training (Easy and Hard). Each participant experienced a training phase and a test phase. Both the training and test phases used a single-interval forced-choice identification task, with a 1 s inter-trial interval. During the training phase, participants heard 80 FM sounds and were asked to press the shift key labeled F if they heard a “fast” sound, and the shift key labeled S if they heard a “slow” sound. Participants were told to guess if they were unsure, and they were given feedback after each response. If participants did not respond within 5 s after the end of the stimulus, the words “no response” appeared on the screen and the program moved to the next trial. If the participant responded within 5 s, the word “correct” (or “wrong”) along with the reaction time appeared on the screen immediately after the response. Participants were informed at the beginning of the experiment that only one sound (12 sweeps/s) would be considered as “fast”, and that the slow sounds might vary during the training phase, and would certainly vary during the test phase. Participants were also informed that half of the sounds would be fast and half slow, randomly intermixed.
Participants in the Easy condition were progressively trained in three blocks. The program immediately moved from one block to the next without any delineation or delay cueing the participant to the change. Within each block, items were presented in a pseudorandom order so that no more than three stimuli requiring the same response appeared in a row. The three blocks contained 20, 20, and 40 trials, respectively, with equal numbers of slow and fast sounds in each block. In order, the slow sounds in the three blocks were 10, 11, and 11.5 sweeps/s, and the fast sound was always 12 sweeps/s. Participants in the Hard condition received a single block of 80 trials, with 40 trials of slow (11.5 sweeps/s) and fast (12 sweeps/s) sounds each. The most similar pair of sounds participants were trained with (11.5 versus 12 sweeps/s) will be referred to as the critical contrast in the rest of the paper.
A message appeared informing the participant that the training had ended after 80 trials, and instructing them to press the space bar to begin the test phase. The test phase consisted of 80 trials (40 fast trials and 40 slow trials) with no feedback, pseudorandomly intermixed in the same way as training. Of the 40 slow sounds, 10 were 10 sweeps/s, 10 were 11 sweeps/s, and 20 were 11.5 sweeps/s. The inter-trial interval and response window were the same as in training. Participants were debriefed at the end of the experiment.
Results
We used an alpha level of .05 for all significance tests. Because Experiment 1 was a conceptual replication of McLaren and Suret (2000), all tests were one-tailed. The percent correct and discriminability index (d′) for each group in both overall test performance and test performance on the critical contrast are presented in Table 1.
Table 1.
Mean Percent Correct (%) and Discriminability Index (d′) for Different Training Conditions in Experiment 1 (N = 20).
| Easy | Hard | |
|---|---|---|
| Overall test | ||
| % | 81 | 71 |
| d′ | 1.97 | 1.23 |
|
| ||
| Critical contrast | ||
| % | 68 | 61 |
| d′ | 1.25 | 0.65 |
To correct for potential response biases, all statistical comparisons used d′ as the dependent measure.1 We made two simple comparisons. First, we compared the overall test performance of participants in the Easy and Hard conditions. Participants in the Easy condition (who received the progressive training) identified sounds significantly better during the test phase, t(18) = 2.47, p = .01, d = 1.16. This is not surprising, because these participants were trained with all of the sounds used in the test phase, whereas participants in the Hard condition were only exposed to the 11.5 and 12 sweep/s sounds during training. The comparison of test performance with the critical contrast was of greater theoretical interest. Replicating the findings of McLaren and Suret (2000), we found significantly better performance when participants experienced easier training examples before being trained on the most difficult contrast, t(18) = 1.91, p = .04, d = 0.90. This was true even though participants in the Hard condition were trained to distinguish the 11.5 sweeps/s sound from the 12 sweeps/s sound for twice as many trials.
Discussion
In Experiment 1, we trained participants either progressively or specifically to distinguish a pair of highly similar complex sounds. Despite less experience with the critical contrast, progressively trained participants were better able to distinguish these sounds than participants trained exclusively on this task. This replicates McLaren and Suret’s (2000) finding of an easy-to-hard effect in humans after a single session of training, even though we used complex auditory stimuli instead of morphed faces, and used progressive training rather than training with a single pair of easily distinguishable stimuli. In conclusion, our results confirm that the complex sounds and progressive training regimen used in the current study can elicit an easy-to-hard effect in a single-session training protocol.
Experiment 2: Multi-Session Training in Humans
The goal of Experiment 2 was to extend the findings of Experiment 1 to multi-session training protocols. Instead of one training session of 80 trials, participants were trained, either progressively or specifically on the critical contrast, over 8 daily training sessions of 300 trials each. Additionally, we hoped to further explore the nature of perceptual learning in human audition. As noted earlier, research on visual and auditory perceptual learning has shown differences in the generalizability of learning in these two modalities. Perceptual learning in the visual domain has shown less generalization across stimuli, with the least generalization seen in difficult tasks. Auditory perceptual learning leads to greater generalization independent of task difficulty. To further examine how different training regimens impact generalization by humans, we used a different task to test participants’ ability to discriminate sounds than that used during training. Specifically, an oddball discrimination test was used the day before training began, and the day after the last training session. We also tested their ability to make distinctions that they had not been explicitly trained to make. This allowed us to examine how participants transferred learning during progressive versus specific training to new tasks and new stimuli, and it also allowed us to examine whether the effects of training lasted longer than had been previously investigated. Using a different test task from training also enabled us to rule out the potential confound that any observed improvement was caused by procedural learning.
We predicted that the multi-session training schedules would lead to auditory perceptual learning and to an easy-to-hard effect, even with a new task (oddball discrimination) and a one day delay between the last training session and the test. We also predicted that individuals trained in the Easy condition would show a greater capacity to distinguish untrained FM sounds than individuals trained in the Hard condition.
Method
Participants
Twenty-four normal hearing adults (mean age = 23.6, range = 19 – 36 years; male: female = 1.18) were recruited through ads. The two training conditions each had 9 participants; an additional Control condition had 6. All participants received monetary compensation for the total time they participated in this experiment.
Acoustic stimuli and apparatus
All FM sounds described in Experiment 1 were used. Stimulus sounds were generated using Tucker Davis Technologies (TDT) software and hardware, and delivered to the left ear at 75 dB SPL through an insert earphone, Etymotic Research (ER-3), in MX-41/AR cushions.
Design
Each participant in the Easy and Hard condition was trained for eight days, and tested the day before (pre-test) and the day after training (post-test). In pre- and post-tests, each participant’s ability to discriminate a 12 sweeps/s sound from other FM sounds with slower sweep rates was measured. For participants in the Control condition, the two days of pre- and post- tests were separated by eight days without any training session in between.
Pre- and post-tests
Pre- and post- tests took place in a sound attenuated booth, and consisted of 600 trials of a 3-alternative forced-choice (3AFC, oddball) task. The inter-trial and inter-stimulus intervals were 1 s and 500 ms, respectively. In each trial, two FM sounds of 12 sweeps/s (the standard sound) were always presented, along with one sound of a slower rate varying from 2 to 11.9 sweeps/s (the deviant sound). These three sounds were presented in random order. As each sound was presented, a red light-emitting diode (LED) above each button on a 3-button response box was lit corresponding to the concurrent interval (first, second, or third). Participants were asked to indicate the interval associated with the deviant sound by pressing the corresponding button; the response window was 10 s. The 15 deviant sounds used included the following sweep rates: 2, 4, 6, 8, 10, 11, 11.1, 11.2, 11.3, 11.4, 11.5, 11.6, 11.7, 11.8, and 11.9 sweeps/s. Each deviant was presented in 40 trials randomly distributed throughout the test. No feedback was provided after responding. Participants could take a break after every 100 trials. Most participants finished the test in less than 1.5 hr.
Training
Training also took place in the sound attenuated booth and lasted for eight daily sessions. Each training session consisted of three 100-trial blocks, and they typically took 30 minutes to complete. Participants could take a break between blocks. The training task was a single-interval, forced-choice identification task, the same as that used in Experiment 1. In each trial, participants were presented with one of the two possible sounds, and were instructed to classify the sound as either “slow” or “fast” by pressing the corresponding button on a response box. The response window was 10 s. A correct response would initiate the next trial, whereas an incorrect response would induce the LED light corresponding to the correct button to flash briefly before proceeding to the next trial. Of the 300 trials, half the sounds presented were “fast” and the other half were “slow”, presented in random order within each block.
Two training conditions were used. In the Easy condition, participants were initially trained to identify easily distinguishable FM sounds with sweep rates of 12 versus 8 sweeps/s (1 session). The sound contrast then became increasingly similar as training progressed across sessions, from 12 versus 10 sweeps/s (2 sessions), to 12 versus 11 sweeps/s (2 sessions), to 12 versus 11.3 sweeps/s (2 sessions), and finally to 12 versus 11.5 sweeps/s (1 session). In the Hard condition, participants were trained with the critical contrast (11.5 versus 12 sweeps/s) throughout the eight sessions. Participants in the Easy condition did not experience this difficult contrast until the last day of training.
Results
Test performance
The mean percent correct for pre- and post-tests at each deviant rate are listed in Table 2. In multiple-alternative forced-choice tasks, such as that used in our behavioral tests, response bias is conventionally assumed to be non-existent (Green & Swets, 1966), hence the proportion of correct responses can be used to measure discriminability. We therefore used percent correct to analyze test performance. Because all participants performed nearly at ceiling with deviant rates up to 8 sweep/s (see Table 2), performance in this range was excluded from all further analyses.
Table 2.
Mean Percent Correct (%) by Deviant Sweep Rate (sweeps/s) for Pre-test and Post-test in Different Conditions.
| Deviant sweep rate | Easy (n = 9) |
Hard (n = 9) |
Control (n = 6) |
|||
|---|---|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | Pre-test | Post-test | |
| 2 | 99 | 94 | 98 | 96 | 100 | 99 |
| 4 | 98 | 94 | 98 | 95 | 98 | 98 |
| 6 | 96 | 92 | 97 | 95 | 98 | 97 |
| 8 | 97 | 89 | 95 | 94 | 100 | 97 |
|
| ||||||
| 10 | 84 | 79 | 85 | 88 | 96 | 89 |
| 11 | 57 | 63 | 56 | 73 | 78 | 69 |
| 11.1 | 49 | 60 | 52 | 70 | 80 | 68 |
| 11.2 | 48 | 61 | 52 | 64 | 69 | 68 |
| 11.3 | 42 | 56 | 49 | 61 | 63 | 64 |
| 11.4 | 43 | 58 | 41 | 58 | 59 | 55 |
| 11.5 | 38 | 58 | 42 | 55 | 54 | 50 |
| 11.6 | 37 | 52 | 39 | 51 | 44 | 49 |
| 11.7 | 36 | 45 | 38 | 40 | 43 | 39 |
| 11.8 | 36 | 43 | 30 | 42 | 35 | 33 |
| 11.9 | 38 | 38 | 37 | 38 | 28 | 39 |
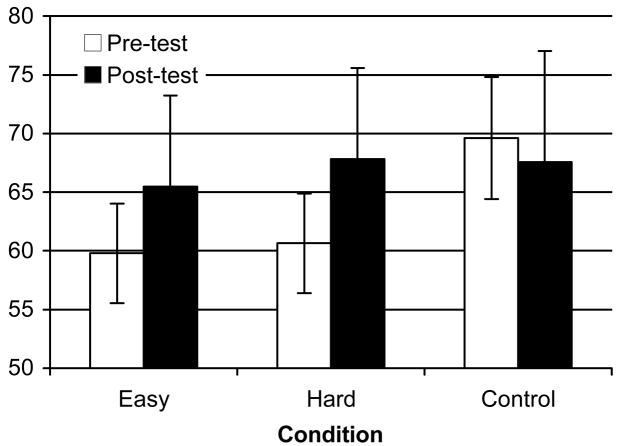
All statistical tests had an alpha level of .05 and were two-tailed. We first asked whether training facilitated performance in the post-test. Three paired-sample t-tests confirmed that post-test performance was significantly better than pre-test in the Easy condition, t(8) = 2.59, p = .03, d = 0.65, and the Hard condition, t(8) = 5.70, p = .00, d = 1.04, but not in the Control condition, t(5) < 1 (see Figure 2). This indicates that training was necessary for test improvement to take place. Therefore, the Control condition was dropped from further comparisons.
Figure 2.
Mean percent correct for pre- and post-tests across deviant sweep rates from 10 to 11.9 sweeps/s in the Easy (n = 9), Hard (n = 9), and Control (n = 6) conditions in Experiment 2. Error bars represent the 95% confidence intervals (CIs).
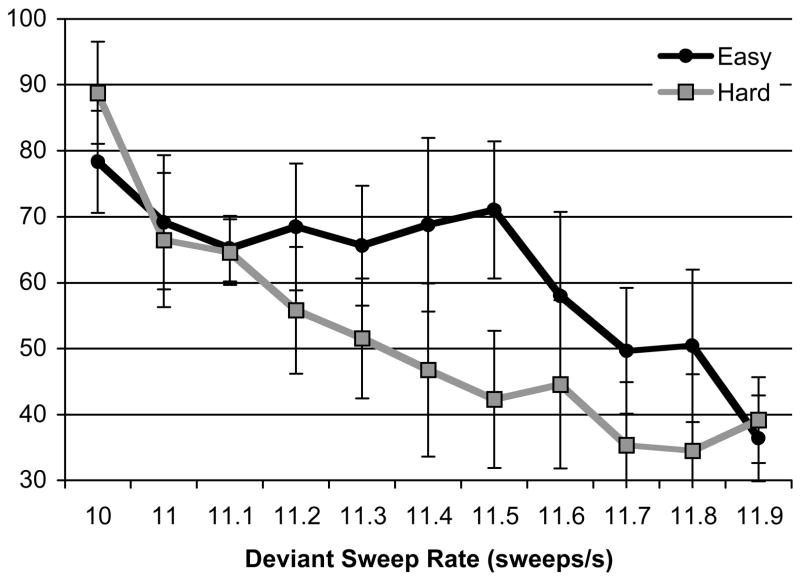
The effect of training was examined by entering individual post-test performances at each deviant rate into a 2 (Condition: Easy, Hard) × (11) (Deviant Rate: 10, 11, 11.1, 11.2, 11.3, 11.4, 11.5, 11.6, 11.7, 11.8, and 11.9 sweeps/s) mixed analysis of covariance (ANCOVA). Corresponding pre-test performances were entered as covariants in the analysis because pre-test significantly correlated with both post-test performances, r(16) = .81, p < .01, and the improvement from pre-test to post-test, r(16) = .47, p = .05. Therefore, simply subtracting pre-test from post-test performances could not fully partial out the influence of pre-test.2 The ANCOVA resulted in significant effects of Condition, F(1, 6) = 8.77, p = .03, ηp2 = .59, and Condition × Deviant Rate interaction, F(10, 60) = 3.42, p < .01, ηp2 = .36, and a non-significant effect of Deviant Rate, F(10, 60) = 1.61, p = .13, ηp2 = .21. In other words, after controlling for pre-test performance, participants in the Easy condition significantly outperformed those in the Hard condition in the post-test, showing an easy-to-hard effect. These results are shown in Figure 3.
Figure 3.
Adjusted mean percent correct in post-test as a function of deviant sweep rate (sweeps/s) in the Easy and Hard conditions (n = 9) in Experiment 2. The effect of pre-test performance was controlled by ANCOVA (percent correct at corresponding deviant rates in pre-test entered as covariates). Covariates appearing in the model for 10, 11, 11.1, 11.2, 11.3, 11.4, 11.5, 11.6, 11.7, 11.8, 11.9 sweeps/s were evaluated at 84.44, 56.25, 50.28, 49.72, 45.56, 42.08, 40.14, 38.06, 36.67, 33.19, and 37.50, respectively. Error bars represent the 95% confidence intervals (CIs).
Two planned contrasts were conducted to assess the generalization of training effects. First we examined performance on the critical contrast. Despite the fact that participants in the Hard condition had seven times more training with the 11.5 sweeps/s sound, in post-tests, participants in the Easy condition still showed better performance (71%) than those in the Hard condition (42%), t(6) = 6.78, p < .01, d = 5.54. This comparison revealed a very strong easy-to-hard effect. Next, we compared the mean performance on all untrained deviant rates (including 11.1, 11.2, 11.4, 11.6, 11.7, 11.8, and 11.9 sweeps/s). Again, participants in the Easy condition showed better performance with these untrained rates (57%) than those in the Hard condition (46%), t(6) = 2.62, p = .04, d = 2.14. This suggests that the easy-to-hard effect generalized to deviant rates that were not part of the training experience.
Training performance
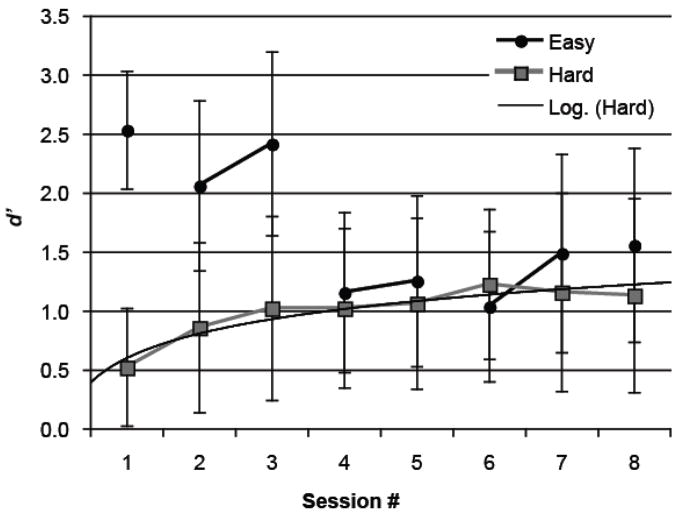
Training performance was analyzed for direct comparisons to experiments with rats. These results are less comparable to those reported in previous human studies because participants received feedback during training. As in Experiment 1, d′ was used to discount potential response biases in the training task (single-interval forced-choice identification). The percent correct data are presented in Table 3 for reference. The mean d′ values for each condition by the eight daily training sessions are presented in Figure 4. A planned comparison showed that, in the Hard condition, d′ was greater in Session 8 (d′ = 1.13) than in Session 1 (d′ = 0.52), t(8) = 4.85, p < .01, d = 3.43, indicating that training increased participants’ sensitivity to the minute differences between the similar FM sounds. Curve fitting for d′ in the Hard condition showed that more variation was accounted for by the logarithmic trend (R2 = .90) than the linear trend (R2 = .71).3 This indicates that improvement of d′ in the Hard condition was approaching asymptote rather than continuing to improve at a constant rate as training proceeded. Participants in the Easy condition showed a similar learning rate for training sessions involving identical stimulus contrasts across two consecutive days.
Table 3.
Mean Percent Correct (%) and the Sweep Rate (sweeps/s) of the Slow Sound by Training Session in Experiment 2 (n = 9).
| Easy |
Hard |
|||
|---|---|---|---|---|
| Training session | Slow sound (sweeps/s) | % | Slow sound (sweeps/s) | % |
| 1 | 8 | 94 | 11.5 | 63 |
| 2 | 10 | 89 | 11.5 | 67 |
| 3 | 10 | 91 | 11.5 | 71 |
| 4 | 11 | 76 | 11.5 | 71 |
| 5 | 11 | 78 | 11.5 | 71 |
| 6 | 11.3 | 75 | 11.5 | 75 |
| 7 | 11.3 | 79 | 11.5 | 73 |
| 8 | 11.5 | 79 | 11.5 | 75 |
Figure 4.
The discriminability index (d′) as a function of training session by condition in Experiment 2. Participants in the Hard condition (n = 9) were trained with the critical contrast (11.5 vs. 12 sweeps/s) throughout the eight sessions, whereas those in the Easy condition (n = 9) were trained with progressively more similar contrasts as training sessions proceeded from 8 vs.12 sweeps/s (Session 1), to 10 vs. 12 sweeps/s (Sessions 2 and 3), 11 vs. 12 sweeps/s (Sessions 4 and 5), 11.3 vs. 12 sweeps/s (Sessions 6 and 7), and finally to the critical 11.5 vs. 12 sweeps/s (Session 8). Sessions with the same sound contrast are connected by a line in the figure. The solid curve labeled as ‘Log. (Hard)’ represents the logarithmic trend fitted to the learning curve of the Hard condition.
To further compare the learning pattern in Session 8, we examined the performance change over the three blocks of training in this session. Because we had a small number of participants with great individual variability in each condition, and a large number of trials for each participant in each training session, we analyzed the data both by participant and by trial. The by-trial analysis enabled us to maximize statistical power by fully taking advantage of the large number of trials that participants engaged in during the training session.
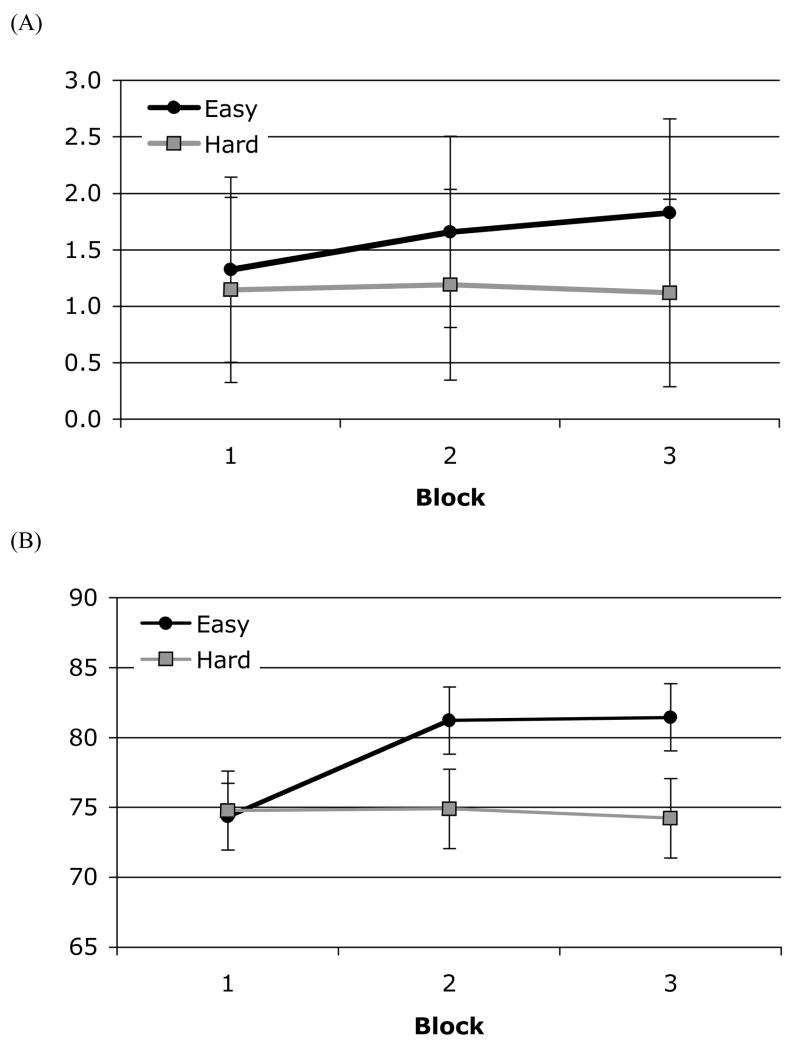
The d′ values in Session 8 were entered into a 2 (Condition: Easy, Hard) × (3) (Block: 1, 2, 3) mixed ANOVA for the by-participant analysis.4 Because individual trials do not have discriminibility or bias, the by-trial analysis used percent correct in a 3 (Block: 1, 2, 3) × (2) (Condition: Easy, Hard) mixed ANOVA. These analyses yielded no significant effect in the by-participants analysis, because of the lack of power, and significant effects in the by-trial analysis; Condition: F(1, 297) = 16.01, p < .01, ηp2 = .05; Block: F(2, 297) = 4.31, p = .01, ηp2 = .03; Block × Condition: F(2, 297) = 4.91, p = .01, ηp2 = .03. Three planned contrasts showed that participants in both conditions were equally accurate for trials in Block 1, t(198) < 1; participants in the Easy condition were significantly more accurate in Block 3 than Block 1, t(198) = 3.84, p < .01, d = 0.55; and participants in the Hard condition were not significantly more accurate in Block 3 than Block 1, t(198) < 1. The results are shown in Figure 5.
Figure 5.
Performance in Session 8 as a function of Block by Condition in Experiment 2. (A) The discrimination index (d′); error bars represents the 95% confidence intervals (CIs) in the by-participant analysis. (B) Percent correct; error bars represents the 95% confidence intervals (CIs) in the by-trial analysis.
In summary, changes in performance during training indicated that participants in the Hard condition approached an asymptote for distinguishing sounds in the critical contrast after 8 days of training. In contrast, progressively trained participants in the Easy condition were able to identify the same sounds at the same accuracy when they encountered the critical contrast for the first time, and their performance continued to improve over the three training blocks in the eighth training session.
Discussion
The results of Experiment 2 support the robustness of the easy-to-hard effect. Extending the results from Experiment 1 and previous studies, progressively trained individuals in the Easy condition showed greater ability to discriminate the critical contrast and more generalization to novel contrasts than did individuals trained in the Hard condition. Most importantly, a strong easy-to-hard effect was observed in the post-test, even though the test task was different from the training task, with a significant delay between the last training session and the test. These results confirm past reports that progressive training with complex sounds can significantly enhance discrimination of novel sounds with similar features (Merzenich et al., 1996), and demonstrate for the first time that the easy-to-hard advantage persists, even when individuals are trained for thousands of trials across several days, and tested a day later.
In terms of performance improvements during training, most participants in the Hard condition were able to gradually improve across sessions from their initial inaccurate performances, roughly following the power law of learning across the eight training sessions (as shown in Figure 4). It was more difficult to track improvements in acuity for participants in the Easy condition, because of the variability in stimulus contrasts across sessions. Whereas participants in the Hard condition were already approaching their learning asymptote, participants in the Easy condition were able to distinguish the critical contrast as well as participants in the Hard condition when they first encountered the contrast. This indicates that progressive training led to improvements in perceptual acuity. Participants in the Easy condition continued to improve their identification accuracy over the three blocks of the final training session, whereas participants in the Hard condition showed no improvement. These results provide the first direct evidence of a clear efficiency advantage of progressive training versus specific training in humans after extensive multi-session training.
It could be argued that the observed increases in performance accuracy reflect procedural learning (i.e., that participants became increasing familiar with the structure of the task during training). However, procedural learning cannot account for the differences in accuracy observed between pre-and post-tests, because the procedures used in these tests were not the same as those used during training, and no changes were observed for participants in the Control condition who had equal experience with the testing procedures. Furthermore, recent studies of auditory learning suggest that improvements during even the earliest stages of auditory training do not depend heavily on procedural learning mechanisms (Hawkey et al., 2004). A more parsimonious explanation for the observed effects is that participants’ ability to distinguish auditory temporal intervals improved during training, as has been observed in individuals trained to discriminate intervals between brief tones (Karmarkar & Buonomano, 2003). It is also possible that participants became more sensitive to differences in the duration of individual sweeps, differences in the rate of frequency modulation within sweeps, or to some combination of differences in acoustic features that varied between sounds. Increases in sensitivity to differences between complex sounds could reflect non-associative stimulus differentiation (E. J. Gibson & Levin, 1975), associative learning leading to acquired distinctiveness (James, 1890; Miller & Dollard, 1941), or both (Hall, 1991).
Experiment 3A: Multi-Session Training in Rats
In Experiment 3A, rats were trained to identify complex sounds varying in sweep repetition rate, using either a progressive or specific training schedule. As in Experiment 2, one group received progressive training that started with an easy identification task and that became more difficult across days, and the other group received training on a difficult identification task across all the days of training. The purpose of this experiment was to investigate whether rats faced with a difficult auditory identification problem would benefit from prior experience with easier stimulus contrasts. Specifically, we predicted that after progressive training, rats in the Easy condition would be able to identify highly similar sounds better than rats that were extensively trained to identify those specific sounds.
Method
Subjects
Subjects were 12 female Long-Evans rats approximately three months of age at the beginning of the experiment. Female rats were used because more is known about how their brains respond to complex sounds (Mercado, Bao, Orduña, Gluck, & Merzenich, 2001), and because females had been used in several similar behavioral experiments (Mercado, Orduna, & Nowak, 2005; Orduña, Mercado, Gluck, & Merzenich, 2005; Sun et al., 2005). Rats were housed two animals per cage under a 12 hr light/12 hr dark cycle (light period from 6:00 a.m. to 6:00 p.m.), and maintained at 90% of their free-feeding weight, with water freely accessible within their home cages.5
Acoustic stimuli
Four FM sounds, a 9 kHz tone, and a 200 Hz click train were chosen as the total stimulus set; all sounds lasted one second. The FM sounds contained sweeps repeated at rates of 2, 4, 8, or 12 sweeps/s. They were similar to those used in Experiment 2, but differed in the starting and ending frequency (from 2 to 16 kHz), and the overlap between 2 consecutive sweeps (33% frequency overlap versus none in the sounds used in Experiments 1 and 2). These acoustic parameters were selected based on the known hearing sensitivities of rats (Orduña et al., 2005).
Apparatus
Six operant chambers (described in Mercado et al., 2005) were enclosed within isolation cubicles. Each chamber contained a speaker, a nosing hole, two low profile retractable levers, a hooded house light, and a drop liquid dispenser. During the final stages of training, the nosing hole and drop receptacle were each located in the center section of an end wall, directly across from each other and the speaker was located directly above the nosing hole. Sounds were broadcasted above the rat’s head whenever the rat put its nose into the hole (breaking an infrared beam). Response levers were positioned to either side of the hole. Rats performed all trials in total darkness. A house light, positioned to the left of the drop receptacle in the upper corner of the chamber, illuminated the chamber only when rats pressed an incorrect lever. The liquid dispenser delivered one drop of condensed milk diluted with water (1:2 dilution) into the receptacle after a correct lever press.
The operant chambers were controlled and monitored using interface cards (MED Associates Model # DIG-712, DIG-726, DIG-700P2), a computer, and MED-PC software. Sound presentation was controlled using a custom-built system (described in Mercado et al., 2005) involving three real-time signal processors (TDT Model # RP2.1). Sounds were presented at ~75 dB SPL.
Training
Rats were first acclimated to the condensed milk by soaking their daily allotment of food pellets in the milk. The rats were then trained to feed from the liquid dispenser using a fixed time (15 – 30 s) schedule of reinforcement, and trained to press both levers using a modified autoshaping procedure. Reinforcement was one drop of milk throughout the experiment.
After stable pressing was established on both levers, rats were given stimulus control training in which a single lever was present, and lever presses were only reinforced when a sound was playing. Finally, rats were trained to identify sounds by pressing levers. Rats initiated trials using the nosing hole, which caused one of the two sounds to play repeatedly until the rat made a response (inter-stimulus interval = 3 s). Left lever presses were reinforced when one sound was presented, and right lever presses were reinforced when the second sound was presented. Incorrect responses terminated sound playback and initiated a 10 s period during which the house light was turned on and the nosing hole was inactivated. Lever presses made in the absence of sound had no programmed consequence. Rats were placed in the chambers for approximately 1.5 hour per daily session; each session consisted of about 300 trials, with a maximum limit of 400 trials. This number was chosen to match the condition in Experiment 2, in which human participants were trained with 300 practice trials daily. Order of sound presentation was pseudo-randomized within each session.
The rats were distributed into two groups of six. Each group received a total of 16 training sessions following either a progressive (the Easy condition) or a specific (the Hard condition) training schedule. Rats in the Easy condition were initially trained to associate left and right lever presses (12 sessions) with a tone or a click train, respectively, followed by identification training involving FM sounds with sweep repetition rates of 12 versus 2 sweeps/s (2 sessions), then 12 versus 4 sweeps/s (1 session), and finally 12 versus 8 sweeps/s (1 session). In the first eight sessions, training was facilitated through the use of correction trials and by biasing the distribution of trial types (cued identification) – these cues were discontinued for subsequent training sessions (un-cued identification). Tone and click trains were chosen for the initial training sessions because rats can identify tones versus clicks at ceiling levels of accuracy (Sun et al., 2005). Rats in the Hard condition were trained for 16 sessions with the most similar pair of sounds (8 versus 12 sweep/s) experienced by the first group. This contrast was chosen because rats are known to have difficulty differentiating these two FM sounds (Orduña et al., 2005). Again, the first eight sessions involved correction trials and trial-type biasing, whereas later sessions did not include these cues. Accurate choice responding in the last eight training sessions constituted the primary evidence for learning. To verify that rats in the Hard condition could hear and learn to identify sounds in the two-choice task, they were trained to identify tones and clicks after completion of the main experiment.
Results
All analyses were two-tailed, and the alpha level for significance was set at the .05 level. The experiment yielded data from 11 rats; one rat in the hard condition did not complete training because it did not learn to press the levers. Rats learned to press both levers after 3 – 10 sessions of autoshaping. Stimulus-control training was completed in 4 or 5 sessions. Rats completed an average of 161 trials (SD = 128) per session during the first eight sessions of cued identification training, and an average of 290 trials (SD = 86) per session during the final eight sessions (un-cued identification training). There was no significant difference in the number of trials per session completed by each group. All rats were able to distinguish tones from click trains with high accuracy, correctly identifying 80 – 90% of sounds on average.
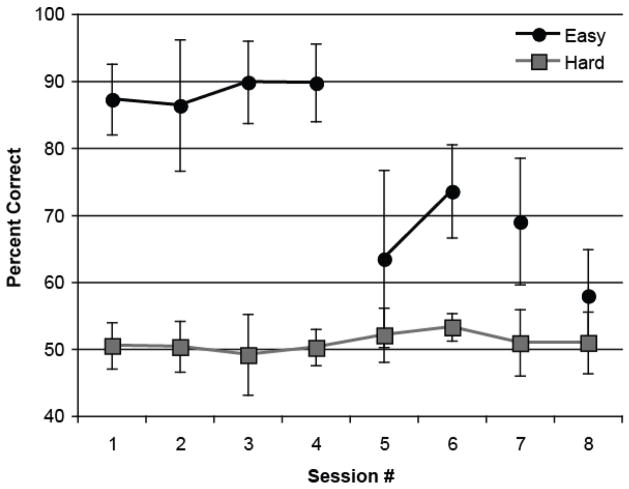
Rats in the Easy condition were able to distinguish 2 versus 12 sweeps/s (74% correct, d′ = 1.1 on average; Session 6) and 4 versus 12 sweeps/s (69% correct, d′ = 1.0 on average; Session 7), at levels significantly above chance; t(5) = 4.70, p = .01, d = 4.20, and t(5) = 4.67, p = .01, d = 4.17, respectively (see Figure 6). They also distinguished the 8 sweeps/s sound from the 12 sweeps/s sound at levels above chance (58% correct, d′ = 0.4 on average, t(5) = 2.60, p = .05, d = 2.33; Session 8). In contrast, rats in the Hard condition showed no evidence of learning during their attempts to distinguish the 8 sweeps/s sound from the 12 sweeps/s sound (51% correct on average; d′ = 0.14 in Session 1, d′ = 0.14 in Session 8), despite more than ten times as much training on this stimulus contrast (Figure 6).
Figure 6.
Identification accuracy as a function of session for rats in the Easy and Hard conditions in Experiment 3A. Rats in the Hard condition were trained with the same two FM sounds (8 vs. 12 sweeps/s) throughout the eight sessions, whereas those in the Easy condition were trained with progressively more similar contrasts as training sessions proceeded from tones vs. clicks (Sessions 1 – 4), to 2 vs. 12 sweeps/s FM sounds (Sessions 5 and 6), 4 vs. 12 sweeps/s (Sessions and 7), and finally to 8 vs. 12 sweeps/s sounds (Session 8). Sessions with the same sound contrast are connected by a line in the figure. Error bars represent the 95% confidence intervals (CIs).
To assess acquisition in Session 8, we divided the session into three blocks of 100 trials, and analyzed the data both by subject and by trial, as in Experiment 2. These analyses revealed no significant effect in the by-subjects analysis, because of the lack of power, and significant effects in the by-trial analysis; Condition: F(1, 297) = 14.65, p = .00, ηp2 = .05; Block: F(2, 297) = 0.02, p = .98; Block × Condition: F(2, 297) = 3.14, p = .04, ηp2 = .02. Three planned contrasts showed that subjects in both conditions were equally accurate during Block 1, t(198) < 1; and that subjects in the Easy condition were significantly more accurate than rats in the Hard condition in Blocks 2 and 3, t(198) = 3.11, p = .00, d = 0.44, and t(198) = 3.25, p = .00, d = 0.46, respectively.
Discussion
In Experiment 3A, rats that were initially trained to identify dissimilar sounds learned to perform a difficult auditory identification task, whereas rats that were extensively trained only on the difficult task failed to learn it. This finding is consistent with Pavlov’s (1927) early reports regarding the facilitation of conditioning associated with progressive training. Pavlov found that extensively training dogs to discriminate between a light gray and a white circle “failed to produce the slightest sign of differentiation” (Pavlov, 1927, p. 122), whereas complete differentiation was evident after only 20 conditioning trials when a much darker circle was initially contrasted with the white circle. Most past reports of easy-to-hard effects in animal discrimination learning simply showed that pre-training with an easier task facilitated acquisition (as first reported by Lawrence, 1952; for review, see Riley, 1968). Subjects in the Hard conditions of these prior experiments invariably learned to perform the difficult discrimination task. They simply did not perform the task as well as subjects with pre-training after equal numbers of training trials (an effect replicated in Experiments 1 and 2).
Support for the idea that progressive training can be necessary for improvement in some difficult perceptual tasks comes from human experiments showing that “impossible” visual discrimination tasks become possible after participants observe one example of an easier, related contrast (Ahissar & Hochstein, 1997). To our knowledge, in non-human species this finding has only been previously replicated in birds (Njegovan & Weisman, 1997). The current results similarly suggest that some auditory perceptual tasks may be impossible for rats to learn without prior exposure to more easily distinguishable contrasts.
It was surprising that rats’ performance in the Hard condition did not improve after thousands of training trials, because past experiments showed that most rats can distinguish an 8 sweeps/s FM sound from a 12 sweeps/s sound in generalization tests (Orduña et al., 2005). One possible explanation for the lack of learning in the current experiment is that the particular rats in the Hard condition had learning deficits or below average hearing capacities. This explanation seems implausible, however, because these same rats rapidly learned to classify tones and clicks, and in later experiments proved to be able to discriminate FM sounds with accuracy levels comparable to those of rats in the Easy condition (unpublished data). Another possibility is that rats in the Hard condition did not know the purpose of the task, because the sounds were highly similar and required attention to detect the difference. This possibility is addressed in Experiment 3B. An important issue that future studies of the easy-to-hard effect need to more directly address is exactly how similar two stimuli need to be before it becomes impossible for an individual to learn to distinguish them despite repeated attempts, and in what circumstances suitable training regimens can overcome such constraints.
Rats learned the two-choice auditory identification task in ways that differed from human participants in Experiments 1 and 2 (e.g., rats’ correct responses led to a food reward, and their errors led to a timeout). Nevertheless, the basic trends in group differences across Easy and Hard conditions during the final training session were similar for both humans and rats, with individuals in the Easy condition performing more accurately in both cases. However, human participants in the Hard condition of Experiment 2 differed from rats in that they made steady progress whereas the rats did not. Thus, the learning experiences of rats in the Hard condition are not directly comparable to those of humans in Experiment 2. Experiment 3B was conducted in an attempt to increase the similarity of learning experiences between rats and humans during training in the Hard condition, and to rule out the possibility that rats in the Hard condition simply did not learn that they would be rewarded for identifying specific sounds.
Experiment 3B
In Experiment 3A, rats that were trained exclusively on a task that required them to identify two highly similar complex sounds failed to learn to identify those sounds. In Experiment 3B, we performed a complementary study in which rats were trained to identify simpler tones varying only in frequency. The goal of this experiment was to decrease the difficulty of the auditory distinctions required to perform the task, thereby increasing the likelihood that rats in the Hard condition would learn to distinguish the sounds. All rats were pre-trained to ensure that they had learned the basic identification task before entering the Easy or Hard condition.
Method
Subjects
Subjects were twelve female Long-Evans rats approximately five months of age at the beginning of the experiment. Rats were housed two animals per cage under a 12 hr light/12 hr dark cycle (light period from 9:00 a.m. to 9:00 p.m.), and maintained at 90% of their free-feeding weight, with water freely accessible within their home cages.
Acoustic stimuli
Six tones (9, 8, 7.5, 2.5, 2, and 1 kHz) and a 200 Hz click train were chosen as the total stimulus set. Click trains and the 9 kHz tone were 1 s in duration during pre-training; during subsequent training sessions, tones played continuously until a response was made. Sounds were presented at ~75 dB SPL.
Training
Rats were divided into two groups and trained to perform the two choice identification task as in Experiment 3A; both groups were trained to distinguish a 9 kHz tone from a click train until performance accuracy reached ceiling levels. Rats in the Hard condition were then trained for two sessions to identify a 7.5 kHz tone and a 2.5 kHz tone (the critical contrast). Rats in the Easy condition were also trained for two sessions, progressing from training with 9 kHz versus 1 kHz (40 trials), to training with 8 kHz versus 2 kHz (~150 trials), before proceeding to training with 7.5 and 2.5 kHz. Subsequent to these two sessions, rats in both conditions were trained for six additional sessions on the critical contrast.
Results
All twelve rats completed training; rats learned to press both levers after 3 sessions of autoshaping, and stimulus-control training was completed in 4 sessions. All rats distinguished tones from clicks with high accuracy (84%, d′ = 2.0 on average).
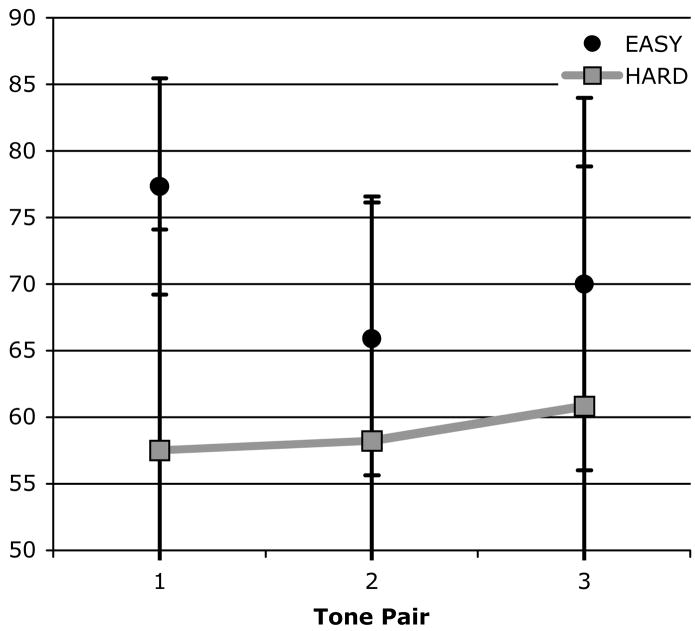
Rats in the Easy condition successfully distinguished 9 kHz from 1 kHz (77%, d′ = 1.6 on average), t(5) = 7.00, p = .00, d = 6.26, and 8 kHz from 2 kHz (66%, d′ = 0.8 on average), t(5) = 4.40, p = .01, d = 3.94. They were also able to distinguish a 7.5 kHz tone from a 2.5 kHz tone (70%, d′ = 1.1 on average), t(5) = 3.30, p = .02, d = 2.95. Rats in the Hard condition were also able to identify a 7.5 kHz and 2.5 kHz tone (61%, d′ = 0.6 on average), t(5) = 3.50, p = .02, d = 3.13. These results are summarized in Figure 7. A planned comparison of the d′ for rats in the Hard condition between the first 40 trials of the first training session and the last 40 trials of the second training session was not significant; i.e., there was no statistical evidence of improvement in the first two training sessions.
Figure 7.
Identification accuracy as a function of tone pairs for rats in the Easy and Hard conditions in Experiment 3B. Rats in the Hard condition identified the same two tones (Pairs 1-3: 7.5 and 2.5 kHz) throughout training, whereas those in the Easy condition were trained with progressively more similar tones as training proceeded (Pair 1: 9 and 1 kHz; Pair 2: 8 and 2 kHz; Pair 3: 7.5 and 2.5 kHz). Sessions with the same sound contrast are connected by a line in the figure. Error bars represent the 95% confidence intervals (CIs).
To assess acquisition, we divided initial training with the critical contrast into three blocks of 100 trials, and analyzed the data both by subject and by trial, as in Experiment 3A. These analyses revealed no significant effect in the by-subjects analysis, because of the lack of power, and a significant effect in the by-trial analysis; Condition: F(1, 297) = 9.61, p = .00, ηp2 = .03; Block: F(2, 297) = 1.19, p = .31; Block × Condition: F(2, 297) = 1.12, p = .33. Planned contrasts showed that for Blocks 1 and 2, rats in the Easy condition were significantly more accurate than those in the Hard condition, t(198) = 2.60, p = .01, d = 0.37, t(198) = 1.99, p = .05, d = 0.28, and that both groups were equally accurate during Block 3. After six additional training sessions with the critical contrast (~1400 trials), rats in the Easy condition were correctly classifying 66% of tones on average, and rats in the Hard condition were correctly classifying 72% of tones; the difference between groups was not significant. A planned comparison of the d′ between Session 1 and 8 in the Hard condition revealed a significant difference, t(5) = 3.85, p = .01, d = 3.44, indicating that rats improved in their ability to distinguish tones (d′ = 0.64 in Session 1, d′ = 1.2 in Session 8).
Discussion
Experiment 3B achieved the intended goal of providing rats in the Hard condition with a pair of sounds that they could identify at levels significantly above chance. Learning by rats in the Hard condition was similar to that of human participants in Experiment 2, who initially classified sounds with an accuracy of 60% correct, and gradually improved their performance to about 70% after thousands of training trials. As in Experiment 3A, rats in the Easy condition distinguished sounds from the critical contrast more accurately than rats in the Hard condition, despite much less experience identifying these sounds. However, in Experiment 3B, this easy-to-hard effect was shown after hundreds of trials rather than after thousands, and with simple tones rather than acoustically complex sounds, making it more comparable to past animal learning experiments examining this phenomenon. For example, Marsh (1969) found that pigeons trained for 350 trials on a difficult color discrimination task improved to about 60% correct, whereas pigeons initially trained for 150 trials on an easy task, followed by 150 trials on a discrimination of intermediate difficulty, performed the difficult discrimination task with an accuracy of about 75% after only 50 training trials.
The findings from Experiment 3A and 3B establish for the first time that the easy-to-hard effects described by both Pavlov (1927) and Lawrence (1952) can be demonstrated using a single task and species. In addition, the current experiment showed that, with extended training, rats in the Hard condition performed the critical contrast as well as those in the Easy condition. Together with Experiment 3A, this suggests that subjects in both conditions could be trained to perform a difficult task equally well with sufficient training, if they are able to distinguish the sounds in early training sessions. The current results also indicate that direct comparisons of auditory learning and generalization between rats and humans are feasible using a single-interval forced-choice identification task, and that progressive training is associated with easy-to-hard effects in both species.
General Discussion
The current study demonstrated that training humans and rats to identify complex sounds can facilitate their ability to differentiate those sounds. In Experiments 1 and 2, human participants that were progressively trained were able to identify highly similar FM sounds more accurately than individuals that were extensively trained to identify those sounds, and they showed greater generalization when tested on untrained distinctions post-training. Similarly, in Experiment 3, rats that were progressively trained on difficult auditory identification tasks were better able to distinguish highly similar sounds than rats that were trained only with those sounds. In the following discussion, we consider how these findings compare to those from past studies of visual learning and generalization, and what cross-species differences and similarities suggest about the mechanisms of perceptual learning and the easy-to-hard effect.
Comparisons Across Modalities
Past work has demonstrated that the easy-to-hard effect occurs in a wide range of situations. For example, Pavlov’s (1927) early reports of the benefits of progressive training include experiments involving complex visual, auditory, and somatosensory stimulus contrasts. Consequently, we were not surprised to find this effect in a complex auditory identification task. Not enough is yet known, however, to conclude that the easy-to-hard effect is modality-independent. Studies of perceptual learning in the visual domain have generated seemingly contradictory results, some of which differ qualitatively from the findings of auditory perceptual learning studies (Karmarkar & Buonomano, 2003). Similarly, a disproportionate number of studies have focused on simple stimulus contrasts that vary along a single unimodal (usually visual) dimension. In fact, the current study is the first since Pavlov’s original work to examine the easy-to-hard effect in non-humans learning to distinguish complex sound patterns. Perception of complex sounds can engage cortical networks that are less critical for discriminating simpler sounds, and that may have species-unique processing features. In the same vein, the neural structures involved in processing sensory inputs can vary substantially across modalities. Such differences in neural processing undoubtedly impact how organisms perceive the world, and may also constrain how perception can be affected by experience.
Past work in the visual domain suggests that task difficulty is a powerful determinant of how perceptual learning transfers (Ahissar & Hochstein, 2000), whereas studies in the auditory domain seem to indicate that good transfer is not dependent on task difficulty (Karmarkar & Buonomano, 2003; Merzenich et al., 1996). The current results lend some support to both of these perspectives. Human participants trained in the Easy condition showed better transfer than those trained in the Hard condition, as would be predicted from visual perceptual learning studies. At the same time, participants trained in the Hard condition showed significant levels of transfer to a wide range of stimulus contrasts in post-tests, as would be predicted from auditory perceptual learning studies. This suggests that the extent to which learning generalizes is not constrained by task difficulty per se, but may instead relate to the nature of the stimulus representations that are involved during learning. For example, training with stimuli that are spatially discrete and temporally transient (such as those often used in visual perceptual learning studies) may transfer less than training with stimuli that are temporally structured and spatially dispersed (such as speech sounds or periodic FM sounds). Additional studies will be needed, however, to tease apart the effects of stimulus dimensions, complexity, similarity, and modality on perceptual learning and the easy-to-hard effect.
Comparisons Across Species
The current experiments are the first to directly compare the effects of progressive training on auditory sensitivities in two species using highly similar methods. Past comparative assessments of the easy-to-hard effect have focused on qualitative similarities in visual learning by pigeons and humans (Mackintosh & Little, 1970; Suret & McLaren, 2003). However, these experiments involved very different stimuli and behavioral tasks across species, making comparisons difficult. In the current study, both humans and rats identified FM sounds that varied along identical acoustic dimensions, in a behavioral task in which all individuals were required to make a pressing response at one of two locations (left or right) to indicate their choice. The identification task required both species to associate particular sounds with highly similar behavioral responses (pressing buttons or levers located at different spatial positions). Trial pacing was self-initiated in all experiments, and both humans and rats completed a comparable number of training trials. The choice of an auditory rather than a visual task also increased the likelihood that both humans and rats were exposed to similar stimulus events, because sound reception is less dependent on head and receptor orientation than is light reception. Although it is impossible to equate the perceptual-motor experiences of humans and non-humans, minimizing procedural differences affords new opportunities for comparing learning capacity and mechanisms across species.
Most work on visual perceptual learning in humans has progressed independently of findings from studies in non-humans. This rift is attributable, at least in part, to methodological differences. Past investigations of discrimination learning in non-humans have focused almost exclusively on tasks in which a subject is conditioned to respond to some stimuli but not others. Consequently, explanations of learning-related changes in behavior during discrimination learning, including the easy-to-hard effect, have often focused on differences in excitatory and inhibitory generalization gradients (Logan, 1966; Riley, 1968; Singer, Zentall, & Riley, 1969). Few human researchers employ this type of differential reinforcement when training humans to discriminate stimulus events. The current approach addresses this methodological divide by removing the requirement that either rats or humans differentially inhibit responding based on stimulus differences. Instead, all subjects are required to spatially segregate their responses based on perceived differences in stimulus events. This further increases the likelihood that both species will engage comparable mechanisms while learning to identify sounds.
Associative Accounts of the Easy-to-Hard Effect
Three different proposals have been advanced to explain the easy-to-hard effect in animal discrimination learning studies. The first is that target and distracter stimuli each induce conditioned excitatory and inhibitory generalization gradients in the individual, and behavior is determined by the sum of the two. Because excitatory and inhibitory generalization gradients overlap more in a hard than an easy task, individuals learn slower due to the cancellation of the gradients (Logan, 1966; Pavlov, 1927). This explanation does a good job of accounting for numerous conditioning experiments, and is generally viewed as the simplest account of the easy-to-hard effect. The second explanation is that individuals learn to selectively attend to relevant dimensions and to ignore irrelevant dimensions (Lawrence, 1952; Mackintosh, 1965; 1975; Mackintosh & Little, 1970; Sutherland & Mackintosh, 1971). Attentional explanations focus on the relative weight given to separate stimulus processing channels independently of specific excitatory and inhibitory responses to stimuli. Finally, it has been suggested that the easy-to-hard effect may result from more general learning effects that are unrelated to stimulus features (Marsh, 1969; Eisenberger, 1992). For example, initial failures on a hard discrimination task could lead to confusion and frustration, causing subjects in the hard condition to lose interest in the task.
All of these proposed mechanisms could potentially contribute to easy-to-hard effects either in parallel, or at different stages of learning, depending on the particular learning context. In the current study, however, humans in the Hard condition improved steadily across training sessions, suggesting that the increased number of errors did not negatively impact their performance or ability to learn. Similarly, rats in the Hard condition voluntarily performed as many or more trials as rats in the Easy condition, which is inconsistent with the idea that they were becoming increasingly frustrated or disinterested in the task. Our findings from Experiment 2 are also inconsistent with the proposal that individuals in the Easy condition improved by learning to attend to a relevant stimulus dimension. If early training in the Easy condition increased the salience of a particular dimension, one would expect that the capacity to distinguish new contrasts varying along that same dimension would increase at a faster rate in later sessions. Contrary to this prediction, analyses of acquisition in the Easy condition of Experiment 2 revealed that learning rates were comparable to those observed for participants in the Hard condition. Riley (1968) similarly noted that progressive training rarely affects the rate at which subjects learn the more difficult discrimination, but instead simply raises initial performance levels at transfer (a stimulus generalization effect). Finally, the stimulus generalization account, which depends on differences between excitatory and inhibitory generalization gradients, predicts that there should be no easy-to-hard effect in any of the current experiments, because the single-interval forced-choice identification task is balanced in terms of excitatory and inhibitory associations.
A Hierarchical Account of the Easy-to-Hard Effect
A fourth possible explanation for the easy-to-hard effect is suggested by recent studies of visual perceptual learning and auditory category learning by humans. This work shows that task difficulty and the very nature of the stimuli may determine what mechanisms are engaged during the learning process (Ahissar & Hochstein, 1997; Guenther, Husain, Cohen, & Shinn-Cunningham, 1999; Lavie, Hirst, de Fockert, & Viding, 2004). For instance, the reverse hierarchy theory of perceptual learning proposes that learning of easy perceptual tasks depends on higher-level cortical areas, and proceeds to lower level cortical areas as task difficulty increases (Ahissar & Hochstein, 1997). Similarly, the load theory of attention proposes that tasks that approach the limits of perceptual capacity engage different attentional mechanisms than low load tasks (Lavie et al., 2004). The basic idea is that different mechanisms are engaged during processing of a particular perceptual event depending on whether fine distinctions between stimulus differences are required. In this framework, acquisition differs between the Easy and Hard conditions because different stimulus representations (with differing levels of detail) are being used to perform each task. Stated another way, the “dimension” that individuals selectively attend to during learning may correspond to levels of representational precision rather than to a particular continuum of stimulus features.
Recent work also suggests that the nature of the perceptual task can influence how learning progresses. For example, auditory categorization tasks lead to different changes in perceptual sensitivities than auditory discrimination tasks (Guenther et al., 1999), and auditory working memory tasks engage different neural processes than auditory reference memory tasks (Sakurai, 1994). The early stages of training in the Easy condition were more conducive to categorization by human participants than initial training in the Hard condition. Specifically, participants in the Easy condition labeled multiple FM sounds as “slow” across sessions, and in early training sessions the repetition rate of “slow” sounds was clearly slower than for the sound labeled as “fast.” In contrast, the two FM sounds experienced by participants in the Hard condition were so similar that participants likely viewed them as particular instances of a category that needed to be differentiated based on more subtle cues than those indicated by the provided labels. The model of Guenther and colleagues (1999) suggests that two different kinds of perceptual changes should occur during training in the Easy condition: compression of perceptual representations of “slow” FM sounds during early sessions, followed by differentiation of auditory representations later in training. Progressive training in the current experiments can thus be thought of as creating a sequential task continuum from category learning to discrimination learning. In contrast, the Hard condition should only lead to differentiation of stimulus representations as the participants learn to distinguish the two sounds. Categorization tends to be associated with activity in cortical regions beyond those that are devoted to differentiating fine details of stimuli, and therefore this model parallels the reverse hierarchy theory of perceptual learning.
These hierarchical frameworks also provide a possible explanation for the species differences observed in the current study. Specifically, if humans and rats differ in the levels of representational resolution that are available, or in their ability to learn categories, then these models predict that the rats and humans should differ in their ability to benefit from progressive training. In fact, it is well known that humans and other primates possess secondary auditory cortical fields with representational capacities not evident in rodents (see Rauschecker, 1998 for review). According to the reverse hierarchy theory of perceptual learning, these additional auditory cortical regions should enable humans to benefit from progressive training in ways that rats cannot. It is not yet known how rats and humans differ in their ability to learn categories, but the few experiments that have been conducted to date suggest that it is more difficult for rats to learn auditory categories than it is for humans (Mercado et al., 2005; Weisman, Williams, Cohen, Njegovan, & Sturdy, 2006). An important direction for future research will be to determine how differences in representational resolution and category learning capacities constrain the dynamics of generalization gradients.
One limitation of hierarchical accounts of perceptual learning is that although they predict when different cortical regions or classification mechanisms are likely to contribute to learning, they do not specify what happens when learning occurs. Perceptual learning often is associated with changes in the spatial organization and response properties of sensory cortical neurons (Buonomano & Merzenich, 1998; Feldman & Brecht, 2005; Weinberger, 2004). Although cortical changes are correlated with increased acuity (Recanzone, Schreiner, & Merzenich, 1993; Blake, Strata, Churchland, & Merzenich, 2002), and can impact generalization gradients (Orduña et al., 2005; Thompson, 1965), it remains unclear whether cortical reorganization is a cause or an effect of behavioral improvements (Talwar & Gerstein, 2001). If cortical plasticity does contribute to perceptual learning and the easy-to-hard effect, then hierarchical accounts predict that reorganization of primary sensory cortices is constrain5ed by processing in higher-level cortical areas.
Conclusion
Cross-species comparisons can provide insights into the minimal conditions necessary for perceptual learning and related phenomena such as the easy-to-hard effect, and can facilitate the characterization of neural mechanisms that mediate these phenomena. Findings from the current and past studies suggest that common learning mechanisms or constraints may underlie behavioral phenomena related to discrimination learning, selective attention, perceptual learning, and categorization, and that these mechanisms can be related to neural function and organization, as originally suggested by Pavlov (1927). Ultimately, a full understanding of the conditions that lead to generalization effects such as the easy-to-hard effect should make it possible to identify the factors that constrain learning in a given organism or individual, which in turn should make it easier to maximize the benefits of training, and may suggest new techniques for overcoming constraints on learning.
Acknowledgments
This research was supported by grants from the National Institute of Mental Heath (Grant MH 67952) and the National Science Foundation (Minority Postdoctoral Research Fellowship 0409087). We thank David Eddins and Ann Eddins for their help in stimulus construction and setting up the human experiment, as well as for providing access to their laboratory equipment. Chong Wang, Rachael Rubin, Patchouly Banks, Matt Wisniewski, Chloe Ruebeck, Julie Garringer, Jeaveen Neaderhiser, and Xiaojun Shan helped with data collection. We are also grateful to David Smith and three reviewers for helpful comments and suggestions on earlier versions of this article.
Footnotes
Hit rates and false alarm values by session were corrected for the calculation of d′. False alarm values of zero were replaced by 1/(twice the number of trials), since the true false alarm rate should be between zero and 1/(the number of trials). For the same reason, we replaced hit rates of 100 by 1 - [1/( twice the number of trials)]. We referred to Wixted and Lee (2004) for the standard correction and the formula used to calculate d′ in Excel.
ANCOVAs were conducted using both percent correct in post-test and the difference score between pre- and post-tests, with covariants of pre-test percent correct at corresponding sweep rates. Both analyses gave exactly the same pattern of significant results.
The function for the linear trend was y = 0.08x + 0.66, R2 = .71, and the function for the logarithmic trend was y = 0.30Ln(x) + 0.60, R2 = .90.
By-participant analyses were conducted using both d′ and percent correct. Analyses using both dependent measures produced exactly the same pattern of results as the by-trial analyses.
All procedures described conformed to NIH guidelines and were approved by the Institutional and Animal Care and Use Committee of the University at Buffalo, SUNY.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not thedefinitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/com.
References
- Ahissar M, Hochstein S. Task difficulty and visual hierarchy: reverse hierarchies in Sensory processing and perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. The spread of attention and learning in feature search: Effects of target distribution and task difficulty. Vision Research. 2000;40:1349–1364. doi: 10.1016/s0042-6989(00)00002-x. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Corbett AT, Koedinger KR, Pelletier R. Cognitive tutors: Lessons learned. The Journal of the Learning Sciences. 1995;4:167–207. [Google Scholar]
- Baker RA, Osgood SW. Discrimination transfer along a pitch continuum. Journal of Experimental Psychology. 1954;48:241–246. doi: 10.1037/h0059962. [DOI] [PubMed] [Google Scholar]
- Blake DT, Strata F, Churchland AK, Merzenich MM. Neural correlates of instrumental learning in primary auditory cortex. Proceedings of the National Academy of Sciences, USA. 2002;99:10114–10119. doi: 10.1073/pnas.092278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annual Review of Neuroscience. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Czerwinski M, Lightfoot N, Shiffrin RM. Automization and training in visual search. American Journal of Psychology. 1992;105:271–315. [PubMed] [Google Scholar]
- deCharms RC, Blake DT, Merzenich MM. Optimizing sound features for cortical neurons. Science. 1998;280:1439–1443. doi: 10.1126/science.280.5368.1439. [DOI] [PubMed] [Google Scholar]
- Eisenberger R. Learned industriousness. Psychological Review. 1992;99:248–267. doi: 10.1037/0033-295x.99.2.248. [DOI] [PubMed] [Google Scholar]
- Fahle M, Poggio T, editors. Perceptual Learning. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005 November 4;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Fetterman JG. Dimensions of stimulus complexity. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:3–18. [PubMed] [Google Scholar]
- Forster KL, Forster JC. DMDX: A windows display program with millisecond accuracy. Behaviour Research Methods, Instruments, and Computing. 2003;35:116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Gibson EJ, Levin H. The Psychology of Reading. Cambridge, MA: MIT Press; 1975. [Google Scholar]
- Gibson EJ, Walk RD. The effect of prolonged exposure to visually presented patterns on learning to discriminate them. Journal of Comparative and Physiological Psychology. 1956;49:239–242. doi: 10.1037/h0048274. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. Perception as a function of stimulation. In: Koch S, editor. Psychology: A Study of A Science. Vol. 1. New York: McGraw-Hill; 1959. pp. 456–501. [Google Scholar]
- Goldstone RL. Influences of categorization on perceptual discrimination. Journal of Experimental Psychology: General. 1994;123:178–200. doi: 10.1037//0096-3445.123.2.178. [DOI] [PubMed] [Google Scholar]
- Goldstone RL. Perceptual learning. Annual Review of Psychology. 1998;49:585–612. doi: 10.1146/annurev.psych.49.1.585. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Guenther FH, Husain FT, Cohen MA, Shinn-Cunningham BG. Effects of categorization and discrimination training on auditory perceptual space. Journal of the Acoustical Society of America. 1999;106:2900–2912. doi: 10.1121/1.428112. [DOI] [PubMed] [Google Scholar]
- Hall G. Perceptual and Associative Learning. New York: Oxford University Press; 1991. [Google Scholar]
- Hawkey DJC, Amitay S, Moore DR. Early and rapid perceptual learning. Nature Neuroscience. 2004;7:1055–1056. doi: 10.1038/nn1315. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. New York: Henry Holt & Co; 1890. [Google Scholar]
- Karmarkar UR, Buonomano DV. Temporal specificity of perceptual learning in an auditory discrimination task. Learning and Memory. 2003;10:141–147. doi: 10.1101/lm.55503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lawrence DH. The transfer of a discrimination along a continuum. Journal of Comparative and Physiological Psychology. 1952;45:511–516. doi: 10.1037/h0057135. [DOI] [PubMed] [Google Scholar]
- Logan FA. Transfer of discrimination. Journal of Experimental Psychology. 1966;71:616–618. doi: 10.1037/h0023012. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. Selective attention in animal discrimination learning. Psychological Bulletin. 1965;71:616–618. doi: 10.1037/h0022347. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Mackintosh NJ, Little L. An analysis of transfer along a continuum. Canadian Journal of Psychology. 1970;24:362–369. doi: 10.1037/h0082872. [DOI] [PubMed] [Google Scholar]
- Marsh G. An evaluation of three explanations for the transfer of discrimination effect. Journal of Comparative and Physiological Psychology. 1969;68:268–275. [Google Scholar]
- McCandliss BD, Fiez JA, Protopapas A, Conway M, McClelland JL. Success and failure in teaching the [r]–[l] contrast to Japanese adults: Tests of a Hebbian model of plasticity and stabilization in spoken language perception. Cognitive, Affective, and Behavioral Neuroscience. 2002;2:89–108. doi: 10.3758/cabn.2.2.89. [DOI] [PubMed] [Google Scholar]
- McLaren IPL, Suret M. Transfer Along a Continuum: Differentiation or Association?; Paper presented at the Twenty-Second Annual Conference of the Cognitive Science Society; NJ: LEA. 2000. [Google Scholar]
- Mercado E, III, Bao S, Orduña I, Gluck MA, Merzenich MM. Basal forebrain stimulation changes cortical sensitivities to complex sound. Neuroreport. 2001;12:2283–2288. doi: 10.1097/00001756-200107200-00047. [DOI] [PubMed] [Google Scholar]
- Mercado E, III, Orduna I, Nowak JN. Auditory categorization of complex sounds by rats. Journal of Comparative Psychology. 2005;119:90–98. doi: 10.1037/0735-7036.119.1.90. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Jenkins WM, Johnston P, Schreiner C, Miller SL, Tallal P. Temporal processing deficits of language-learning impaired children ameliorated by training. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- Miller NE, Dollard J. Social Learning and Imitation. New Haven: Yale University Press; 1941. [Google Scholar]
- Morris CD, Bransford JD, Franks JJ. Levels of processing versus transfer-appropriate processing. Journal of Verbal Learning and Verbal Behavior. 1977;16:519–533. [Google Scholar]
- Njegovan M, Weisman R. Pitch discrimination in field- and isolation-reared blackcapped chickadees (Parus atricapillus) Journal of Comparative Psychology. 1997;111:294–301. [Google Scholar]
- Orduña I, Mercado E, Gluck MA, Merzenich MM. Spectrotemporal sensitivities in rat auditory cortical neurons. Hearing Research. 2001;160:47–57. doi: 10.1016/s0378-5955(01)00339-2. [DOI] [PubMed] [Google Scholar]
- Orduña I, Mercado E, Gluck MA, Merzenich MM. Cortical responses in rats predict perceptual sensitivities to complex sounds. Behavioral Neuroscience. 2005;119:256–264. doi: 10.1037/0735-7044.119.1.256. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Clarendon Press; 1927. [Google Scholar]
- Poggio T, Fahle M, Edelman S. Fast perceptual learning in visual hyperacuity. Science. 1992;256:1018–1021. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiology and Neurotology. 1998;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. Journal of Neuroscience. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley DA. Discrimination Learning. Boston: Allyn and Bacon; 1968. [Google Scholar]
- Sakurai Y. Involvement of auditory cortical and hippocampal neurons in auditory working memory and reference memory in the rat. Journal of Neuroscience. 1994;14:2606–2623. doi: 10.1523/JNEUROSCI.14-05-02606.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups AA, Orban GA. Interocular transfer in perceptual learning of a pop-out discrimination task. Proceedings of the National Academy of Sciences of the United States of America, USA. 1996;93:7358–7362. doi: 10.1073/pnas.93.14.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B, Zentall T, Riley DA. Stimulus generalization and the easy-to-hard effect. Journal of Comparative and Physiological Psychology. 1969;69:528–535. [Google Scholar]
- Sun W, Mercado E, III, Wang P, Shan X, Te-Chung L, Salvi RJ. Changes in NMDA receptor expression in auditory cortex after learning. Neuroscience Letters. 2005;374:63–68. doi: 10.1016/j.neulet.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Suret MB, McLaren IPL. Representation and discrimination along an artificial dimension. The Quarterly Journal of Experimental Psychology. 2003;56B:30–42. doi: 10.1080/02724990244000142. [DOI] [PubMed] [Google Scholar]
- Sutherland NS, Mackintosh J. Mechanisms of Animal Discrimination Learning. New York: Academic Press; 1971. [Google Scholar]
- Talwar SK, Gerstein GL. Reorganization in awake rat auditory cortex by local microstimulation and its effect on frequency-discrimination behavior. Journal of Neurophysiology. 2001;86:1555–1572. doi: 10.1152/jn.2001.86.4.1555. [DOI] [PubMed] [Google Scholar]
- Thompson RF. The neural basis of stimulus generalization. In: Mostofsky DI, editor. Stimulus generalization. Stanford, CA: Stanford University Press; 1965. pp. 154–178. [Google Scholar]
- Tremblay K, Kraus N. Auditory training induces asymmetrical changes in cortical neural activity. Journal of Speech, Language, and Hearing Research. 2002;45:564–572. doi: 10.1044/1092-4388(2002/045). [DOI] [PubMed] [Google Scholar]
- Walker MM, Lee Y, Bitterman ME. Transfer along a continuum in the discriminative learning of Honeybees (Apis mellifera) Journal of Comparative Psychology. 1990;104:66–70. [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nature Reviews Neuroscience. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman RG, Williams MT, Cohen JS, Njegovan MJ, Sturdy CB. The comparative psychology of absolute pitch. In: Wasserman EA, Zentall TR, editors. Comparative Cognition: Experimental Explorations of Animal Intelligence. New York: Oxford; 2006. [Google Scholar]
- Wixted J, Lee K. Signal detection theory. 2004 Retrieved October 12, 2006, from University of California, San Diego, Department of Psychology Web site: http://psy.ucsd.edu/~kang/sdt/sdt.htm.