Abstract
Cutaneous allodynia is common in migraine. In the majority of previous studies on allodynia in migraine, only patients with episodic migraine (EM) were included. Little is known on patterns of allodynia in chronic migraine (CM). Since the presence of allodynia is associated with a poor response to triptans, a clinically practical method to test migraine patients for allodynia would be useful to the clinician. The aim of this study was to assess the prevalence of dynamic mechanical (brush) allodynia (BA) in CM, using a clinically practical method. Eighty-nine CM patients were prospectively recruited. Patients were given a structured questionnaire regarding demographic data and migraine characteristics. Allodynia was tested using a 10 × 10-cm gauze pad to brush various areas of the skin lightly. The prevalence of BA in the entire study population and in different patient subgroups was calculated. BA was present in 42.7% (38/89) of the patients. The presence of allodynia was unrelated to age, disease duration or to the occurrence of an acute headache exacerbation at the time of testing. Allodynia was positively associated with a history of migraine aura. BA was most common in the cephalic area, but was also seen in cervical dermatomes. BA is common in CM and, unlike in EM, is not significantly affected by the occurrence of an acute headache exacerbation. This suggests that central trigeminovascular neurons are chronically sensitized in patients experiencing migraine headache >15 days per month. The testing of BA in the clinical setting is possible using a simple and brief approach. It allows the clinician to determine whether the patient is sensitized, a diagnosis that affects treatment decisions.
Keywords: Allodynia, migraine, sensitization, trigeminal
Introduction
Cutaneous allodynia is the perception of pain when a non-noxious stimulus is applied to normal skin (1). Scalp and muscle tenderness have been recognized in migraine, and 60–80% of migraine patients have cutaneous allodynia during an acute attack (2–6). The underlying mechanism of cutaneous allodynia in migraine is thought to be sensitization of second-order neurons in the trigeminal nucleus caudalis (TNC) (7–10). Migraine patients experience both mechanical and thermal allodynia. Mechanical allodynia can be detected using light brush (dynamic) or steady pressure (static) cutaneous stimuli. Thermal allodynia can be detected using heat or cold stimulation. Patients’ hypersensitivity to heat, cold or mechanical stimulation can be selective (i.e. heat allodynia only) or non-selective (i.e. heat, cold and mechanical allodynia), suggesting that the different sensory modalities are mediated differently in the dorsal horn (5, 11). Allodynia in migraine is usually found in cephalic areas; however, it may also occur in extracephalic regions, suggesting sensitization of trigeminovascular neurons that process sensory information arising from skin of various body regions (10, 12).
The presence of cutaneous allodynia during an acute migraine attack is associated with significantly reduced efficacy of treatment with the 5HT1B/1D agonists known as triptans (6). In allodynic patients, triptans were significantly more effective in relieving headache when given before, rather than after, cutaneous allodynia was established. In non-allodynic patients, however, treatment with triptans was equally effective when given early (1 h after the onset of headache) or late (4 h after headache onset) during an acute attack. This observation has been supported by an electrophysiological study in a rat model of migraine. After the dura was irritated by inflammatory mediators, sumatriptan prevented the sensitization of neurons in the TNC when given early, but failed to do so when given late (13). Cutaneous allodynia is therefore an important phenomenon not only from the pathophysiological aspect but also therapeutically. Recognizing allodynia can help clinicians select the optimal treatment for migraine patients. In the few studies of allodynia in migraine, patients were evaluated using either quantitative sensory testing (QST) (5) or a questionnaire (14, 15). QST is a lengthy process which requires sophisticated equipment, whereas a questionnaire is subjective. A practical objective tool is therefore needed to assess cutaneous allodynia in migraine in a clinical setting. The current study describes a simple, brief and reliable way to assess migraine patients for dynamic mechanical (brush) allodynia in the clinic.
Methods
This study was approved by the Institutional Review Board for Studies in Human Subjects of Thomas Jefferson University Hospital. We recruited men or women, ≥16 years old, with International Headache Society (IHS)-defined chronic migraine (CM), from our out-patient clinic (16). Included patients were allowed to be on migraine-preventive drugs. Patients with neurological diseases that could cause sensory abnormalities (e.g. peripheral neuropathy, multiple sclerosis) and patients with dermatological diseases that could cause abnormal skin sensation were excluded. Patients who had been treated with botulinum toxin during the 3-month period prior to allodynia testing were also excluded. All patients provided informed consent prior to enrolment in the study.
Patients were given a structured questionnaire to obtain demographic data, migraine history (age of onset, disease duration), average attack frequency and duration, headache characteristics (severity, location, pain character) and associated symptoms. Questions regarding a history of IHS-defined aura of any type were included.
The assessment of allodynia was made at a single point in time. Some patients were evaluated during an acute headache exacerbation and others were evaluated between exacerbations, when having the baseline headache. Brush allodynia (BA) was tested by lightly brushing a 10 × 10-cm gauze pad over a 50-mm area of skin, at a rate of 2/s, for a total of 10 times (17, 18). Six skin areas were stimulated: right frontal (V1), left frontal, right posterior cervical (C2—C3), left posterior cervical, right medial forearm (C8) and left medial forearm. For each site, patients were asked to assess the degree of pain (if any) evoked by the gauze pad application to the skin, using a 100-mm visual analogue scale (VAS). The allodynia score at each site ranged from 0 to 100. In a pilot study, 10 healthy subjects who did not suffer from headaches were tested for BA using the procedure described above. All subjects had a total allodynia score of zero. Therefore, in our study, patients who recorded a VAS allodynia score greater that zero at one or more sites were considered to be allodynic. A total allodynia score was calculated as the sum of the six site-specific VAS scores (maximum possible total score: 6 × 100 = 600). The prevalence of allodynia in the entire study population, as well as in different patient subgroups, was calculated. Subgroups were defined based on the presence or absence of a history of aura and on the presence or absence of an acute headache exacerbation at the time of allodynia testing. The intensity of headache pain at the time of testing (current headache pain), as measured using an 11-point verbal scale, was documented.
Statistical analysis
χ2 tests were used to compare the prevalence of allodynia among different subgroups of chronic migraine patients. Spearman correlation was used to evaluate the association between the total allodynia score and current headache pain. Fisher’s exact test was used to study the relationship between the laterality of allodynia and the laterality of the current headache pain. For those patients who had allodynia at all three dermatomes (at either right or left, or both) (i.e. V1, C2—C3 and C8), the Friedman test was used to compare the median maximal allodynia score among dermatomes. For a given dermatome, the median maximal score was defined as the median of the maximum allodynia scores at that dermatome. All statistical tests were performed using Stata/SE 9.0 and with two-tailed α = 0.05.
Results
Eighty nine patients (75 women and 14 men) were included in the study. Their mean (±SD) age was 44.5 ± 11.9 years. Thirty-five patients were tested for allodynia while they were having an acute headache exacerbation and 54 were tested when they had their baseline headache. Thirty-five patients (39%) reported experiencing auras in the past, whereas 54 patients (61%) did not. There was no significant difference in age or in disease duration between allodynic and non-allodynic patients [mean (±SD) age: allodynic 43.3 ± 12.2 years, non-allodynic 45.4 ± 11.7 years (P = 0.49, Wilcoxon rank sum test); mean (±SD) disease duration: allodynic 23.7 ± 16.9 years, non-allodynic 25.0 ± 13.0 years (P = 0.57, Wilcoxon rank sum test)].
Seventy-one (80%) patients were taking headache-preventive drugs at the time of the study (Table 1). Twenty-nine (33%) patients were on a single preventive drug and 42 (47%) were on multiple preventives. The most commonly used preventive drugs were anticonvulsants [49 patients (55%)] and antidepressants [40 patients (45%)]. The prevalence of allodynia in patients who used preventives was 39% vs. 55% allodynic patients among those who did not use preventives (P = 0.22). Allodynia score was not related to the number of preventive drugs used (P = 0.6, Kruskal—Wallis test) (Table 2). Allodynia was also not related to the type of headache preventive drug used (data not shown).
Table 1.
Types of headache-preventive medications used by the patients
| Preventive medication class | No. of patients (%) |
|---|---|
| Anticonvulsants (topiramate, valproic acid, lamotrigine, zonisamide, gabapentin) | 49 (55%) |
| Antidepressants (nortriptyline, amitriptyline, desipramine, venlafaxine, fluoxetine) | 40 (45%) |
| Antipsychotics (quetiapine, olanzapine, ziprasidone, aripiprazole) | 13 (15%) |
| Vitamins and minerals (riboflavin, magnesium, coenzyme Q 10) | 13 (15%) |
| β-Blockers (propranolol, timolol, metoprolol) | 7 (8%) |
| Non-steroidal anti-inflammatory drugs (indomethacin, celecoxib) | 6 (7%) |
| Calcium channel blockers (verapamil, diltiazem) | 5 (6%) |
| Muscle relaxants (tizanidine, carisoprodol) | 3 (3%) |
| Antiserotonergics (methylergonovine) | 2 (2%) |
Percentages sum to greater than 100% since patients may have used more than one preventive drug.
Table 2.
Mean allodynia score by number of preventive drugs
| No. of preventives |
No. (%) of patients |
Allodynia VAS score (mean ± SD) |
|---|---|---|
| 0 | 18 (20.2%) | 46.5 ± 74.2 |
| 1 | 29 (32.6%) | 31.7 ± 60.2 |
| 2 | 25 (28.1%) | 55.1 ± 127.8 |
| 3 | 11 (12.4%) | 34.6 ± 53.5 |
| 4+ | 6 (6.7%) | 10.3 ± 16.3 |
VAS, Visual analogue scale.
The presence of allodynia was neither associated with gender nor with the presence of photophobia or phonophobia .
Allodynia during an acute exacerbation and in between exacerbations
Overall, 38 (43%) patients exhibited brush allodynia. Figure 1 depicts the prevalence of BA in patients that were tested during an acute headache exacerbation relative to that of patients tested between exacerbations. Sixteen (45.7%) of the 35 patients who were tested during an acute exacerbation were allodynic, compared with 22 (40.7%) allodynic patients of those who were tested when they had their baseline headache. There was no significant difference in the prevalence of allodynia between these groups .
Figure 1.
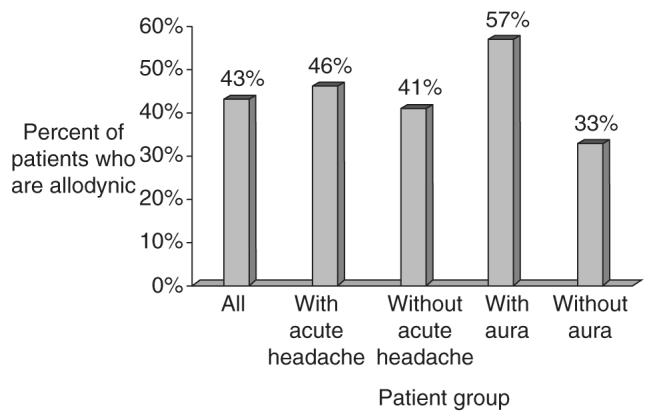
Allodynia prevalence in the entire study population and in the different patient subgroups.
Cutaneous allodynia and history of aura
Figure 1 also depicts the relationship between the prevalence of allodynia and history of migraine aura. Twenty (57.1%) of the 35 patients who reported a history of aura had BA. In comparison, 18 (33.3%) of the 54 patients who did not report a history of aura were allodynic. The difference in the prevalence of allodynia between these two groups was statistically significant (of note, our study was not designed to analyse the presence of allodynia in those patients who actually experienced aura during allodynia testing). There was no correlation between a history of aura and being tested during acute headache exacerbation .
The spatial distribution of allodynia
Table 3 presents the distribution of cephalic and extracephalic BA in the study population. Cephalic allodynia was found in 33 patients (86.8% of allodynic patients, 37.1% of the entire study population), whereas extracephalic BA was found in 25 patients (65.8% of allodynic patients, 28.1% of the entire study population). Of those patients with extracephalic allodynia, all had BA at the C2—C3 area, while 14 (56.0% of patients with extracephalic allodynia; 36.8% of allodynic patients; 15.7% of the entire study population) had BA at the C8 area.
Table 3.
No. (%) of patients with cephalic or extracephalic allodynia*
| Site | No. of patients |
Percent of allodynic patients |
Percent of total study population |
|---|---|---|---|
| Cephalic | 33 | 86.8 | 37.1 |
| Extracephalic: all | 25 | 65.8 | 28.1 |
| Extracephalic: C2—C3 | 25 | 65.8 | 28.1 |
| Extracephalic: C8 | 14 | 36.8 | 15.7 |
| Total | 38 | 100.0 | 42.7 |
Numbers in columns do not add up to 100% since patients may have had allodynia at more than one site.
Table 4 presents the different patterns of spatial distribution of BA in the study population. The most common pattern was cephalic with extra-cephalic, which was found in 20 patients (52.6% of allodynic patients; 22.5% of the entire study population). Twelve of these patients (31.6% of allodynic patients; 13.5% of the entire study population) had BA at all three sites. Thirteen patients (34.2% of allodynic patients; 14.6% of the entire study population) had cephalic BA alone. Only five patients (13.2% of allodynic patients; 5.6% of the entire study population) had extracephalic BA with no cephalic allodynia.
Table 4.
No. (%) of patients with allodynia at single and multiple sites*
| Site | No. of patients |
Percent of allodynic patients |
Percent of total study population |
|---|---|---|---|
| Cephalic and extracephalic (V1 with C2—C3 and/or C8) | 20 | 52.6 | 22.5 |
| Cephalic only (V1) | 13 | 34.2 | 14.6 |
| All tested sites (V1 and C2—C3 and C8) | 12 | 31.6 | 13.5 |
| Extracephalic only (C2—C3 and/or C8) | 5 | 13.2 | 5.6 |
| Total | 38 | 100.0 | 42.7 |
Numbers in columns do not add up to 100% since patients with allodynia at all three sites are a subset of those with cephalic and extracephalic allodynia.
Allodynia score in relation to headache and other migraine symptoms
Figure 2 presents a scatterplot of total allodynia scores against current headache pain ratings along with a fitted regression line. There existed a positive rank-order correlation between total allodynia scores and headache pain ratings (ρ = 0.21, P = 0.048). Although the magnitude of this association changed slightly with the exclusion of one patient with a total allodynia score of 582, the association was no longer statistically significant at the conventional level (ρ = 0.18, P = 0.09). No association was found between the laterality (right, left or bilateral) of allodynia and the laterality of current headache pain (P = 0.09).
Figure 2.
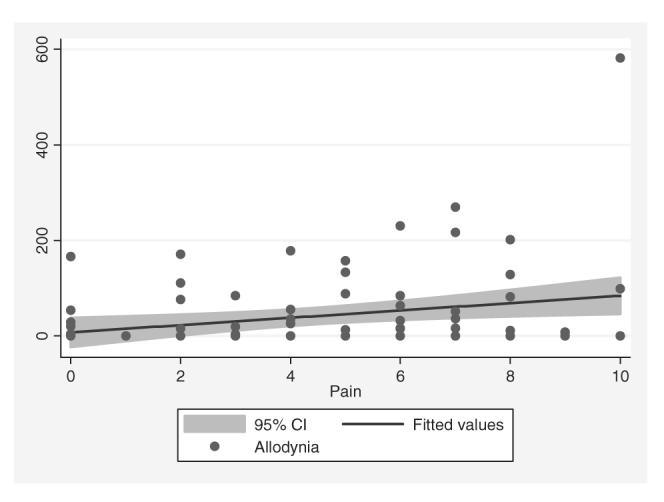
Correlation between total allodynia score and current headache pain rating.
Discussion
In most previous studies, allodynia in migraine was assessed in patients with the episodic type, showing that allodynia occurred during acute migraine attacks but not between attacks (5, 10). In addition, in previous studies allodynia was assessed using either time-consuming QST or a questionnaire alone (5, 14, 15). In this study, we used a practical method to evaluate CM patients for the presence of allodynia.
There is scant data in the literature regarding the prevalence of allodynia in chronic (or transformed) migraine. Creach et al. found that extracranial, but not facial, heat allodynia was significantly more common in patients with transformed migraine (TM) compared with those with episodic migraine (EM) (19). Sorbino used a questionnaire to identify CM patients with cutaneous allodynia. Of 135 patients with CM in his study, 56% were allodynic (20). This figure is somewhat higher than ours. However, no sensory testing was used in that study to verify the presence of allodynia. Kitaj and Klink tested patients with TM and EM for pain thresholds, using QST. They found that patients with TM had lower pain thresholds compared with those with EM (21).
This study demonstrated a high prevalence of cutaneous allodynia in patients with CM, both during an acute headache exacerbation and between exacerbations. In contrast to the findings in studies of patients with EM, allodynia prevalence in patients with CM was not significantly affected by the presence of acute headache exacerbation at the time of testing. These observations support the hypothesis that a state of ongoing sensory neuronal sensitization in the trigeminal, and possibly extratrigeminal, pain pathways exists in CM. Sensory neurons in the trigeminovascular system that are chronically sensitized may not be affected by the physiological and chemical events that occur during an acute migraine attack to the same degree as neurons that are not chronically sensitized. Our results may also explain why patients with CM are more difficult to treat than those with EM. Burstein et al. have shown that the presence of allodynia is associated with a dramatically reduced efficacy of triptans in migraine treatment (6). The high prevalence of allodynia in CM patients may offer an explanation for the refractoriness to triptans which many of these patients exhibit.
We found that brush allodynia was 1.7 times as common in patients who had a history of aura with their headache exacerbation compared with those who did not. These data are consistent with results of previous studies (5, 6). There is currently no satisfactory explanation for the association between cutaneous allodynia and a history of aura. There was no correlation between the presence of aura and the likelihood of being tested during an acute headache exacerbation. Therefore, the higher prevalence of allodynia in patients who had a history of aura compared with those who did not, could not be explained by a higher proportion of patients with aura being tested during acute exacerbation.
We found that BA was most common in the V1 area, followed by the C2—C3 dermatome, and was least common at the C8 dermatome. These results confirm data from a previous smaller study, in which migraine patients were tested for brush allodynia (12, 17). The frequent occurrence of allodynia in the C2—C3 dermatomes may be explained by the presence of a large number of trigeminovascular neurons in the dorsal horn at these spinal levels (22–24). Liu et al. have recently shown that the sensory information from the superior sagittal sinus is transmitted to central neurons located in an area that extends from the trigeminal interpolar nucleus down to the C3 level (23). Therefore, allodynia at C2—C3 dermatomes may be mediated by sensitization of second-order neurons that receive convergent input from trigeminal and cervical fibres, rather than by sensitization of third-order neurons in the thalamus.
The most common spatial distribution of allodynia in the present study was cephalic +extracephalic. Similar results have been found in studies using QST. Burstein et al. found that allodynia was always present at the referred head pain area, and it was also found in extracephalic areas in the majority (28/33) of allodynic patients (5). This spatial distribution supports the hypothesis that allodynia initially occurs at the referred head pain area and then spreads to extracephalic areas, possible through sequential sensitization of higherorder trigeminovascular neurons. This pattern of allodynia spread has been shown clinically in a patient with migraine with aura (MA), who was tested for allodynia at several time points during an acute migraine attack (10). Allodynia at extracephalic areas only was rare.
There was a weak positive correlation between allodynia and headache intensities in this study. The correlation between allodynia and headache severity has not been extensively studied before. In a smaller preliminary study, we found a correlation between allodynia score and migraine score (a measure of migraine severity and duration), which was not statistically significant (17). This correlation is expected, since allodynia is a manifestation of neuronal sensitization in pain pathways that are responsible for the headache generation. The laterality of headache and that of allodynia were not correlated in this study, suggesting a more complex relationship between the two. Indeed, we recently reported on a migraine patient who exhibited cephalic allodynia that was contralateral to the side of head pain, a phenomenon we called referred allodynia (25).
The results of this study should be interpreted with the following reservations: (i) since it was a clinic-based study, the prevalence of allodynia may differ in the general migraine population; (ii) of all sensory modalities, only brush allodynia was tested; (iii) the physician who tested the patients for allodynia was not blinded to their diagnosis; (iv) the inclusion of patients who were taking migraine-preventive drugs during the study period may have affected our ability to detect the presence of BA. Experimental and clinical evidence suggest that gabapentin, which is occasionally used for migraine prevention, reduces cutaneous allodynia in humans and in a rat model of postherpetic neuralgia (26, 27); (v) the observed spatial distribution of allodynia in this study may have been affected by the order in which the different skin areas were stimulated.
In summary, we used a clinically practical method to assess the prevalence of brush allodynia in CM and to define its patterns in these patients. Our findings suggest a high prevalence of BA in CM which may interfere with patients’ response to acute migraine treatment. Our proposed method for allodynia testing can help clinicians identify patients with allodynia and select the appropriate treatment for them.
Acknowledgements
We thank Linda A. Kelly for her hard and skilful work in the preparation of this manuscript.
References
- 1.Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann NY Acad Sci. 2001;933:142–56. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- 2.Tfelt-Hansen P, Lous I, Olesen J. Prevalence and significance of muscle tenderness during common migraine attacks. Headache. 1981;21:49–54. doi: 10.1111/j.1526-4610.1981.hed2102049.x. [DOI] [PubMed] [Google Scholar]
- 3.Drummond PD. Scalp tenderness and sensitivity to pain in migraine and tension headache. Headache. 1987;27:45–50. doi: 10.1111/j.1526-4610.1987.hed2701045.x. [DOI] [PubMed] [Google Scholar]
- 4.Selby G, Lance JW. Observation on 500 cases of migraine and allied vascular headaches. J Neurol Neurosurg Psychiatry. 1960;23:23–32. doi: 10.1136/jnnp.23.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–24. [PubMed] [Google Scholar]
- 6.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the developing allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 7.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–4. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 8.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brainstem trigeminal neurons. J Neurophysiol. 1998;79:964–82. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 9.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–10. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 10.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123:1703–9. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 11.Lopinto C, Ashkenazi A, Young WB. Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine. Cephalalgia. 2004;24:1093. doi: 10.1111/j.1468-2982.2006.01121.x. (Abstract) [DOI] [PubMed] [Google Scholar]
- 12.Bove ME, Anjum MW, Ashkenazi A, Young WB. Brush (dynamic mechanical) allodynia in headache: the mapping study. Neurology. 2004;62:A336. (Abstract) [Google Scholar]
- 13.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- 14.Jakubowski M, Silberstein S, Ashkenazi A, Burstein R. Can allodynic migraine patients be identified interictally using a questionnaire? Neurology. 2005;65:1419–22. doi: 10.1212/01.wnl.0000183358.53939.38. [DOI] [PubMed] [Google Scholar]
- 15.Mathew NT, Kailasam J, Seifert T. Clinical recognition of allodynia in migraine. Neurology. 2004;63:848–52. doi: 10.1212/01.wnl.0000137107.27585.f7. [DOI] [PubMed] [Google Scholar]
- 16.Headache Classification Committee The International Classification of Headache Disorders, 2nd edition. Cephalalgia. 2004;24:1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 17.Ashkenazi A, Young WB. The effects of greater occipital nerve block and trigger point injection on brush allodynia and pain in migraine. Headache. 2005;45:350–4. doi: 10.1111/j.1526-4610.2005.05073.x. [DOI] [PubMed] [Google Scholar]
- 18.Ashkenazi A, Young WB. Dynamic mechanical (brush) allodynia in cluster headache. Headache. 2004;44:1010–2. doi: 10.1111/j.1526-4610.2004.04195.x. [DOI] [PubMed] [Google Scholar]
- 19.Creach C, Radat F, Laffitau M, Irachabal S, Henry P. Cutaneous allodynia in transformed migraine with medication overuse. Cephalalgia. 2003;23:656–7. (Abstract) [Google Scholar]
- 20.Sobrino FE. Cutaneous allodynia in chronic migraine. Cephalalgia. 2003;23:750. (Abstract) [Google Scholar]
- 21.Kitaj MB, Klink M. Pain thresholds in daily transformed migraine versus episodic migraine headache patients. Headache. 2005;45:992–8. doi: 10.1111/j.1526-4610.2005.05179.x. [DOI] [PubMed] [Google Scholar]
- 22.Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994;14:3725–35. doi: 10.1523/JNEUROSCI.14-06-03725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Broman J, Edvinsson L. Central projections of sensory innervation of the rat superior sagittal sinus. Neuroscience. 2004;129:431–7. doi: 10.1016/j.neuroscience.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 24.Yamamura H, Malick A, Chamberlin NL, Burstein R. Cardiovascular and neuronal responses to head stimulation reflect central sensitization and cutaneous allodynia in a rat model of migraine. J Neurophysiol. 1999;81:479–93. doi: 10.1152/jn.1999.81.2.479. [DOI] [PubMed] [Google Scholar]
- 25.Ashkenazi A, Lopinto C, Young WB. Referred cutaneous allodynia in a migraine patient without simultaneous headache. Cephalalgia. 2005;25:75–8. doi: 10.1111/j.1468-2982.2004.00821.x. [DOI] [PubMed] [Google Scholar]
- 26.Berry JD, Petersen KL. A single dose of gabapentin reduces acute pain and allodynia in patients with herpes zoster. Neurology. 2005;65:349–50. doi: 10.1212/01.wnl.0000168259.94991.8a. [DOI] [PubMed] [Google Scholar]
- 27.Chen SR, Pan HL. Effect of systemic and intrathecal gabapentin on allodynia in a new rat model of postherpetic neuralgia. Brain Res. 2005;1042:108–13. doi: 10.1016/j.brainres.2005.02.024. [DOI] [PubMed] [Google Scholar]


