Abstract
Objective:
The authors estimated the prevalence and severity of cutaneous allodynia (CA) in individuals with primary headaches from the general population.
Methods:
We mailed questionnaires to a random sample of 24,000 headache sufferers previously identified from the population. The questionnaire included the validated Allodynia Symptom Checklist (ASC) as well as measures of headache features, disability, and comorbidities. We modeled allodynia as an outcome using headache diagnosis, frequency and severity of headaches, and disability as predictor variables in logistic regression. Covariates included demographic variables, comorbidities, use of preventive medication, and use of opioids.
Results:
Complete surveys were returned by 16,573 individuals. The prevalence of CA of any severity (ASC score ≥3) varied with headache type. Prevalence was significantly higher in transformed migraine (TM, 68.3%) than in episodic migraine (63.2%, p < 0.01) and significantly elevated in both of these groups compared with probable migraine (42.6%), other chronic daily headaches (36.8%), and severe episodic tension-type headache (36.7%). The prevalence of severe CA (ASC score ≥9) was also highest in TM (28.5%) followed by migraine (20.4%), probable migraine (12.3%), other chronic daily headaches (6.2%), and severe episodic tension-type headache (5.1%). In the migraine and TM groups, prevalence of CA was higher in women and increased with disability score. Among migraineurs, CA increased with headache frequency and body mass index. In all groups, ASC scores were higher in individuals with major depression.
Conclusions:
Cutaneous allodynia (CA) is more common and more severe in transformed migraine and migraine than in other primary headaches. Among migraineurs, CA is associated with female sex, headache frequency, increased body mass index, disability, and depression.
Central sensitization, an increased excitability of spinal and medullary dorsal horn neurons resulting from ongoing input from C-fiber nociceptors, may lead to cutaneous allodynia (CA), a neurologic condition characterized by pain elicited by ordinary nonnociceptive stimulation of the skin.1,2 During migraine, facial CA is likely to be a clinical manifestation of sensitization at the level of the trigeminal nucleus caudalis.3,4 In clinic-based studies, most migraineurs develop CA during the course of an attack.5-8 In migraine, CA has been proposed as a predictor of poor response to triptan therapy8 as well as a risk factor for disease progression.9
The gold standard for the assessment of CA at particular points in time is quantitative sensory testing (QST). Using QST, pain thresholds for heat, cold, and pressure stimuli are measured. However, routine QST is not practical in the clinical setting and not feasible in large population studies. Therefore, questionnaires that assess CA are of importance in clinical practice and research. Based on a previously published questionnaire,10 we developed and validated the 12-item Allodynia Symptom Checklist (ASC) and tested it in a large population sample of migraineurs participating in the American Migraine Prevalence and Prevention (AMPP) study.11 We showed that the questionnaire is psycho-metrically sound. Additionally, severe CA, as measured by the ASC, correlates with frequency and severity of migraine attacks, with typical migraine symptoms as well as with migraine disability.10
Here, we used the ASC-12 to assess the prevalence and severity of CA in subjects with various types of primary headaches in the general population. Because CA has been recently suggested as a risk factor for migraine progression, we hypothesized that the prevalence and severity of CA would be higher in transformed migraine (TM) than in migraine and in migraine than in other primary headache disorders. We also hypothesized that CA would be associated with frequency of attacks and disability. We assessed and controlled for the influence of demographic features, pain, frequency and severity of headache, psychiatric comorbidity, and use of medication. Finally, because obesity and depression have both been suggested as risk factors for migraine progression, we investigated the association of CA with these variables.
METHODS
Study population
The AMPP is a longitudinal study following a cohort of severe headache sufferers selected from a representative sample of the general population.12,13 One of the main objectives of the project is to establish risk factors for headache progression (e.g., risk factors for chronic daily headaches [CDH]).
The AMPP is composed of two major phases. In Phase 1, we screened a nationwide sample to identify headache sufferers using a validated self-administered questionnaire. The questionnaire was mailed (July–August 2004) to a stratified random sample of 120,000 US households drawn from a 600,000-household national panel maintained by the National Family Opinion, Inc. Of 162,576 individual respondents, 30,721 reported at least one severe headache in the past year. In Phase 2, a random sample of 24,000 individuals with headache was enrolled in 5 years of follow-up study. Like in Phase 1, headache categories were determined. Episodic headache sufferers (<15 days of headache per month) were classified as having migraine, probable migraine (PM), or severe episodic tension-type headaches (S-ETTH). Individuals with CDH (≥15 days of headache per month) were subdivided into TM and other CDH (O-CDH). The reason we report on S-ETTH and not ETTH is because the screening question was based on severe headaches in the prior year.
The first follow-up survey was conducted in July–August 2005. It has been described elsewhere as well as the validation of the allodynia questionnaire.10,12
Description of the survey
The questionnaire consisted of 82 questions assessing headache diagnosis, CA, comorbidities, headache-related impact, health-related quality of life, and demographics. We used a validated headache questionnaire consisting of 21 questions to capture the features of up to three headache types.12,13 Diagnoses of migraine and PM were assigned based on the criteria proposed by the second edition of the International Classification of Headache Disorders.14 Diagnosis of CDH and TM followed the Silberstein and Lipton criteria.15 The survey had been previously shown to have a sensitivity of 100% and specificity of 82.3% for the diagnosis of migraine.16 The questionnaire has a sensitivity of 93% and a specificity of 85% for the diagnosis of TM.17
The questionnaire also captured information on demographic features, duration of headache (number of years with headache), weight and height, and comorbid disorders (arthritis, asthma, and chronic pain). Disability was assessed with the Migraine Disability Assessment (MIDAS) questionnaire.18 MIDAS was used to divide patients into four grades using previously validated and well-accepted scores based on lost time attributable to headaches. Depression was screened with the Patient Health Questionnaire from the Prime-MD questionnaire.19
Assessment of allodynia
Allodynia symptoms and score on CA severity were obtained by using the ASC questionnaire.10 The ASC included 12 questions about the frequency of various allodynia symptoms in association with headache attacks. For individuals with more than one type of headache, questions were directed to the “most severe type of headache” based on the prior evidence indicating that the most severe type was likely to be migraine.20-22 Instead of using a dichotomous option (yes or no), the response categories were never, rarely, less than half the time, and half the time or more.
ASC items were scored as 0 (i.e., never or rarely or does not apply to me), 1 (less than half the time), and 2 (half the time or more), yielding scores that ranged from 0 to 24. In the development of ASC, alternative scoring strategies were evaluated but did not alter the results.23 The validation process defined the following categories based on the ASC CA scores: no allodynia (0–2), mild (3–5), moderate (6–8), and severe (9 or higher).
Analyses
Analyses were performed using SAS. Data were summarized using frequency counts and descriptive statistics. ASC scores were assessed as explained previously.
The χ2 test was used to compare proportions among groups. We modeled headache status as well as frequency of headaches among the different headache status and disability as measured by the MIDAS questionnaire as dependent variables using CA as an independent variable after adjusting for covariates in three different models of logistic regression. In model 1, adjustment was conducted for demographic variables (age, sex, race, income, and so on). In model 2, we used the same adjustments for model 1 and also headache frequency, severity, and duration of illness. In model 3, we used the same adjustments for model 2 and also included comorbidities, use of preventive medication, and use of opioids.
Our sample size allowed running the three models for migraine and model 1 for the other headache types. Logistic regression was used to estimate the odds and prevalence ratios for each explanatory variable. Ninety-five percent CIs are provided for all ratios. All CIs not containing the value 1 indicate that the effect is a significant predictor.
Finally, we also treated CA as a continuous variable and compared the mean CA scores in individuals with and without major depressive disorder (as assessed by the Patient Health Questionnaire) after stratifying by the body mass index category (normal weight, overweight, obese, and morbidly obese) using parametric statistics.
RESULTS
Study population
Of 24,000 headache sufferers surveyed, 16,573 returned complete questionnaires (69.0% response rate). All of them had at least one severe headache in the previous year. A total of 11,094 had migraine; 1,491 had PM, 1,151 had S-ETTH, 643 had TM, and 152 had O-CDH. A total of 2,042 had unclassified headaches. Table 1 displays the demographic features of our sample by headache type.
Table 1.
Demographic characteristics of our sample stratified by headache diagnosis
| Episodic headaches |
Chronic daily headaches |
|||||
|---|---|---|---|---|---|---|
| Mean | PM | S-ETTH | TM | O-CDH | ||
| Sample size | 11,094 | 1,491 | 1,151 | 643 | 152 | |
| Sex | ||||||
| Men | 2,192 (19.8) | 577 (38.7) | 350 (30.4) | 135 (20.9) | 60 (39.5) | |
| Women | 8,902 (80.2) | 914 (61.3) | 801 (69.6) | 508 (79.1) | 92 (60.5) | |
| Race | ||||||
| No answer | 275 (2.4) | 40 (2.7) | 33 (2.9) | 15 (2.3) | 2 (1.3) | |
| White | 9,696 (87.4) | 1,263 (84.7) | 978 (85) | 583 (90.6) | 135 (81.9) | |
| Black | 784 (7.1) | 148 (9.9) | 106 (9.2) | 26 (4.0) | 11 (7.2) | |
| Asian | 86 (0.7) | 19 (1.3) | 14 (1.2) | 1 (0.1) | 0 | |
| Native American | 96 (0.8) | 5 (0.3) | 7 (0.6) | 9 (1.4) | 1 (0.7) | |
| Other | 157 (1.4) | 16 (1.1) | 13 (1.1) | 9 (1.4) | 3 (1.9) | |
| Income | ||||||
| <$22,500 | 2,225 (23.4) | 361 (24.2) | 316 (27.5) | 164 (31.5) | 42 (27.6) | |
| $22,500–39,999 | 1,891 (19.9) | 281 (18.8) | 218 (18.9) | 111 (21.3) | 31 (20.4) | |
| $40,000–59,999 | 1,803 (19.0) | 284 (19.0) | 219 (19.0) | 81 (15.6) | 32 (21.1) | |
| $60,000–89,999 | 1,755 (18.5) | 276 (18.5) | 185 (16.1) | 92 (17.7) | 24 (15.8) | |
| $90,999–124,999 | 1,820 (19.2) | 289 (19.4) | 213 (18.5) | 72 (13.8) | 23 (15.1) | |
| $125,000+ | 636 (6.7) | 99 (6.6) | 87 (7.6) | 12 (2.3) | 9 (5.9) | |
| Urbanization | ||||||
| <100,000 | 1,654 (17.4) | 206 (13.8) | 170 (14.8) | 92 (17.7) | 19 (12.5) | |
| 100,000–499,999 | 1,714 (18.1) | 239 (16.0) | 198 (17.2) | 110 (21.2) | 23 (15.1) | |
| 500,000–,999,999 | 2,230 (23.5) | 368 (24.7) | 292 (25.4) | 114 (21.9) | 41 (27.0) | |
| 2,000,000+ | 3,896 (41.0) | 678 (45.5) | 491 (42.7) | 204 (39.2) | 69 (45.4) | |
| Weight (pounds) | ||||||
| Mean (SD) | 180.2 (50.3) | 184 (48.7) | 183.9 (49.9) | 187.2 (57.7) | 184.9 (49.2) | |
Values are n (%).
PM = probable migraine; S-ETTH = severe episodic tension-type headache; TM = transformed migraine; O-CDH = other chronic daily headaches.
Prevalence and severity of allodynia by headache diagnosis
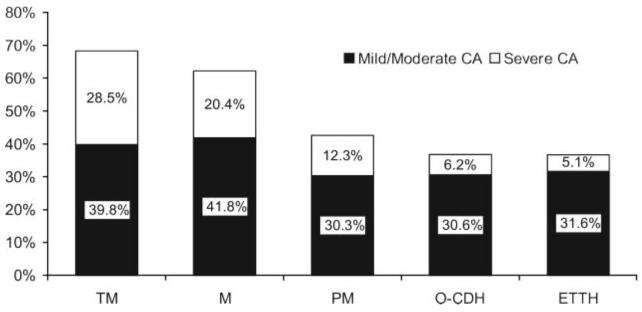
The prevalence of CA during headache attacks (defined as an ASC-12 score of 3 or higher) was higher in those with TM (68.3%) than in episodic migraine (63.2%, p < 0.01) and in both of these groups compared with PM (42.6%, p < 0.001), O-CDH (36.8%, p < 0.001), and S-ETTH (36.7%, p < 0.001) (figure 1). The relative frequency of mild and moderate CA was remarkably similar among the headache groups, whereas the prevalence of severe CA varied markedly (figure 1). In TM sufferers, 23.7% had mild, 16.1% moderate, and 28.5% severe CA. Figure 1 illustrates the severity of CA for the other headache subtypes.
Figure 1.
Relative frequency and severity of cutaneous allodynia according to headache subtype
CA = cutaneous allodynia; TM = transformed migraine; M = migraine; PM = probable migraine; O-CDH = other chronic daily headaches; ETTH = episodic tension-type headache.
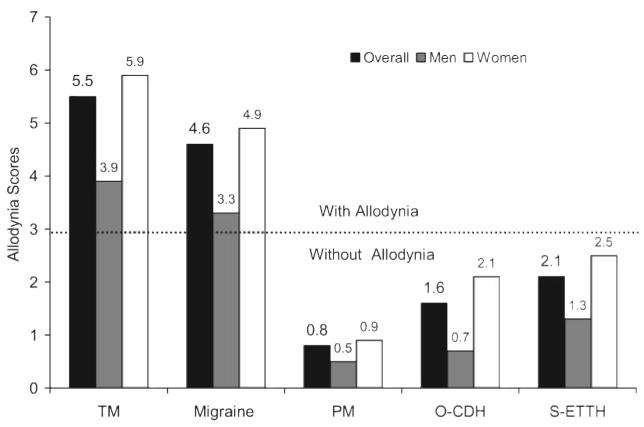
Figure 2 presents the mean ASC-12 scores per each headache diagnostic group. Overall, the scores were higher in TM (5.5 ± 5.0) than in migraine (4.6 ± 4.5, p < 0.001) and in both TM and migraine than in O-CDH (1.59 ± 2.5), PM (0.8 ± 1.9), and S-ETTH (2.15 ± 2.99) (p < 0.001 for all comparisons). The ASC-12 scores did not differ significantly among PM, O-CDH, and S-ETTH. For all the primary headaches, the overall ASC scores were higher in women than in men (figure 2). Results of logistic modeling are presented subsequently.
Figure 2.
Mean allodynia symptom checklist score, overall and by gender, in individuals with primary headache disorders
TM = transformed migraine; PM = probable migraine; O-CDH = other chronic daily headaches; S-ETTH = severe episodic tension-type headache.
Predictors of allodynia by headache type.
Migraine (n = 11,094)
Table 2 summarizes the predictors of allodynia by headache type adjusting by demographic features. The description of the CA sub-types as well as the association among allodynia, demographics, and headache features in persons with migraine were described in details in the validation of the instrument.10 Among episodic migraineurs, CA was more common in women than in men (prevalence ratio [PR] = 1.7, 95% CI = 1.55–1.82). Contrasted to white subjects, CA was more prevalent in black subjects (PR = 1.14, 05% CI = 1.04–1.25). The prevalence of CA decreased with educational level (graduated vs less than high school, PR = 0.68, 95% CI = 0.55–0.83). The prevalence of CA decreased with age. Contrasting migraineurs 74 years or older with those aged 18–24, the PR was 0.76 (95% CI = 0.61–0.95). It increased with attack frequency (from 24.9% in those with <6 migraine attacks per year to 48% in those with 2–3 attacks per week, PR = 1.96, 95% CI = 1.7–2.26) and higher disability (MIDAS IV vs I, PR = 1.98, 95% CI = 1.85–2.11). Finally, CA was more common in obese individuals (1.21, 95% CI = 1.13–1.30).
Table 2.
Prevalence of allodynia and adjusted prevalence ratios (PR) in individuals with specific primary headaches
| Adjusted PR (model 1) |
|||||
|---|---|---|---|---|---|
| Migraine (n = 11,094 | PM(n = 1,491) | S-ETTH (n = 1,151) | TM (n = 643) | ||
| Sex | |||||
| Men | 1 | 1 | 1 | 1 | |
| Women | 1.68 (1.55–1.82) | 2.95 (1.98–4.40) | 2.68 (1.37–5.22) | 1.71 (1.00–2.91) | |
| Race | |||||
| White | 1 | 1 | 1 | 1 | |
| Black | 1.14 (1.04–1.25) | 1.09 (0.70–1.70) | NOT-EST | 1.03 (0.49–2.20) | |
| Asian | 1.07 (0.82–1.40) | 2.12 (0.80–5.62) | NOT-EST | 0.92 (0.29–2.99) | |
| Native American | 1.37 (1.11–1.68) | 1.55 (0.27–9.02) | NOT-EST | 0.81 (0.24–2.66) | |
| Other | 1.20 (1.00–1.44) | 1.41 (0.58–3.47) | NOT-EST | NOT-EST | |
| Illness duration, y | |||||
| <10 | 1 | 1 | 1 | 1 | |
| 10–19 | 1.13 (1.04–1.23) | 1.67 (1.01–2.74) | NOT-EST | 0.88 (0.65–1.19) | |
| 20–29 | 1.08 (0.98–1.19) | 1.69 (0.84–3.40) | NOT-EST | 1.04 (0.76–1.41) | |
| 30–39 | 1.01 (0.88–1.16) | 1.19 (0.46–3.09) | NOT-EST | 0.79 (0.49–1.28) | |
| 40–49 | 1.08 (0.90–1.31) | 1.15 (0.27–4.82) | NOT-EST | 0.96 (0.42–2.18) | |
| 50–59 | 0.96 (0.88–1.04) | 0.86 (0.47–1.57) | NOT-EST | 1.04 (0.80–1.35) | |
| >60 | 0.98 (0.70–1.37) | 0.00 (0.00–.) | NOT-EST | 0.96 (0.24–3.88) | |
| Attack frequency/y | |||||
| <6 | 1 | 1 | 1 | — | |
| 6–12 | 1.41 (1.23–1.62) | 1.39 (0.81–2.40) | 1.1 (0.41–2.40) | — | |
| 13–24 | 1.49 (1.30–1.71) | 1.46 (0.80–2.64) | 1.9 (0.8–.3–1–8) | — | |
| 24–51 | 1.59 (1.40–1.82) | 1.71 (1.00–2.91) | 1.77 (1.06—2.06) | — | |
| 52–103 | 1.82 (1.59–2.08) | 1.81 (1.00–3.27) | 1.85 (1.0—2.2) | — | |
| 104–179 | 1.96 (1.70–2.26) | 1.83 (0.92–3.65) | 1.81 (0.90–4.2) | — | |
| 180–241 | — | — | 1 | ||
| 242–303 | — | — | 1.09 (0.87–1.37) | ||
| 304+ | — | — | 0.96 (0.78–1.19) | ||
| Disability | |||||
| No | 1 | 1 | 1 | 1 | |
| Mild | 1.46 (1.36–1.56) | 1.74 (1.22–2.49) | NOT-EST | 0.93 (0.42–2.06) | |
| Moderate | 1.60 (1.49–1.72) | 2.39 (1.63–3.52) | NOT-EST | 1.61 (0.94–2.77) | |
| Severe | 1.98 (1.85–2.11) | 2.87 (1.95–4.23) | NOT-EST | 2.49 (1.73–3.59) | |
| Body mass index | |||||
| Normal | 1 | 1 | Reference | 1 | |
| Overweight | 1.05 (0.98–1.12) | 0.91 (0.66–1.27) | NOT-EST | 0.91 (0.71–1.16) | |
| Obese | 1.12 (1.04–1.20) | 0.65 (0.42–1.00) | 1.04 (0.45–2.40) | 1.00 (0.77–1.29) | |
| Morbidly obese | 1.21 (1.13–1.30) | 0.93 (0.64–1.36) | 1.38 (0.55–3.43) | 0.95 (0.74–1.22) | |
Each value is followed by the 95% CI.
Adjustments were conducted by age, sex, race, income, headache frequency, severity and duration of illness, use of preventive medication, and use of opioids.
PM = probable migraine; S-ETTH = severe episodic tension-type headache; TM = transformed migraine; PR = prevalence ratio; NOT-EST = not estimated as a result of sample size limitations.
In table 2, the prevalence of CA in migraine is adjusted by demographic variables (model 1), and in table 3, it is further adjusted by comorbidities (model 2) and use of medication (model 3). The more strict models of adjustment confirmed the findings and also suggested that CA increased with illness duration.
Table 3.
Adjusted prevalence ratios in individuals with migraine
| Model 2* | Model 3* | ||
|---|---|---|---|
| Race | |||
| White | 1 | 1 | |
| Black | 1.07 (0.98–1.17) | 1.08 (0.97–1.20) | |
| Asian | 1.18 (0.91–1.54) | 1.28 (1.02–1.61) | |
| Native American | 1.33 (1.08–1.63) | 1.32 (1.07–1.64) | |
| Other | 1.15 (0.96–1.37) | 1.10 (0.88–1.39) | |
| Sex | |||
| Men | 1 | 1 | |
| Women | 1.68 (1.54–1.82) | 1.43 (1.28–1.59) | |
| Highest education level | |||
| Junior high or less | 1 | 1 | |
| Some high school | 0.82 (0.67–1.01) | 0.88 (0.67–1.16) | |
| High school diploma or graduation equivalence diploma |
0.68 (0.57–0.82) | 0.80 (0.62–1.02) | |
| Some college | 0.73 (0.61–0.88) | 0.85 (0.66–1.08) | |
| Bachelor's degree | 0.58 (0.48–0.69) | 0.69 (0.53–0.89) | |
| Graduate | 0.61 (0.50–0.75) | 0.71 (0.55–0.92) | |
| Age, y | |||
| 18–24 | 1 | 1 | |
| 25–34 | 0.99 (0.88–1.13) | 1.02 (0.87–1.20) | |
| 35–44 | 0.97 (0.86–1.09) | 0.96 (0.82–1.14) | |
| 45–54 | 0.95 (0.84–1.07) | 0.90 (0.77–1.07) | |
| 55–64 | 0.90 (0.79–1.02) | 0.86 (0.72–1.02) | |
| 65–74 | 0.78 (0.67–0.92) | 0.78 (0.62–0.97) | |
| >74 | 0.66 (0.53–0.84) | 0.64 (0.43–0.95) | |
| Illness duration, y | |||
| <10 | 1 | 1 | |
| 10–19 | 1.16 (1.07–1.27) | 1.15 (1.06–1.25) | |
| 20–29 | 1.22 (1.10–1.35) | 1.19 (1.08–1.32) | |
| 30–39 | 1.21 (1.05–1.41) | 1.22 (1.05–1.43) | |
| 40–49 | 1.31 (1.04–1.66) | 1.28 (1.01–1.64) | |
| 50–59 | 0.95 (0.88–1.04) | 0.95 (0.87–1.03) | |
| >60 | 1.46 (0.95–2.26) | 1.12 (0.63–1.98) | |
| Attack frequency/y | |||
| <6 | 1 | 1 | |
| 6–12 | 1.37 (1.19–1.58) | 1.21 (1.00–1.46) | |
| 13–24 | 1.41 (1.22–1.62) | 1.19 (0.98–1.44) | |
| 24–51 | 1.51 (1.32–1.72) | 1.15 (0.96–1.39 | |
| 52–103 | 1.67 (1.46–1.92) | 1.19 (0.98–1.44) | |
| 104–179 | 1.40 (1.31–1.51) | 1.22 (1.00–1.50) | |
| Disability | |||
| No | 1 | 1 | |
| Mild | 1.51 (1.41–1.62) | 1.32 (1.21–1.45) | |
| Moderate | 1.60 (1.49–1.72) | 1.88 (1.76–2.00) | |
| Severe | 1.26 (1.17–1.37) | 1.61 (1.46–1.78) | |
| Body mass index | |||
| Normal | 1 | 1 | |
| Overweight | 1.15 (1.07–1.24) | 1.11 (1.03–1.20) | |
| Obese | 1.22 (1.14–1.31) | 1.16 (1.06–1.26) | |
| Morbidly obese | 1.40 (1.32–1.48) | 1.11 (1.02–1.21) | |
Total n = 11,094. Values arePR (95% CI).
For description of adjustments, see Methods.
PR = prevalence ratio.
Probable migraine (n = 1,491)
Like the results in migraine, for PM, CA was more common in women than in men (PR = 2.95, 95% CI = 1.98–4.4), in those with higher headache frequency, and in those with increased disability (MIDAS IV vs I, PR = 2.87, 95% CI = 1.95–4.23). It did not vary as a function of the other demographics or variables.
Severe episodic tension-type headache (n = 1,151)
In S-ETTH, the prevalence of CA was also higher in women than in men (PR = 2.68, 95% CI = 1.37–5.22) and in those with higher headache frequency and disability. Compared with those with less than six headache attacks per year, those with 24–50 had a PR of 1.77 (95% CI = 1.06–2.95). Contrasting severe disability vs no disability, after adjustments, the PR was 1.79 (95% CI = 0.86–3.71). It did not vary as a function of the other demographics.
Transformed migraine (n = 643)
Like in the other groups, CA was more common in women than in men (PR = 1.71, 95% CI = 1.00–2.91) and in those with severe disability (Midas IV vs I, PR = 2.49, 95% CI = 1.73–3.59). CA did not vary as a function of race, education level, illness duration, attack frequency, or body mass index.
Other chronic daily headaches (n = 152)
Individuals from Asian origin with O-CDH were more likely to have CA than whites after adjustments (PR = 5.50, 95% CI = 1.23–24.55). For all the other variables, the differences were not significant, probably as a result of the smaller sample size of the group and the low prevalence of CA on individuals with O-CDH (36.8%). Therefore, adjustments of CA in the O-CDH group are not presented in table 3.
Depression, allodynia, and headache type
For migraine and PM, increased body mass index was associated with an increased prevalence of CA. In addition, individuals with major depression had higher ASC scores than individuals without depression after stratifying by body mass index (table 4). In migraineurs with normal weight without depression, the mean ASC scores were 4.05 vs 6.65 in those with depression (p < 0.01). Similar findings were seen for migraineurs who were overweight, obese, and morbidly obese. In adjusted analyses, depression had an incremental influence on CA after accounting for headache frequency. Compared with those with no depression, migraineurs with mild depression had a PR for CA of 1.22 (95% CI = 1.10–1.35). Those with moderate depression had a PR of 1.4 (95% CI = 1.23–1.58); in those with moderately severe depression, the PR was 1.51 (95% CI = 1.27–1.79). Finally, in those with severe depression, the PR was 1.62 (95% CI = 1.34–1.96). Similar findings were seen for all other primary headaches (table 4).
Table 4.
Allodynia scores, as measured by the ASC-12, in individuals with primary headaches from the population according to the presence of depression and the body mass index
| Migraine (n = 11,094) |
TM (n = 643) |
PM (n = 1,491) |
S-ETTH (n = 1,151) |
O-CDH (n = 152) |
|
|---|---|---|---|---|---|
| Normal weight | |||||
| Major depression | 6.65 (5.41) | 7.23 (5.14) | 2.36 (3.61) | 5.04 (5.82) | 0.83 (1.33) |
| No major depression | 4.05 (4.19) | 4.89 (4.98) | 0.96 (2.22) | 1.89 (2.79) | 2.00 (3.39) |
| Overweight | |||||
| Major depression | 6.26 (4.94) | 5.95 (5.53) | 1.26 (2.58) | 3.20 (3.47) | 1.43 (1.62) |
| No major depression | 4.12 (4.22) | 4.93 (4.95) | 0.73 (1.79) | 2.08 (3.00) | 1.12 (1.62) |
| Obese | |||||
| Major depression | 6.04 (5.05) | 7.06 (6.08) | 1.33 (2.33) | 3.42 (2.50) | 1.00 (1.73) |
| No major depression | 4.41 (4.40) | 5.27 (4.37) | 1.01 (2.22) | 2.03 (2.33) | 1.22 (2.04) |
| Morbidly obese | |||||
| Major depression | 6.49 (5.35) | 7.07 (5.47) | 1.27 (1.57) | 4.32 (3.56) | 2.89 (2.85) |
| No major depression | 4.77 (4.50) | 4.73 (4.29) | 1.06 (1.77) | 1.99 (2.99) | 1.72 (2.32) |
TM = transformed migraine; PM = probable migraine; S-ETTH = severe episodic tension-type headache; O-CDH = other chronic daily headaches.
DISCUSSION
Our data can be summarized as follows. 1) The prevalence of CA is highest in TM and episodic migraine, intermediate in PM, and lower in S-ETTH and O-CDH. CA appears to map onto migraine biology and to the migraine spectrum (TM, migraine, PM). Within a headache type, attack frequency is certainly one determinant of CA, but it is not the most important across headache types; otherwise, the prevalence of CA in O-CDH would be higher than in the episodic headaches (episodic migraine and PM). 2) The severity of CA is also highest in TM and migraine followed by PM and lower in S-ETTH and O-CDH, even after adjustments that account for the severity of the headache. Therefore, it is not that compared with migraine, the lower severity of ETTH attacks account for the lower severity of CA seen in the later group. Once more, this finding supports that CA maps onto migraine biology. 3) In logistic regression, CA is more common in women than in men for all primary headaches. Therefore, sex (female) and CA are associated. 4) In migraineurs, CA is associated, after adjustments, with female sex, high attack frequency, long disease duration, and obesity. The relative frequency of CA decreases with age. 5) Depression is independently associated with higher CA scores for all headache types.
Identifying factors that map onto disease biology as well as risk factors for clinical and anatomic progression for diseases have emerged as a very important public health priority because it may provide a foundation for more aggressive preventive intervention.24 Furthermore, identifying risk factors that are specifically related to one disease progression (e.g., from migraine to transformed migraine or from episodic tension-type headache to chronic tension-type headache) may provide valuable insights into disease pathophysiology.24-26
Although available evidence is limited, risk factors for headache progression can be divided into nonmodifiable and modifiable based on the prospects for addressing them with behavioral or medical interventions.27 For migraine, examples of factors nonmodifiable by medical intervention include age, sex, and socioeconomic status. Medically addressable risk factors include frequency of migraine attacks, obesity, acute medication overuse, caffeine overuse, stressful life events, depression, and sleep disorders.28,29
Our data suggest that CA maps onto the migraine biology. Although the first neurologic event leading to migraine pain is a matter of debate, it has been suggested that dysfunction of brainstem involved in the modulation of craniovascular afferents may lead to activation of ascending and descending pathways with initiation of a perimeningeal vasodilatation and neurogenic inflammation.30 In this context, the pain is understood as a combination of altered perception (as a result of peripheral or central sensitization) of stimuli that are usually not painful as well as the activation of a feed-forward neurovascular dilator mechanism in the first (ophthalmic) division of the trigeminal nerve. Alternative view proposes that cortical spreading depression, the presumed substrate of migraine aura, is the first neurologic event that would lead to inflammation of meningeal blood vessels and activation of the trigeminal nucleolus caudalis.31 Cortical spreading depression would also be modulated by brainstem structures.
CA is a frequent symptom experienced with migraineurs,4-7 reflecting central sensitization at the level of the trigeminal nucleus caudalis, a structure that is in close relation with the periaqueductal gray area.32-34 Speculating on the progression of migraine, it may be hypothesized that repetitive activation of trigeminovascular neurons and consequently repetitive activation of modulatory pain pathways involving the periaqueductal gray may lead to impairment of function or partial neuronal cell damage, through the liberation of free radicals, in the periaqueductal gray (involved with migraine modulation) or eventually in areas involved with migraine generation.30,31 If that is true, CA would be associated with the migraine spectrum, but not with all primary headache disorders, which was the primary hypothesis of our study.
Female sex and depression seem to be nonspecific proallodynic factors. For the first, the importance of sexual hormones may be speculated. In a human model of capsaicin-induced trigeminal sensitization, pain area significantly changed across the menstrual cycle from a maximum at the menstrual and a minimum at the luteal phase (p < 0.001). The areas of pain were significantly larger in both phases for females compared with males. Area of brush-evoked allodynia was also larger at the menstrual phase compared with the luteal phase (p < 0.0001) and males (p < 0.0001).35 For depression, to our knowledge, this is the first study to report it as being independently associated with CA throughout a gamut of headache disorders. Because several antidepressants act in the substantia nigra pars compacta and ventral tegmental area,36 suggesting the importance of these structures that are closely related with the trigeminal nucleus caudalis,37 in the etiology of depression, this may at least partially explain our findings.
Our data also suggest that CA increases with increased weight and decreases with age. Obesity is associated with increased plasmatic concentration of several proinflammatory mediators and of calcitonin gene-related protein.38,39 Furthermore, relative to normal weight, obese migraineurs have attacks of increased frequency and severity,29 which may explain the higher prevalence of CA in obese individuals. Of note is the age-related decrease in CA. The reasons for this are unknown, but previous studies have shown that migraine attack frequency and severity decreases with age.40 Perhaps the activation of pain pathways declines as attacks become less frequent and severe. Perhaps headaches improve with age as a result of involution of the neurotransmitter systems that mediate head pain.41,42
Our data must be interpreted with caution. First, although we used a questionnaire that has been validated for the assessment of CA in the population, the validation happened in migraineurs only.10 Although we have no reasons to assume that the psychometric properties of the questionnaire would be substantially different in the other headache groups, analogous problems were seen with other headache questionnaires such as MIDAS and HIT-6 that have been validated for migraine but are often used for the assessment of other primary headaches such as chronic daily headaches or cluster headaches.43-45 Second, the ASC classification of severity of CA has not been compared with classification based on QST. Although QST is the gold standard for determining whether a patient has allodynia at a particular point in time, it is subject to temporal sampling error.1,2 Finally, although our data suggest that the prevalence and severity of allodynia varies among different primary headaches, we have not yet determined if allodynia as measured by ASC predicts headache progression. Strengths of our study include the robust sample size and the use of validated questionnaires in a cohort of subjects previously identified and representative of the US population.
ACKNOWLEDGMENT
The authors thank Suzanne Simons of the NHF, Kristina M. Fanning, PhD, and Kathy Ward for help with data management and statistical analyses.
Disclosure: This study was sponsored by the National Headache Foundation (NHF) through a grant from Ortho-McNeil Neurologics, Inc. (OMP). Dr. Bigal and Dr. Lipton received grant support from OMP for research projects other than this study. Dr. Bigal and Dr. Lipton are on the advisory board and give lectures supported by OMP. Dr. Bigal is currently an employee of Merck Research Laboratories.
GLOSSARY
- AMPP
American Migraine Prevalence and Prevention
- ASC
Allodynia Symptom Checklist
- CA
cutaneous allodynia
- CDH
chronic daily headaches
- MIDAS
Migraine Disability Assessment
- O-CDH
other chronic daily headaches
- PM
probable migraine
- PR
prevalence ratio
- QST
quantitative sensory testing
- S-ETTH
severe episodic tension-type headache
- TM
transformed migraine
APPENDIX
The AMPP Advisory Group: Richard B. Lipton, MD (principal investigator), Marcelo E. Bigal, MD, PhD, Dawn Buse, MD, Michael L. Reed, PhD, Walter Stewart, PhD, Merle Diamond, MD, Frederick Freitag, DO, Elisabeth Hazard, PhD, Jonothan Tierce, CPhil, Elizabeth Loder, MD, Paul Winner, MD, Stephen Silberstein, MD, Suzanne Simons, and Seymour Diamond, MD.
REFERENCES
- 1.Woolf CJ, Wall PD. Relative effectiveness of C primary afferent fibers of different origins in evoking a prolonged facilitation of the flexor reflex in the rat. J Neurosci. 1986;6:1433–1442. doi: 10.1523/JNEUROSCI.06-05-01433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 3.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123:1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 4.Sessle BJ, Hu JW, Amano N, Zhong G. Convergence of cutaneous, tooth pulp, visceral, neck and muscle afferents onto nociceptive and non-nociceptive neurones in trigeminal subnucleus caudalis (medullary dorsal horn) and its implications for referred pain. Pain. 1986;27:219–235. doi: 10.1016/0304-3959(86)90213-7. [DOI] [PubMed] [Google Scholar]
- 5.Wolff HG, Tunis MM, Goodell H. Studies on migraine. Arch Intern Med. 1953;92:478–484. doi: 10.1001/archinte.1953.00240220026006. [DOI] [PubMed] [Google Scholar]
- 6.Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatry. 1960;23:23–32. doi: 10.1136/jnnp.23.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]
- 8.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 9.Bigal ME, Lipton RB. Modifiable risk factors for migraine progression. Headache. 2006;46:1334–1343. doi: 10.1111/j.1526-4610.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 10.Jakubowski M, Silberstein S, Ashkenazi A, Burstein R. Can allodynic migraine patients be identified interic-tally using a questionnaire? Neurology. 2005;65:1419–1422. doi: 10.1212/01.wnl.0000183358.53939.38. [DOI] [PubMed] [Google Scholar]
- 11.Lipton RB, Bigal ME, Burstein R, et al. Cutaneous allodynia in the migraine population. Ann Neurol Epub. 2007 Dec 4; doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, AMPP Advisory Group Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 13.Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention Study. Headache. 2007;47:355–363. doi: 10.1111/j.1526-4610.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 14.Headache Classification Subcommittee of the International Headache Society . Cephalgia. suppl 1. ed 2. Vol. 24. 2004. The International Classification of Headache Disorders; pp. 1–15. [DOI] [PubMed] [Google Scholar]
- 15.Silberstein SD, Lipton RB, Sliwinski M. Classification of daily and near-daily headaches: field trial of revised IHS criteria. Neurology. 1996;47:871–875. doi: 10.1212/wnl.47.4.871. [DOI] [PubMed] [Google Scholar]
- 16.Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache. 2001;41:638–645. doi: 10.1046/j.1526-4610.2001.041007638.x. [DOI] [PubMed] [Google Scholar]
- 17.Liebestein M, Bigal ME, Sheftell FD, et al. Validation of the chronic daily headache questionnaire. Neurology. 2007;68(suppl 1):369. [Google Scholar]
- 18.Stewart WF, Lipton RB, et al. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. 2000;88:41–52. doi: 10.1016/S0304-3959(00)00305-5. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- 20.Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 21.Lipton RB, Stewart WF, Simon D. Medical consultation for migraine: results from the American Migraine Study. Headache. 1998;38:87–96. doi: 10.1046/j.1526-4610.1998.3802087.x. [DOI] [PubMed] [Google Scholar]
- 22.Bigal ME, Sheftell FD, Rapoport AM, et al. Chronic migraine is the early stage of transformed migraine in adults. Neurology. 2005;65:1556–1561. doi: 10.1212/01.wnl.0000184477.11569.17. [DOI] [PubMed] [Google Scholar]
- 23.Samejima F. Estimating latent ability using a pattern of graded scores. The William Byrd Press; Richmond, VA: 1969. p. 14. (Psychometrika Monograph Supplement, No. 17). [Google Scholar]
- 24.Lipton RB, Pan J. Is migraine a progressive brain disease? JAMA. 2004;291:493–494. doi: 10.1001/jama.291.4.493. [DOI] [PubMed] [Google Scholar]
- 25.Bigal ME, Lipton RB. When migraine progresses: transformed or chronic migraine. Expert Rev Neurother. 2006;6:297–306. doi: 10.1586/14737175.6.3.297. [DOI] [PubMed] [Google Scholar]
- 26.Scher AI, Stewart WF, Ricci JA, Lipton RB. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain. 2003;106:81–89. doi: 10.1016/s0304-3959(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 27.Bigal ME, Lipton RB. Modifiable risk factors for migraine progression. Headache. 2006;46:1334–1343. doi: 10.1111/j.1526-4610.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 28.Poceta JS, Dalessio DJ. Identification and treatment of sleep apnea in patients with chronic headache. Headache. 1995;35:586–589. doi: 10.1111/j.1526-4610.1995.hed3510586.x. [DOI] [PubMed] [Google Scholar]
- 29.Bigal ME, Lieberman JN, Lipton RB. Obesity and migraine. A population study. Neurology. 2006;66:545–550. doi: 10.1212/01.wnl.0000197218.05284.82. [DOI] [PubMed] [Google Scholar]
- 30.Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–1017. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- 31.Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev. 1993;5:159–177. [PubMed] [Google Scholar]
- 32.Hoheisel U, Mense S. Long-term changes in discharge behavior of cat dorsal horn neurones following noxious stimulation of deep tissues. Pain. 1989;36:239–247. doi: 10.1016/0304-3959(89)90029-8. [DOI] [PubMed] [Google Scholar]
- 33.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 34.Welch KMA, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41:629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 35.Gazerani P, Andersen OK, Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization: gender-specific differences. Pain. 2005;118:155–163. doi: 10.1016/j.pain.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Sekine Y, Suzuki K, Ramachandran PV, Blackburn TP, Ashby CR., Jr Acute and repeated administration of fluoxetine, citalopram, and paroxetine significantly alters the activity of midbrain dopamine neurons in rats: an in vivo electrophysiological study. Synapse. 2007;61:72–77. doi: 10.1002/syn.20349. [DOI] [PubMed] [Google Scholar]
- 37.Matharu MS, Goadsby PJ. Functional brain imaging in hemicrania continua: implications for nosology and pathophysiology. Curr Pain Headache Rep. 2005;9:281–288. doi: 10.1007/s11916-005-0038-z. [DOI] [PubMed] [Google Scholar]
- 38.Herishanu Y, Rogowski O, Polliack A, Marilus R. Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. Eur J Haematol. 2006;76:516–520. doi: 10.1111/j.1600-0609.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 39.Zelissen PM, Koppeschaar HP, Lips CJ, Hackeng WH. Calcitonin gene-related peptide in human obesity. Peptides. 1991;12:861–863. doi: 10.1016/0196-9781(91)90147-h. [DOI] [PubMed] [Google Scholar]
- 40.Bigal ME, Liberman JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology. 2006;67:246–251. doi: 10.1212/01.wnl.0000225186.76323.69. [DOI] [PubMed] [Google Scholar]
- 41.Hasselbalch SG, Madsen K, Svarer C, et al. Reduced 5-HT(2A) receptor binding in patients with mild cognitive impairment. Neurobiol Aging Epub. 2007 Jun 1; doi: 10.1016/j.neurobiolaging.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Togsverd M, Werge TM, Tanko LB, et al. Association of a dopamine beta-hydroxylase gene variant with depression in elderly women possibly reflecting noradrenergic dysfunction. J Affect Disord Epub. 2007 Aug 13; doi: 10.1016/j.jad.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Gesztelyi G, Bereczki D. Disability is the major determinant of the severity of depressive symptoms in primary headaches but not in low back pain. Cephalalgia. 2005;25:598–604. doi: 10.1111/j.1468-2982.2005.00937.x. [DOI] [PubMed] [Google Scholar]
- 44.Dando WE, Branch MA, Maye JP. Headache disability in orofacial pain patients. Headache. 2006;46:322–326. doi: 10.1111/j.1526-4610.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 45.Bigal ME, Rapoport AM, Lipton RB, Tepper SJ, Sheftell FD. Assessment of migraine disability using the migraine disability assessment (MIDAS) questionnaire: a comparison of chronic migraine with episodic migraine. Headache. 2003;43:336–342. doi: 10.1046/j.1526-4610.2003.03068.x. [DOI] [PubMed] [Google Scholar]