To the Editor: Transient apical ballooning (TAB) probably comprises a spectrum of transient ventricular dysfunctional patterns that lead to typical apical ballooning or midventricular, basal, or biventricular dyskinesia.1-4 Herein, I introduce a theory regarding the pathophysiologic mechanism of the midcavity variant on the basis of a recent case.
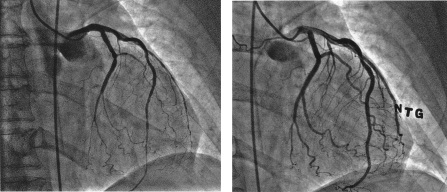
A 71-year-old African American woman was admitted to a hospital elsewhere because of severe chest pain. Coronary angiography revealed mild atherosclerotic plaques. Ventriculography showed akinesia of the mid left ventricle, with well-preserved apical contractility (Video 1). Carvedilol and losartan were prescribed for hypertension. While continuing to have episodes of resting chest pain relieved by nitroglycerin, the patient came to our hospital for endoscopic evaluation of severe abdominal pain and chest pain with ST-T changes. Echocardiography (Video 2) revealed recurrent mid left ventricular (LV) akinesia, with preserved apical wall motion (ejection fraction, 30%). Troponin levels peaked at 1.5 ng/mL (to convert to μg/L [SI unit], multiply by 1.0). Chest pain quickly diminished after sublingual nitroglycerin. Two days later, repeated heart catheterization showed near-normal LV function (ejection fraction, 60%) and no coronary obstruction. Infusion of intracoronary acetylcholine (50 μg for 2 minutes) to detect endothelial dysfunction produced severe chest pain, T-wave changes, and diffuse narrowing of the diagonal, ramus medianus, and 2 marginal branches (Figure, left), with a Thrombolysis in Myocardial Infarction (TIMI) 0 flow pattern. The left anterior descending artery had TIMI-2 flow (Figure, left). Intracoronary nitroglycerin, 100 μg, promptly resolved these changes (Figure, right). Five minutes later, intracoronary acetylcholine (50 μg) was administered, with continuous echocardiographic monitoring. This infusion reproduced chest pain, electrocardiographic changes, and ventricular dysfunction of midcavity TAB (Video 3, clips 1 and 2). After repeated intracoronary nitroglycerin administration, these changes resolved in less than 30 seconds (Video 3, clip 3). Three days after her condition stabilized, the patient was discharged home with a treatment regimen of extended-release diltiazem (240 mg), nitrates, and L-arginine (1000 mg every 8 hours). At 6-month follow-up examination, she was symptom free; electrocardiography and LV contractility were normal.
FIGURE.
Coronary angiograms of the left side obtained during coronary infusion of acetylcholine (50 μg) (left) and after intracoronary nitroglycerin (NTG) administration (right) (see text).
Previously, in a small pilot study involving typical TAB,5 I found that acetylcholine stimulation produced severe spasm of all coronay branches. I suggest that the midcavity variant results from similar intense, spontaneous spasticity that affects the diagonal, ramus, and circumflex branches and to a much lesser extent the left anterior descending artery. The patient's dominant circumflex pattern may have been important in causing the inferior/mid LV contractile changes.
Indeed, TAB involving any distribution seems to feature severe coronary reactivity to acetylcholine testing, characterized by sustained, diffuse spasticity that can induce myocardial stunning, unlike short-lived spontaneous spasm in Prinzmetal angina.5 Intracoronary nitroglycerin can quickly resolve such episodes.5 This degree of spasticity is unlike that seen in common coronary endothelial dysfunction. TAB variants seem to involve selective, not generalized, endothelial dysfunction.
The current case provides important evidence (although not final proof) that in any form of TAB, early echocardiographic LV monitoring can document acetylcholine-related reproduction of contractile dysfunction and its normalization after intracoronary nitroglycerin infusion.5 This case supports my general theory regarding TAB5 while explaining the midcavity variant. Generally, TAB seems to involve a spectrum of varying clinical patterns that depend on precipitating factors (which could be overwhelming in the presence of relatively “normal” endothelial function) and baseline endothelial dysfunction (which, when generalized, would be associated with recurrent apical TAB, but, when selective, would lead to atypical TAB variants).
Footnotes
Supplemental videos are linked to the full-text version of this article at www.mayoclinicproceedings.com.
VIDEO 1. Left ventricular cineangiograms.
VIDEO 2. Left ventricular video-echocardiograms of a recurrent episode.
VIDEO 3. Motion echocardiogram of the left ventricle at baseline (clip 1), after acetylcholine administration (clip 2), and after nitroglycerin infusion (clip 3), showing the onset of transient midventricular akinesia without apical ballooning.
References
- 1.Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol. 2003;41(5):737-742 [DOI] [PubMed] [Google Scholar]
- 2.Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858-865 [DOI] [PubMed] [Google Scholar]
- 3.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548 [DOI] [PubMed] [Google Scholar]
- 4.Kurowski V, Kaiser A, von Hof K, et al. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest 2007September;132(3):809-816 Epub 2007 Jun 15 [DOI] [PubMed] [Google Scholar]
- 5.Angelini P. Transient left ventricular apical ballooning: a unifying pathophysiologic theory at the edge of Prinzmetal angina. Catheter Cardiovasc Interv. 2008;71(3):342-352 [DOI] [PubMed] [Google Scholar]