Abstract
OBJECTIVE: To conduct in vitro studies as well as a phase 2 clinical trial in patients with smoldering or indolent multiple myeloma to determine if interleukin 1 (IL-1) inhibitors can delay or prevent active myeloma.
PATIENTS AND METHODS: Stromal cells were cocultured with IL-1β-expressing myeloma cells in the presence of dexamethasone, IL-1 receptor antagonist (IL-1Ra), or both. Levels of interleukin 6 (IL-6) and of apoptosis were also quantified. Between November 19, 2002, and May 24, 2007, 47 patients were enrolled in the study and subsequently treated with IL-1Ra. In 25 (53%) of the 47 study patients, low-dose dexamethasone (20 mg/wk) was added. The primary end point was progression-free survival (PFS).
RESULTS: In vitro, IL-1Ra was superior to dexamethasone at inhibiting IL-6 production; maximal IL-6 inhibition and apoptosis induction were achieved by addition of both IL-1Ra and dexamethasone. In the clinical trial, 3 patients achieved a minor response to IL-1Ra alone; 5 patients achieved a partial response and 4 patients a minor response after addition of dexamethasone. Seven patients showed a decrease in the plasma cell labeling index that paralleled a decrease in high-sensitivity C-reactive protein (hs-CRP) levels. The median overall PFS was 37.5 months. The median PFS for patients without (n=12) or with (n=35) a greater than 15% decrease in 6-month vs baseline hs-CRP levels was 6 months and more than 3 years, respectively (P=.002). Disease stability was maintained in 8 patients who received therapy for more than 4 years.
CONCLUSION: In patients with smoldering or indolent multiple myeloma who were at risk of progression to active myeloma, treatment with IL-1 inhibitors decreased the myeloma proliferative rate and hs-CRP levels in those who responded, leading to a chronic disease state and an improved PFS.
Trial Registration: clinicaltrials.gov identifier: NCT00635154
In patients with smoldering or indolent multiple myeloma at risk of progression to active myeloma, treatment with interleukin 1 inhibitors decreased the myeloma proliferative rate and high-sensitivity C-reactive protein levels in those who responded, leading to a chronic disease state and an improved progression-free survival.
cDNA = complementary DNA; FI = fold increase; hs-CRP = high-sensitivity C-reactive protein; IL-1 = interleukin 1; IL-6 = interleukin 6; IL-1Ra = IL-1 receptor antagonist; IMM = indolent multiple myeloma; ISR = injection site reaction; MGUS = monoclonal gammopathy of undetermined significance; MR = minor response; PCLI = plasma cell labeling index; PFS = progression-free survival; PR = partial response; SMM = smoldering multiple myeloma
Multiple myeloma is a universally fatal B-cell malignancy characterized by the progressive accumulation of monoclonal plasma cells.1 Multiple myeloma has clinically benign precursor conditions, termed monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM) (bone lesions absent), and indolent multiple myeloma (IMM) (bone lesions present).2,3 Patients with SMM and 20% or more bone marrow plasma cells have a median time to progression to active myeloma of 26 months; patients with IMM have an even shorter time to progression of 8 to 10 months.4-8 The current standard of care for patients with SMM or IMM is observation; however, many patients, concerned that the rate of progression could increase, are uncomfortable with this approach.2 Because some patients with SMM may have a stable disease state for many years despite the presence of an increased number of monoclonal plasma cells, subsets of patients in whom disease is likely to progress rapidly should be characterized, and effective treatments with minimal adverse effects should be developed for this stage of disease.
For editorial comment, see page 105
Interleukin 6 (IL-6) has been shown to be a central growth factor for myeloma cells.9-12 Animal studies using IL-6 knockout mice have shown that IL-6 is essential for the development of myeloma.13 Although many cytokines can stimulate IL-6 production, in myeloma interleukin 1 (IL-1) β appears to be one of the major cytokines responsible for the paracrine production of IL-6 by marrow stromal cells.14 Although IL-1β is expressed by the plasma cells of virtually all patients with myeloma, it is not produced by normal plasma cells.15-17 Bone marrow cells from patients with active myeloma induce higher levels of stromal cell IL-6 production than do those from patients with MGUS.14 The aberrant IL-1β produced by the myeloma cells induces IL-6 production by bone marrow stromal cells, which in turn supports the growth and survival of the myeloma cells.14 We hypothesized that inhibition of the IL-1β/IL-6 pathway would decrease levels of inflammatory cytokines, affecting multiple biomarkers in the myeloma microenvironment and leading to modulation of myeloma cell growth.
On the basis of these findings, we performed in vitro laboratory studies using IL-1β-transduced myeloma cell lines14 and a phase 2 clinical trial. We investigated the biology, efficacy, and safety of the IL-1 receptor antagonist (IL-1Ra) and low-dose dexamethasone and the possibility of using them to delay or prevent active myeloma in patients with SMM or IMM. In addition to the standard M-protein measurements, we found that individual serial measurements of high-sensitivity C-reactive protein (hs-CRP), a surrogate marker for serum IL-6 levels, and the plasma cell labeling index (PCLI), a marker of myeloma cell growth, were useful in showing that IL-6 and the myeloma proliferative component were inhibited in vivo and in identifying responsive patients with an improved progression-free survival (PFS).
PATIENTS AND METHODS
In Vitro Studies
Transduction of Myeloma Cell Lines. KAS-6/1 myeloma cells were retrovirally transduced with a mature IL-1β complementary DNA (cDNA) construct or vector alone using the RetroXpress System available from Clontech (Palo Alto, CA). A human mature IL-1β cDNA was isolated using polymerase chain reaction with appropriate IL-1β oligonucleotide primers and cDNA from normal bone marrow mononuclear cells. An ATG start site was added to the 5′ end of the mature IL-1β construct using polymerase chain reaction.18 Both the KAS-6/1 parent line and the IL-1β-transduced KAS-6/1 myeloma cell lines have been extensively characterized elsewhere.14,19
Stromal Cell Coculture Assay. Normal stromal cells (Clonetics, Walkersville, MD) were plated in a 24-well plate at 0.5 to 1.0 × 105 cells/mL and incubated at 37°C for 48 hours. The stromal cells were composed predominantly of CD38-CD45-CD44+ fibroblasts. Subsequently, the stromal cells were washed and cocultured with myeloma cells with or without dexamethasone (10 μM) (Sigma, St Louis, MO) and/or IL-1Ra (1 μg/mL) (Amgen, Thousand Oaks, CA) for another 48 hours at 37°C. The stromal cells were directly cocultured with 1 million transduced KAS-6/1 myeloma cells. Supernatants were harvested and frozen at -80°C and subsequently assayed for IL-6 using a human IL-6 enzyme-linked immunosorbent assay (ELISA) kit (Biosource, Carlsbad, CA) according to the manufacturer's specifications. Apoptosis was determined using Annexin V/propidium iodide and flow cytometry, as previously described.20,21
Phase 2 Clinical Trial
Between November 19, 2002, and May 24, 2007, 47 patients were enrolled in the study. Inclusion criteria included a new or preexisting diagnosis of SMM or IMM that did not require immediate therapy, 10% or more bone marrow plasma cells, a monoclonal IgG or IgA protein level of 3.0 g/dL or more, an Eastern Cooperative Oncology Group performance status of 0 to 1, a leukocyte count of 3.5 × 109/L or more, an absolute neutrophil count of 1.7 × 109/L or more, a creatinine level 1.5 times or less the upper limit of normal, and a signed consent form. Patients were excluded if they were pregnant or lactating or if they had active myeloma or primary amyloidosis, prior treatment with any agent that could affect M protein within 30 days, acute or chronic infection, malignancy within past 5 years except for basal cell or carcinoma in situ of the cervix, New York Heart Association class III or IV heart failure, asthma, or any other illness that would interfere with participation in the study. This study was approved by the Mayo Clinic Institutional Review Board in accordance with federal regulations and with the Declaration of Helsinki and all patients provided written informed consent.
Treatment Plan. Anakinra (100 mg/d), an IL-1Ra, was subcutaneously administered to all patients for 6 months. Patients with evidence of clinical improvement (ie, reduction in M-protein levels) continued receiving IL-1Ra alone. Patients with stable disease after 6 months of IL-1Ra therapy or those with clinical features worrisome for progression (ie, increasing M-protein levels) received low-dose dexamethasone (20 mg/wk) in conjunction with the IL-1Ra. Patients were evaluated monthly for the first 3 months, every 6 weeks for the remainder of the first year, and then every 3 months thereafter.
Laboratory Testing. The PCLI, a measure of myeloma cell proliferation as determined by bromodeoxyuridine incorporation, was performed as previously described.22 Interleukin 1 levels were determined by coculturing supernatants harvested from unsorted patient bone marrow cells with normal stromal cells and measuring IL-6 production (IL-6 fold increase fi), as previously described.14 Levels of hs-CRP, measured in milligrams per liter, are a surrogate marker for serum IL-6 levels.23
Response and Toxicity Criteria. Response was defined as a reduction in serum M-protein levels of at least 25% (minor response [MR]) or at least 50% (partial response [PR]), confirmed on 2 consecutive evaluations at least 4 weeks apart. Disease progression was defined as a 25% increase (at least 1 g/dL) in M-protein levels. Hypercalcemia (calcium levels >11.5 mg/dL; to convert to mmol/L, multiply by 0.25), a 50% increase in the size of lytic lesions or soft-tissue plasmacytomas, or the appearance of new lytic lesions constituted progression. Disease that did not satisfy the criteria for PR, MR, or progression was considered stable.
Patient safety was monitored using patient diaries that were evaluated according to the Common Toxicity Criteria, versions 2 and 3. For grade 3 to 4 neutropenia, the dosage of IL-1Ra was decreased to 100 mg every other day. Adjustment of the dexamethasone dosage was also allowed if needed to avoid toxicity.
Statistical Analyses. The primary end point of this trial was to assess the PFS of patients with SMM or IMM. Sample size was based on a 0.8 power to detect an increase in PFS from 1 to 2 years with an α level of 0.05, resulting in a sample size of 47 patients who were to receive treatment. Progression-free survival was measured from the date of study entry to the date of last follow-up and was determined using the Kaplan-Meier method. Confidence intervals were calculated using the standard error estimated from the Greenwood formula. Any result of P<.05 was considered to be statistically significant. In addition to clinical outcomes, other correlative markers were evaluated. To determine the cutoff for C-reactive protein and M-protein levels, a partitioning algorithm was used.
RESULTS
In Vitro Studies
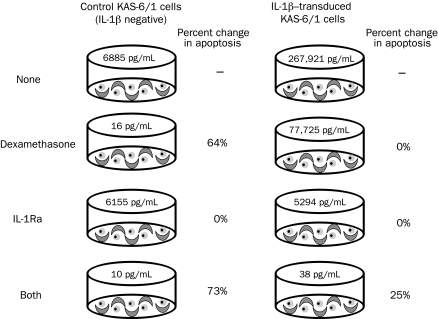
We investigated the effects of dexamethasone and IL-1Ra on stromal cell IL-6 production and myeloma cell apoptosis using an in vitro myeloma cell/stromal cell coculture assay. Control vector-transduced, IL-1β-negative KAS-6/1 cells were cocultured with stromal cells and dexamethasone (10 μM), IL-1Ra (1 μg/mL), or both (or, for controls, with neither) for 48 hours, and the percentage of apoptotic cells was quantified by flow cytometry (Figure 1). Results in Figure 1 are expressed as the percent increase in apoptotic cells above levels in control cultures without drugs. The numbers in pg/mL represent the amount of IL-6 generated in the cocultures of myeloma and stromal cells. Because the KAS-6/1 cells are IL-1β negative, very little paracrine IL-6 was generated (6885 pg/mL). Addition of IL-1Ra alone had little effect on IL-6 production and was unable to induce apoptosis. However, administration of dexamethasone alone induced substantial apoptosis (64%) above control, and this increased apoptosis was minimally enhanced with the addition of IL-1Ra (73%). However, no detectable levels of IL-1β were found in these cultures, and therefore they are not representative of the myeloma microenvironment. When IL-1β-transduced KAS-6/1 cells were cocultured with stromal cells, large amounts of IL-6 (267,921 pg/mL) were generated. In this situation, dexamethasone decreased IL-6 production but caused no change in apoptosis above control. The large amount of remaining paracrine IL-6 (77,725 pg/mL) was sufficient to completely inhibit dexamethasone-induced apoptosis. Alone, IL-1Ra was unable to induce apoptosis, but it was more efficient than dexamethasone at inhibiting paracrine IL-6 production, thereby returning levels to the vector control baseline. When both agents were administered, dexamethasone was again able to increase apoptosis substantially (25%) in the presence of IL-1Ra, thereby inhibiting paracrine IL-6 production (38 pg/mL). These in vitro results suggest that IL-1Ra alone does not induce apoptosis; however, its ability to inhibit paracrine IL-6 production is superior to that of dexamethasone at the concentrations tested. Furthermore, the combination of IL-1Ra and dexamethasone appear to complement each other in the inhibition of IL-6 production and induction of myeloma cell apoptosis.
FIGURE 1.
Effects of an interleukin 1 receptor antagonist (IL-1Ra) and dexamethasone on myeloma cell apoptosis and interleukin 6 (IL-6) production in myeloma cell-stromal cell cocultures. Normal stromal cells (1 × 105 cells/mL) were cocultured directly with either an empty vector control or IL-1β-expressing myeloma cells (1 × 106 cells/mL) in the absence or presence of dexamethasone, IL-1Ra, or both for 48 h at 37°C. Supernatants were assayed for IL-6, and apoptosis was determined using Annexin V/propidium iodide staining and flow cytometry. “None” refers to control cultures without drug, and data are expressed as the percent increase in apoptotic cells above levels in control cultures. Numbers in pg/mL refer to the amount of IL-6 detected by enzyme-linked immunosorbent assay in cocultures of myeloma and stromal cells.
Phase 2 Clinical Trial
On the basis of these preclinical findings, we conducted a phase 2 clinical trial in patients with SMM or IMM to determine the biologic activity of IL-1Ra and dexamethasone and to assess their potential toxicity and their effect on PFS in patients with SMM or IMM. Because IL-1β induces paracrine IL-6, we hypothesized that IL-1Ra would inhibit IL-6 production and myeloma cell growth. Patients were deemed eligible for the study if they had 10% or more bone marrow plasma cells and/or an IgG or IgA M-spike of 3 g/dL or greater and did not require immediate chemotherapy. Patients received subcutaneous administrations of 100 mg/d of IL-1Ra for 6 months. If they showed signs of clinical improvement (ie, reduction in M-protein levels), they continued receiving IL-1Ra alone. However, if their disease was stable after 6 months of IL-1Ra or if they had clinical features worrisome for progression (ie, rising M-protein levels), they began receiving low-dose dexamethasone (20 mg/wk) in conjunction with the IL-1Ra. As a surrogate for IL-6 production, hs-CRP levels were monitored. Forty-seven patients were included for analysis on the basis of intent to treat at diagnosis.
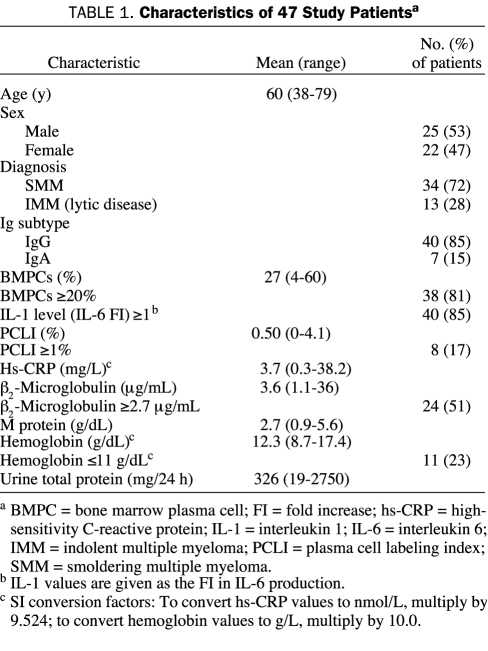
The characteristics of the study patients are summarized in Table 1. Of the 47 patients, 34 had SMM and 13 had IMM with lytic bone disease. Almost all patients (46/47) had 10% or more bone marrow plasma cells, and 85% generated IL-1 levels (IL-6 FI) consistent with myeloma (≥1).
TABLE 1.
Characteristics of 47 Study Patientsa
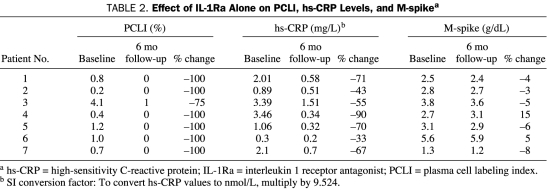
IL-1Ra Alone Inhibits Paracrine IL-6 Production and Myeloma Cell Growth. When administered alone, IL-1Ra appeared to target the proliferative myeloma fraction, resulting in a decrease in the PCLI (a measure of the myeloma cell proliferative rate). As shown in Table 2, 7 patients with a baseline PCLI greater than zero who were treated with IL-1Ra alone had a 75% to 100% reduction from their baseline PCLI value after 6 months of therapy. The change in the PCLI paralleled a decrease in hs-CRP levels in all cases (33%-90% decrease; Table 2). Although an attempt was made to measure IL-6 levels, they were found to be too low in most patients to be reproducible, and the hs-CRP was judged to be more informative. These findings suggest that IL-1Ra inhibited IL-6 production in the myeloma microenvironment, as evidenced by a reduction in hs-CRP levels, resulting in suppression of myeloma cell proliferation. Notably, proliferation was suppressed independently of a reduction in M-protein production.
TABLE 2.
Effect of IL-1Ra Alone on PCLI, hs-CRP Levels, and M-spikea
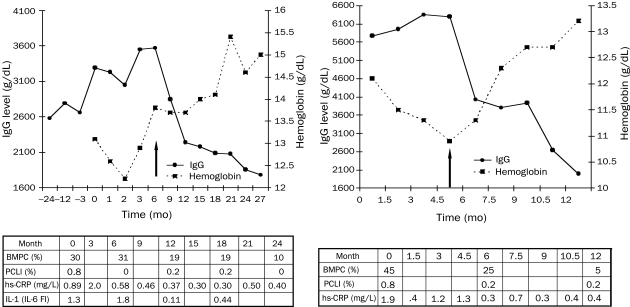
Induction of a Chronic Disease State in Patients at High Risk of Active Myeloma. In our phase 2 clinical trial, we combined IL-1Ra with low-dose dexamethasone on the basis of our in vitro findings that IL-1Ra inhibits paracrine IL-6 production and dexamethasone decreases IL-1β levels through myeloma cell apoptosis. Data for 2 representative patients treated with combined IL-1Ra and low-dose dexamethasone therapy are provided in Figure 2. One patient (Figure 2, left) had worsening anemia and an increasing IgG level during the 2 years before participation in this clinical trial. After 6 months of IL-1Ra alone, the patient's PCLI decreased from 0.8% to 0%, and his hs-CRP level decreased from 0.89 to 0.58 mg/L (to convert to nmol/L, multiply by 9.524).
FIGURE 2.
Clinical effects of the combination of interleukin 1 (IL-1) receptor antagonist (IL-1Ra) and low-dose dexamethasone. Left, This patient received IL-1Ra alone at month 0 and the combination of IL-1Ra and low-dose dexamethasone at month 6. Right, This patient received IL-1Ra alone at month 0 and the combination of IL-1Ra and low-dose dexamethasone at month 4.5. Arrows indicate the initiation of dexamethasone. Levels of IL-1 are determined using interleukin 6 (IL-6) fold increase (see Patients and Methods). BMPC = bone marrow plasma cell; hs-CRP = high-sensitivity C-reactive protein; PCLI = plasma cell labeling index. SI conversion factors: To convert hs-CRP values to to nmol/L, multiply by 9.524.
At 6 months, when dexamethasone was added to the IL-1Ra, the M-spike and percentage of bone marrow plasma cells decreased while hemoglobin levels returned to normal (Figure 2, left). The osteolytic lesions in the right radius of this patient have remained stable, as evidenced by findings on positron emission tomography and bone survey. He continues to be asymptomatic with stable disease after 4.5 years of participation in this trial.
The other patient (Figure 2, right), who was anemic when enrolled in the study, had 45% bone marrow plasma cells and an elevated PCLI (0.8%). The IgG levels continued to increase, and dexamethasone was added after 4.5 months. Response to the combined treatment was noted 1.5 months after addition of low-dose dexamethasone. Both the PCLI and hs-CRP levels decreased at 6 months (Figure 2, right) and have remained low on combination therapy. Levels of IgG decreased by 65%, and hemoglobin increased to near normal levels. The patient remains asymptomatic and stable after more than 3 years of therapy.
Serial IL-1 levels were determined by coculturing supernatants harvested from unsorted patient bone marrow cells with normal stromal cells and measuring IL-6 production (IL-6 FI), as previously described.14 Levels of IL-1 (IL-6 FI) of 1.0 or more were consistent with active/progressive myeloma, and values less than 1.0 were typical of MGUS.14 For the patient described in Figure 2, left, the IL-1 levels at baseline (1.3) and at 6-month follow-up (1.8) remained stable while he was taking IL-1Ra alone. However, after dexamethasone was added, the IL-1 levels decreased to 0.11 at 1 year and remained low (0.44) at 18 months. Because dexamethasone therapy was initiated at month 4.5, the effect of IL-1Ra alone on IL-1 levels cannot be determined for the patient described in Figure 2, right; however, like the patient described in Figure 2, left, this patient's IL-1 levels decreased while receiving combination therapy, from 4.8 to 1.5. In summary, serial measurements showed that dexamethasone, but not IL-1Ra, can lower IL-1 levels.
All 47 patients received IL-1Ra initially; 25 (53%) of 47 patients subsequently received combined IL-1Ra and dexamethasone therapy. The median follow-up was 40 months. The overall PFS was 37.5 months (95% confidence interval, 9.6-~). In the clinical trial, 3 patients achieved an MR to IL-1Ra alone; 5 patients achieved a PR and 4 patients an MR after addition of dexamethasone.
Eight patients have now maintained a chronic disease state for more than 4 years while receiving therapy. One of these patients had an initial high PCLI of 1.2% and an increasing M-protein level. He was the only patient enrolled in the study with less than 10% bone marrow plasma cells but an M-spike of 3 g/dL or greater. His disease continues to be controlled by IL-1Ra alone. Disease control has also been achieved in other patients with less than 20% bone marrow plasma cells by IL-1Ra alone. Another patient with an exceptionally high PCLI of 6.4% has remained stable while receiving combined IL-1Ra and dexamethasone therapy for more than 4 years.
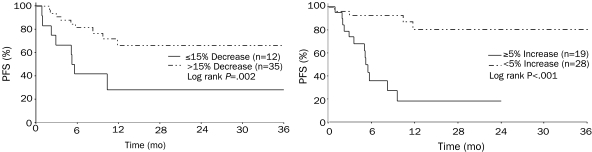
Reduction in Baseline hs-CRP Levels Predicts Stability of Disease. In addition to clinical outcomes, we also evaluated changes in other correlative markers from baseline to 6 months. Because hs-CRP levels are often used as a surrogate marker for IL-6 levels, we investigated whether hs-CRP levels can be used to identify patients who can maintain stable disease while receiving this treatment. Statistical analysis using a partitioning algorithm showed that the median PFS for patients without (n=12) or with (n=35) a greater than 15% decrease in 6-month vs baseline levels of hs-CRP was 6 months and more than 3 years, respectively (P=.002) (Figure 3, left). Disease progression was more likely in patients with IMM. Of the 35 patients with a decrease in hs-CRP levels, 20% presented with IMM, whereas 50% of the 12 patients without a decrease in hs-CRP levels had IMM. Stability of the M protein also distinguished the 2 groups. The median PFS for patients with (n=19) and without (n=28) a 5% or greater increase in 6-month vs baseline M-protein levels was 6 months and more than 3 years, respectively (P<.0001) (Figure 3, right).
FIGURE 3.
Progression-free survival (PFS). Left, The median PFS for patients without (n=12) and with (n=35) a decrease at 6 mo of greater than 15% from the baseline hs-CRP level was 6 mo and more than 3 y, respectively (P=.002). Right, The median PFS for patients with (n=19) and without (n=28) an increase at 6 mo of 5% or greater in the M-protein level was 6 mo and more than 3 y, respectively (P<.001).
Toxicity of Therapy. Injection site reactions (ISRs) from the IL-1Ra were the most common side effects, occurring in 86% of patients. Two patients were withdrawn from the study because of ISRs in the first months of treatment. Grade 4 adverse events occurred in 5 patients, and grade 3 adverse events occurred in 16. The most common grade 3 to 4 toxicity was asymptomatic neutropenia (8 patients) due to the IL-1Ra; this toxicity was managed by decreasing the IL-1Ra dosage to every other day. Thrombosis was infrequent in this trial, occurring in only 3 patients (6%). Two patients (4%) developed grade 4 infections (pneumonias), and 5 (11%) experienced grade 3 infections. In patients with infections requiring antibiotics, IL-1Ra and dexamethasone were withheld until the illness resolved. Two patients (4%) developed a grade 3 increase in creatinine level. Other potentially dexamethasone-related toxicities were colon perforation (1 patient), myocardial infarction (1 patient), and cataracts (2 patients). Four patients ended active treatment because of adverse events (2 from ISRs within the first month of therapy). No grade 5 events were reported.
DISCUSSION
The results of the clinical trial demonstrated that IL-1Ra in vivo targets the myeloma proliferative component. Interleukin 1 inhibitors can be successfully used in the treatment of patients with SMM or IMM to prevent the progression to active myeloma. In patients with SMM or IMM and an elevated on-study PCLI, IL-1Ra led to a decrease in both the levels of hs-CRP, a surrogate marker for plasma cell IL-6 levels, and correspondingly, the PCLI, a measure of the myeloma cell proliferative rate in responsive patients. These results, obtained after treatment with IL-1Ra alone, were found to be independent of a reduction in M-protein levels. The importance of IL-1 lies in the fact that small amounts of IL-1β can induce large amounts of IL-6 in a paracrine fashion.14,24 Because IL-6 is a central myeloma growth factor, it appears that the myeloma proliferative component can be inhibited with IL-1Ra in early-stage disease.
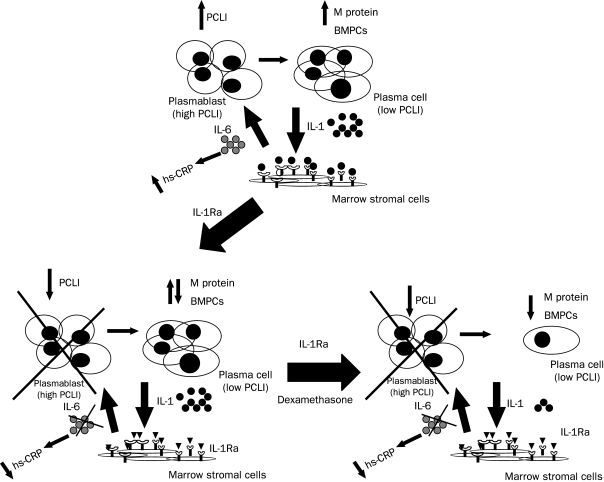
Interleukin-1Ra also suppressed the IL-6 production that inhibited the dexamethasone-induced apoptosis of myeloma cells in vitro. In general, IL-1Ra and dexamethasone appear to have biologic effects that complement each other. Dexamethasone decreases IL-1 levels via myeloma cell apoptosis, thereby increasing the effectiveness of IL-1Ra (Figure 4). This was shown clinically in the patient described in Figure 2, right; the patient's M-spike and hs-CRP levels both started to decrease simultaneously with the addition of low-dose dexamethasone after 4.5 months. Dexamethasone reduced the nonproliferating, IL-1β-producing myeloma compartment, as evidenced by a decrease in serial IL-1 levels (Figures 2 and 4), in addition to secreted M-protein levels. Interleukin 1Ra reduced the elevated IL-6 levels in the microenvironment and inhibited the IL-6-responsive proliferating myeloma cell subset identified by the PCLI (Figure 4).
FIGURE 4.
Schematic of the role of an interleukin 1 receptor antagonist (IL-1Ra) and dexamethasone in the myeloma microenvironment. Bone marrow plasma cells (BMPCs) from patients with progressive smoldering multiple myeloma (SMM) or indolent multiple myeloma (IMM) produce IL-1β that stimulates stromal cells to make interleukin 6 (IL-6), which can be monitored by the high-sensitivity C-reactive protein (hs-CRP) levels (upper panel). The IL-6 can then stimulate the growth of the proliferative myeloma component, resulting in an elevated plasma cell labeling index (PCLI). Interleukin 1Ra selectively targets the proliferative myeloma component, resulting in a decrease in the hs-CRP levels and the PCLI. The proliferative component is crossed out (lower left) because it is unknown whether these cells are induced into a nonproliferative state or eliminated. Dexamethasone complements IL-1Ra biologic activity by inducing myeloma cell apoptosis and decreasing the percentage of BMPCs, M-protein levels, and myeloma cell-produced IL-1 levels (lower right).
Although several studies have attempted to treat patients with SMM or IMM, 25-27 to our knowledge our study is the first to take an anticytokine approach. In this clinical trial, IL-1Ra and dexamethasone induced a chronic disease state, resulting in decreased hs-CRP levels and PCLIs in responsive patients with SMM or IMM. Disease could be controlled in patients with low numbers of plasma cells by IL-1Ra alone, whereas low-dose dexamethasone was typically required for disease control in patients with 20% or greater plasma cells. Because the goal of this study was to delay or prevent the development of active myeloma, MRs or the induction of stable disease are important findings in this disease group. Of the 47 study patients, 8 (17%) have now exceeded 4 years with a chronic disease state while receiving therapy. The combination of IL-1Ra and low-dose dexamethasone induces a chronic disease state similar to that in patients with a less aggressive SMM who have a long PFS. Although such patients usually do not show a decrease in their M-spike, they can have stable disease for years. One may speculate that these patients with stable disease release less IL-1β and therefore stimulate less IL-6. Targeting the myeloma proliferative component with IL-1Ra leads to stable disease by reducing the growth rate of the proliferating plasma cells and potentially slowing the acquisition of harmful genetic changes.
Several different adverse prognostic factors have been shown to assist in the identification of patients with SMM or IMM who may be at risk of disease progression. Kyle et al4 reported on 276 patients with SMM and found that patients with 10% or greater bone marrow plasma cells had the greatest probability of disease progression when compared with patients with less than 10% plasma cells and an M-spike of greater than 3 g/dL. More specifically, patients with 20% or greater bone marrow plasma cells had a median time to disease progression of 26 months. Other adverse factors in asymptomatic myeloma include an elevated PCLI, an increase in the number of circulating plasma cells, a serum M-protein level greater than 3 g/dL, IgA subtype, urinary M-protein level greater than 50 mg/d, and free light chain ratio.5,28-30 Patients with IMM and bone lesions have an even shorter median time to progression of approximately 8 to 10 months.6-8 Using an IL-1 bioassay, we have shown that 2 groups of patients with SMM or IMM may be distinguished: those with IL-1 levels (IL-6 FI) of 1 or more (similar to those of patients with active myeloma) and those with IL-1 levels less than 1 (comparable to patients with MGUS).14 On the basis of multiple prognostic factors for increased risk to progression in patients with SMM or IMM, most of the 47 patients enrolled in this trial were at high risk of progression to active MM, with 98% having 10% or greater bone marrow plasma cells (81% with ≥20% plasma cells), 85% generating IL-1 levels (IL-6 FI) consistent with myeloma (≥1), and 28% having bone lesions.
Future clinical trials are warranted that use the adverse prognostic factors previously discussed and treatments with low toxicity that reduce both the proliferative and nonproliferative myeloma compartments in patients with SMM or IMM. Controlling the myeloma proliferative component is critical in managing both patients with SMM or IMM and those with active myeloma. In several myeloma studies, Greipp et al22,28,31-33 have clearly shown that patients with high PCLIs had shortened overall survival, highlighting the importance of the PCLI as a prognostic factor. Furthermore, it has been suggested that myeloma remains incurable because the stem cell/proliferative component is not adequately eliminated by current therapies.34 The results from the clinical trial reported here show that IL-1 inhibitors can be used to target the myeloma proliferative component in patients with SMM or IMM and suggest the possibility that these inhibitors may also be used for the same purpose in patients with newly diagnosed myeloma.
CONCLUSION
Many patients with SMM or IMM are at high risk of progression to active myeloma. We investigated whether the development of active myeloma could be delayed in patients with SMM or IMM by performing in vitro laboratory studies with IL-1β-transduced myeloma cell lines and a phase 2 clinical trial using IL-1Ra and low-dose dexamethasone. The results of the clinical trial showed that IL-1Ra targeted the proliferative myeloma fraction in vivo, resulting in a decrease in hs-CRP levels, a surrogate marker for plasma cell IL-6 levels, and correspondingly, the PCLI, a measure of the myeloma cell proliferative rate in responsive patients. Dexamethasone suppressed the nonproliferating, IL-1β-producing myeloma compartment, as evidenced by a decrease in serial IL-1 levels, in addition to the secreted M protein. In patients with SMM or IMM at risk of progression to active myeloma, treatment with IL-1Ra and low-dose dexamethasone can induce a chronic disease state by targeting IL-1β-induced IL-6 production and the myeloma proliferative component.
Footnotes
This work was supported by the Multiple Myeloma Research Foundation, the Robert A. Kyle Hematologic Malignancies Fund, and the National Institutes of Health (P0I CA62242).
Dr Lust received travel support from Amgen, and Dr Greipp is a member of the Amgen Advisory Board.
REFERENCES
- 1.Kyle RA, Rajkumar SV. Treatment of multiple myeloma: an emphasis on new developments. Ann Med. 2006;38(2):111-115 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Hematol Oncol Clin North Am. 2007;21(6):1093-1113 [DOI] [PubMed] [Google Scholar]
- 3.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749-757 [PubMed] [Google Scholar]
- 4.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590 [DOI] [PubMed] [Google Scholar]
- 5.Weber DM, Dimopoulos MA, Moulopoulos LA, Delasalle KB, Smith T, Alexanian R. Prognostic features of asymptomatic multiple myeloma. Br J Haematol. 1997;97(4):810-814 [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Moulopoulos A, Smith T, Delasalle KB, Alexanian R. Risk of disease progression in asymptomatic multiple myeloma. Am J Med. 1993;94(1):57-61 [DOI] [PubMed] [Google Scholar]
- 7.Facon T, Menard JF, Michaux JL, et al. Groupe d'Etudes et de Recherche sur le Myelome (GERM) Prognostic factors in low tumour mass asymptomatic multiple myeloma: a report on 91 patients. Am J Hematol. 1995;48(2):71-75 [DOI] [PubMed] [Google Scholar]
- 8.Wisloff F, Andersen P, Andersson TR, et al. Incidence and follow-up of asymptomatic multiple myeloma: the myeloma project of health region I in Norway. II. Eur J Haematol. 1991;47(5):338-341 [DOI] [PubMed] [Google Scholar]
- 9.Kawano M, Hirano T, Matsuda T, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 1988;332(6159):83-85 [DOI] [PubMed] [Google Scholar]
- 10.Schwab G, Siegall CB, Aarden LA, Neckers LM, Nordan RP. Characterization of an interleukin-6-mediated autocrine growth loop in the human multiple myeloma cell line, U266. Blood 1991;77(3):587-593 [PubMed] [Google Scholar]
- 11.Klein B, Zhang XG, Jourdan M, et al. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood 1989;73(2):517-526 [PubMed] [Google Scholar]
- 12.Portier M, Rajzbaum G, Zhang XG, et al. In vivo interleukin 6 gene expression in the tumoral environment in multiple myeloma. Eur J Immunol. 1991;21(7):1759-1762 [DOI] [PubMed] [Google Scholar]
- 13.Hilbert DM, Kopf M, Mock BA, Köhler G, Rudikoff S. Interleukin 6 is essential for in vivo development of B lineage neoplasms. J Exp Med. 1995;182(1):243-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Y, Donovan KA, Kline MP, et al. Identification of two groups of smoldering multiple myeloma patients who are either high or low producers of interleukin-1. J Interferon Cytokine Res. 2006;26(2):83-95 [DOI] [PubMed] [Google Scholar]
- 15.Donovan KA, Lacy MQ, Kline MP, et al. Contrast in cytokine expression between patients with monoclonal gammopathy of undetermined significance or multiple myeloma. Leukemia 1998;12(4):593-600 [DOI] [PubMed] [Google Scholar]
- 16.Lust JA, Donovan KA. The role of interleukin-1 beta in the pathogenesis of multiple myeloma. Hematol Oncol Clin North Am. 1999;13(6):1117-1125 [DOI] [PubMed] [Google Scholar]
- 17.Lacy MQ, Donovan KA, Heimbach JK, Ahmann GJ, Lust JA. Comparison of interleukin-β1 expression by in situ hybridization in monoclonal gammopathy of undetermined significance and multiple myeloma. Blood 1999;93(1):300-305 [PubMed] [Google Scholar]
- 18.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 1989;77(1):61-68 [DOI] [PubMed] [Google Scholar]
- 19.Westendorf JJ, Ahmann GJ, Greipp PR, Witzig TE, Lust JA, Jelinek DF. Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin-6. Leukemia 1996;10(5):866-876 [PubMed] [Google Scholar]
- 20.Feinman R, Koury J, Thames M, Barlogie B, Epstein J, Siegel DS. Role of NF-κB in the rescue of multiple myeloma cells from glucocorticoid-induced apoptosis by bcl-2. Blood 1999;93(9):3044-3052 [PubMed] [Google Scholar]
- 21.Witzig TE, Timm M, Larson D, Therneau T, Greipp PR. Measurement of apoptosis and proliferation of bone marrow plasma cells in patients with plasma cell proliferative disorders. Br J Haematol. 1999;104(1):131-137 [DOI] [PubMed] [Google Scholar]
- 22.Greipp PR, Witzig TE, Gonchoroff NJ, et al. Immunofluorescence labeling indices in myeloma and related monoclonal gammopathies. Mayo Clin Proc. 1987;62(11):969-977 [DOI] [PubMed] [Google Scholar]
- 23.Bataille R, Boccadoro M, Klein B, Durie B, Pileri A. C-reactive protein and β-2 microglobulin produce a simple and powerful myeloma staging system. Blood 1992;80(3):733-737 [PubMed] [Google Scholar]
- 24.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 1996;87(6):2095-2147 [PubMed] [Google Scholar]
- 25.Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rödjer S, Westin J, Myeloma Group of Western Sweden Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I—a randomized study. Eur J Haematol. 1993;50(2):95-102 [DOI] [PubMed] [Google Scholar]
- 26.Rajkumar SV, Gertz MA, Lacy MQ, et al. Thalidomide as initial therapy for early-stage myeloma. Leukemia 2003;17(4):775-779 [DOI] [PubMed] [Google Scholar]
- 27.Musto P, Petrucci MT, Bringhen S, et al. Final analysis of a multicenter, randomised study comparing zoledronate vs observation in patients with asymptomatic myeloma [abstract 534]. Blood 2007;110(11, pt 1):164a [Google Scholar]
- 28.Greipp PR, Kyle RA. Clinical, morphological, and cell kinetic differences among multiple myeloma, monoclonal gammopathy of undetermined significance, and smoldering multiple myeloma. Blood 1983;62(1):166-171 [PubMed] [Google Scholar]
- 29.Witzig TE, Kyle RA, O'Fallon WM, Greipp PR. Detection of peripheral blood plasma cells as a predictor of disease course in patients with smouldering multiple myeloma. Br J Haematol. 1994;87(2):266-272 [DOI] [PubMed] [Google Scholar]
- 30.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering multiple myeloma [abstract 1487]. Blood 2007;110(11, pt 1):445a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greipp PR, Katzmann JA, O'Fallon WM, Kyle RA. Value of β2-microglobulin level and plasma cell labeling indices as prognostic factors in patients with newly diagnosed myeloma. Blood 1988;72(1):219-223 [PubMed] [Google Scholar]
- 32.Greipp PR, Lust JA, O'Fallon WM, Katzmann JA, Witzig TE, Kyle RA. Plasma cell labeling index and β2-microglobulin predict survival independent of thymidine kinase and C-reactive protein in multiple myeloma. Blood 1993;81(12):3382-3387 [PubMed] [Google Scholar]
- 33.Greipp PR, Lust JA. Pathogenetic relation between monoclonal gammopathies of undetermined significance and multiple myeloma. Stem Cells 1995;13(suppl 2):10-21 [PubMed] [Google Scholar]
- 34.Huff CA, Matsui W, Smith BD, Jones RJ. The paradox of response and survival in cancer therapeutics. Blood 2006January;107(2):431-434 Epub 2005 Sep 8 [DOI] [PMC free article] [PubMed] [Google Scholar]