Abstract
Antiplatelet therapy is an evidence-based, guideline-recommended, worldwide standard of care for treatment of patients with atherothrombosis. However, clinical implementation of the guidelines is suboptimal, in part because of physician and patient nonadherence. The increased risk of bleeding associated with antiplatelet therapy is often the reason for nonadherence, and several programs have been created to increase adherence to guideline treatment recommendations. Despite the relative success of such initiatives, including Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines, Guidelines Applied in Practice, and the American Heart Association's Get With the Guidelines and a Science Advisory, a current estimate is that less than 50% of atherothrombotic patients are taking antiplatelet therapies as recommended by national guidelines. A PubMed and MEDLINE search of the literature (January 1, 1983-May 15, 2008) was performed to examine the bleeding risks associated with various antiplatelet therapies. Relevant clinical trials, observational registry data, and other studies relevant to treatment and guideline recommendations were selected from articles generated through specific search terms. This comprehensive review contributes to the understanding of the benefit-to-risk ratio of antiplatelet therapy for patients with atherothrombosis.
ACC = American College of Cardiology; AHA = American Heart Association; CABG = coronary artery bypass grafting; CI = confidence interval; CLARITY = Clopidogrel as Adjunctive Reperfusion Therapy; COMMIT = Clopidogrel and Metoprolol in Myocardial Infarction Trial; COX-2 = cyclooxygenase 2; CURE = Clopidogrel in Unstable Angina to Prevent Recurrent Events; DISPERSE-2 = Dose Confirmation Study Assessing Antiplatelet Effects of AZD6140 vs. Clopidogrel in non-ST-segment Elevation Myocardial Infarction; ER = extended release; GUSTO = Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries; HR = hazard ratio; MI = myocardial infarction; NSAID = nonsteroidal antiinflammatory drug; PCI = percutaneous coronary intervention; TIA = transient ischemic attack; TIMI = Thrombolysis in Myocardial Infarction; TRITON = Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel
Substantial evidence exists for the benefit of secondary prevention with antiplatelet therapy for patients who have experienced an acute atherothrombotic event.1 Long-term antiplatelet therapy significantly reduces the risk of major cardiovascular events across a wide range of atherothrombotic syndromes.1 Accordingly, the American Heart Association (AHA) and American College of Cardiology (ACC) have published evidence-based guidelines to provide physicians with a framework for secondary prevention in patients at risk of ischemic or cardiovascular events.2-5 Although these recommendations increase use of risk-reducing medications,6 physician and patient adherence to the guidelines needs to improve.
Several hospital-based quality initiatives have improved adherence with consensus guideline recommendations. For instance, the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines (CRUSADE) initiative, the Guidelines Applied in Practice, and the AHA's Get With the Guidelines Program6-8 have improved adherence to secondary prevention guidelines such that more than 90% (up from approximately 81%) of US patients are discharged with an antiplatelet agent prescription after an acute coronary event.6,8,9
However, a prescription at discharge does not necessarily translate into long-term use or therapy adherence by the patient. Findings from an ambulatory care database indicated that only 30% of patients with a history of atherothrombotic events were receiving aspirin as part of their routine care in 2002,10 and data from the Duke Databank for Cardiovascular Disease in the same year showed that almost 30% of patients who were prescribed aspirin were not consistently taking it.11 This information is disconcerting because poor adherence with antiplatelet therapy as secondary preventive therapy is associated with a substantially worse outcome.12-15
Many factors can influence prescribing practices and patient adherence. One of these factors may be an overestimation of the bleeding risk in relationship to cardiovascular benefit of antiplatelet therapy. A PubMed and MEDLINE search of the literature (January 1, 1983-May 15, 2008) was performed to examine the bleeding risks associated with various antiplatelet therapies. Relevant clinical trials, observational registry data, and other studies relevant to treatment and guideline recommendations were selected from articles generated through specific search terms, including antiplatelet therapy (and specific agents), safety, tolerability, bleeding classification, cardiovascular risk, benefit to risk, and treatment recommendations. This article reviews the risk of bleeding with antiplatelet therapy, including classification schemes used to stratify bleeding severity and the influence of bleeding on adherence. In light of this discussion, strategies for maintaining long-term adherence in primary care are explored.
BLEEDING RISK: QUANTIFICATION, CLASSIFICATION, AND ASSOCIATION WITH OUTCOMES
All antiplatelet agents are associated with an increased risk of bleeding complications; however, no universally accepted definition exists for major bleeding. As shown by a recent analysis of large randomized clinical trials, there is considerable heterogeneity among the measurement scales used to define bleeding complications.16 This often leads to differences in reporting rates of major bleeding among studies and disparity in the estimated impact of bleeding on clinical outcomes.
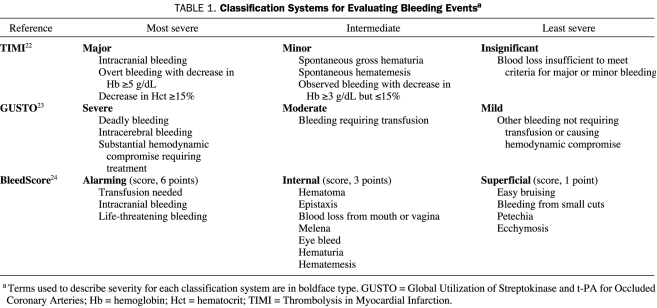
During the thrombolytic era, before the widespread use of percutaneous coronary intervention (PCI) or the advent of long-term antithrombotic therapy, including low-molecular-weight heparins, clopidogrel, or dipyridamole, 2 main bleeding scales were developed: Thrombolysis in Myocardial Infarction (TIMI) and Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO)17-21 (Table 122-24). The TIMI scale uses objective laboratory criteria (eg, hemoglobin levels) in the assessment of major and minor bleeding,22 whereas the GUSTO scale relies more on clinical assessments (eg, hemodynamic compromise requiring treatment, bleeding requiring transfusion) and is therefore more subjective.23
TABLE 1.
Classification Systems for Evaluating Bleeding Eventsa
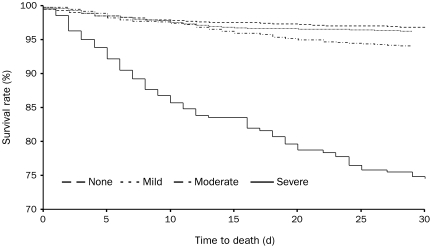
Although bleeding complications are associated with poorer outcomes (death or myocardial infarction [MI]) compared with no bleeding, the effect on outcomes is most important for severe bleeding; both 30-day and 6-month mortality rates increase with increasing severity of bleeding as classified by the GUSTO scale (Figure 1).21 This trend was also apparent in patients with mild bleeding, although to a lesser extent. At 30 days and at 6 months, mild bleeding was associated with a significant increase in the adjusted hazard ratios (HRs) for death and death or MI in patients with GUSTO-classified mild bleeding.21 Similarly, in a retrospective analysis of patients undergoing PCI, 1-year mortality rates were also higher in patients with major bleeding according to the TIMI criteria compared with those with minor or no bleeding.18
FIGURE 1.
Kaplan-Meier survival curves for 30-day mortality by severity of bleeding using Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries criteria in an analysis of data from 26,452 patients with acute coronary syndrome participating in 4 randomized controlled trials.21 Log rank P values are P=.20 for mild bleeding vs no bleeding, P<.001 for mild vs moderate bleeding, and P<.001 for moderate vs severe bleeding. Reprinted from Am J Cardiol, 21 with permission from Elsevier, ©2005.
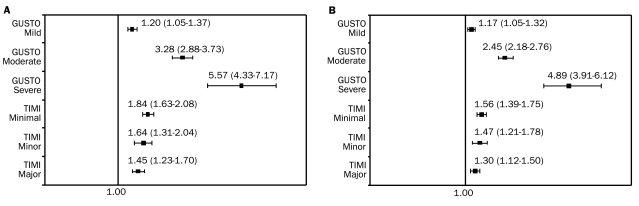
When both scales were used to analyze the association between bleeding and outcomes, there was a stepwise increase in the risk of death or MI at 30 days or at 6 months as bleeding severity increased according to the GUSTO scale, but the risk of death or MI was similar for all grades of TIMI bleeding (Figure 2, A and B).25 Separate models were constructed for each grade. Adjustments were made for age, sex, weight, site, diabetes mellitus, smoking status, prior angina, peripheral arterial disease, prerandomization therapy, MI at enrollment, systolic and diastolic blood pressure at randomization, heart rate at randomization, Killip class, and assigned treatment. This association remained consistent for each scale when applied in a model incorporating bleed severity as a time-dependent covariate,25 suggesting that a bleeding measure based on observational factors (GUSTO) may be a better prognostic indicator than a laboratory-based scale (TIMI), hence indirectly emphasizing the importance of physician involvement in the monitoring of patients with atherothrombosis.
FIGURE 2.
Adjusted hazard ratios (95% confidence intervals) of death or myocardial infarction at 30 days (A) and at 6 months (B) by worsening grade of bleeding using the Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO) and Thrombolysis in Myocardial Infarction (TIMI) criteria for bleeding severity in a pooled analysis of data from the Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrelin Therapy and the Platelet Glycoprotein IIb/IIIa Antagonism for the Reduction of Acute Coronary Syndrome Events in a Global Organization Network studies.25 Separate models were constructed for each grade. Adjustments were made for age, sex, weight, site, diabetes mellitus, smoking status, prior angina, peripheral vascular disease, prerandomization therapy, myocardial infarction at enrollment, systolic and diastolic blood pressure at randomization, heart rate at randomization, Killip class, and assigned treatment. Reprinted from J Am Coll Cardiol,25 with permission from Elsevier, ©2006.
Despite these findings, the TIMI and GUSTO scales are not universally used to assess bleeding complications. Among 13 trials reviewed by Steinhubl et al,16 9 used trial-specific bleeding scales. Although there were some similarities among these scales, there were also important differences, namely, the hemoglobin decrease or number of blood transfusion units that qualified a bleed as major and the subcategorization of major bleeding into severe and life-threatening.16 Such differences in the definition of bleeding events lead to difficulties in comparing results across various clinical trials, particularly with respect to the efficacy of different therapies and the associated relative risk of complications.26
Recently, a single-site, retrospective analysis of 17,901 consecutive patients who underwent PCI showed that major femoral bleeding complications (ie, major hematoma, major femoral bleeding, and retroperitoneal hemorrhage) are strong predictors of 30-day mortality (HR, 9.96; 95% confidence interval [CI], 6.94-14.30; P<.001).27 This study also identified blood transfusion within 7 days of PCI as an independent predictor of 30-day mortality, the risk of which increased with the number of units transfused.
Another type of bleeding event that commonly accompanies antiplatelet therapy is “nuisance bleeding,” such as that from shaving cuts or purpura. Although not life-threatening, this type of bleeding is important because it may lead to nonadherence. A rebound platelet activation effect has been documented after cessation of antiplatelet therapy in a nonadherent patient,28 putting the patient at increased risk of recurrent ischemic events. Because the TIMI and GUSTO scales are not sensitive to such nuisance bleeding events, they may overestimate the benefit-to-risk ratio of antiplatelet therapy.29 Recently, another bleeding assessment scale, the BleedScore, has been introduced to address the cumulative effect of minor bleeding in patients undergoing long-term antiplatelet therapy (Table 1).24 Although providing additional tools to quantify bleeding, the new scale has not yet been validated in relationship to clinical outcomes and may complicate comparisons among trials. A recommended standard bleeding scale is needed, particularly taking into account nuisance bleeding effects that may occur with long-term antiplatelet administration.
THE BENEFIT-TO-RISK PROFILE OF ANTIPLATELET THERAPY
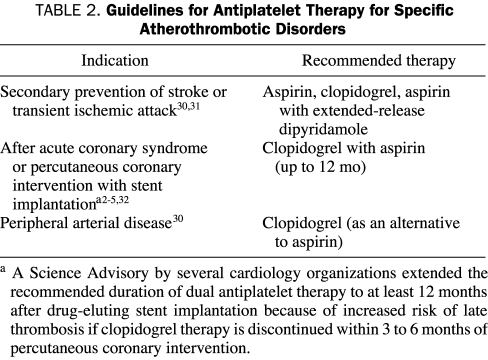
A collaborative meta-analysis of randomized trials of antiplatelet therapy showed an almost 25% reduction in the risk of serious vascular events (ie, nonfatal MI, nonfatal stroke, or vascular death) in patients with unstable angina, acute MI, stroke, transient ischemic attack (TIA), coronary artery disease, peripheral arterial disease, and/or high risk of embolism.1 Although the proportional reduction in serious vascular events varied among different categories of patient risk, the absolute risk of fatal and major nonfatal bleeding with antiplatelet therapy was small, and overall mortality was significantly reduced, suggesting that the cardiovascular benefit of antiplatelet therapy outweighs the risk of major bleeding.1 Accordingly, current evidence-based guidelines strongly recommend using antiplatelet therapy, particularly aspirin, for patients with atherothrombotic disease.5,30,31 Furthermore, dual antiplatelet therapy (aspirin plus clopidogrel or aspirin plus extended-release [ER] dipyridamole) is recommended for various atherothrombotic patient groups, unless the patient has a very low risk or cannot tolerate one of the agents (Table 2).
TABLE 2.
Guidelines for Antiplatelet Therapy for Specific Atherothrombotic Disorders
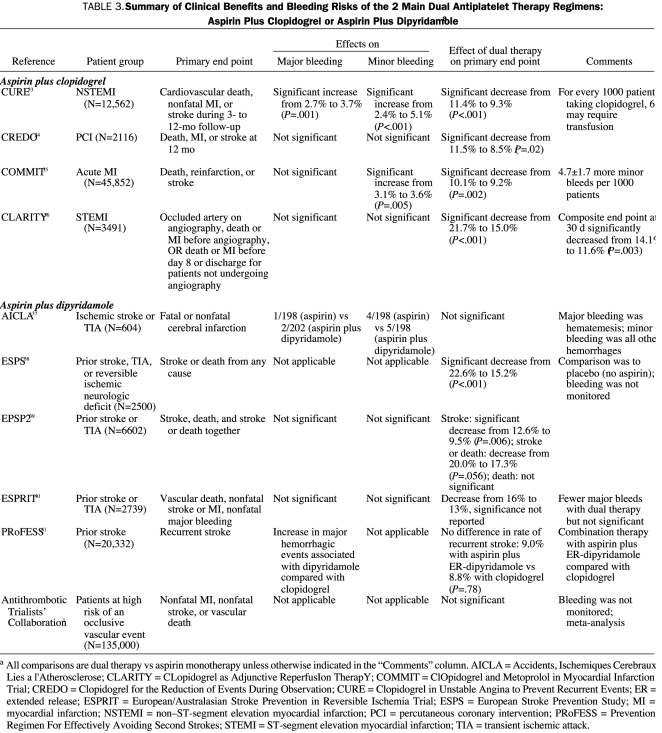
Although the risk of bleeding increases when a combination of antiplatelet agents is used, for most patients, the benefit-to-risk ratio still favors combination therapy32 (Table 333-41). In the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial, the combination of clopidogrel plus aspirin significantly reduced the risk of death from cardiovascular causes, nonfatal MI, or stroke in patients with acute coronary syndrome (relative risk of 0.80 compared with patients receiving aspirin alone; P<.001).33 Although significantly more bleeding events were reported in the clopidogrel group than in the placebo group (3.7% vs 2.7%; P=.001), the occurrence of life-threatening bleeding was not increased (2.2% vs 1.8%; P=.13), suggesting a favorable benefit-to-risk profile.33 In contrast to CURE, results from the CLopidogrel as Adjunctive ReperfusIon TherapY (CLARITY) trial36 and ClOpidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT)35 showed no statistically significant increases in the risk of major bleeding among patients who received clopidogrel and aspirin compared with aspirin alone. Similar to CURE, the combination significantly improved outcomes (in CLARITY, the composite of an occluded infarct-related artery on angiography or death or recurrent MI before angiography; in COMMIT, the composite of in-hospital death, reinfarction, and stroke and in-hospital all-cause death). Similarly, in the European/Australasian Stroke Prevention in Reversible Ischaemia Trial, ischemic events were less frequent in patients with cerebral ischemia of arterial origin receiving aspirin plus dipyridamole than in patients receiving aspirin alone, with an absolute risk reduction of 1% per year.40 In addition, patients receiving combination aspirin-dipyridamole therapy had fewer major bleeding complications and an equal number of minor bleeding complications compared with patients taking aspirin alone.40
TABLE 3.
Summary of Clinical Benefits and Bleeding Risks of the 2 Main Dual Antiplatelet Therapy Regimens: Aspirin Plus Clopidogrel or Aspirin Plus Dipyridamolea
However, this favorable benefit-to-risk ratio does not span all patient populations or all antiplatelet therapy combinations. Recent results of the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) highlight the difficulties of balancing the benefit with risk in patients.42 Although there was an overall decrease in cardiovascular death, nonfatal MI, and nonfatal stroke (HR, 0.81; 95% CI, 0.73-0.90; P<.001) in patients who received prasugrel plus aspirin, overall there was also a statistically significant increase in bleeding compared with clopidogrel plus aspirin (HR, 1.32; 95% CI, 1.03-1.68; P=.03) in patients undergoing PCI.42 In the subset of patients who underwent coronary artery bypass grafting (CABG), the risk of bleeding increased substantially (HR, 4.73; 95% CI, 1.90-11.82; P<.001). The risk of bleeding associated with prasugrel is most likely underestimated because patients in TRITON were only randomized after performance of coronary angiography, thus decreasing the chance a patient would require CABG. Bleeding events correlated with approximately 1 additional episode of fatal bleeding per prevention of 1 cardiovascular death in patients taking prasugrel compared with clopidogrel.43
Although the risk of major bleeding was increased in the overall TRITON patient population, the risk seems even greater for older patients with multiple coexisting conditions and other patient subpopulations.43 Subgroup analyses in TRITON showed lower net clinical benefit with prasugrel compared with clopidogrel in patients 75 years or older or weighing less than 60 kg (HR, 0.99; 95% CI, 0.81-1.21; P=.89; and HR, 1.03; 95% CI, 0.69-1.53; P=.89; respectively) because of a reduction in clinical efficacy and greater absolute levels of bleeding.42 Patients with previous stroke or TIA who received prasugrel exhibited a greater rate of TIMI major bleeding (P=.06) and intracranial hemorrhage (P=.02) than those with prior cerebrovascular events who received clopidogrel.42 In addition, the statistically significant increase in death from any cause, nonfatal MI, nonfatal stroke, or non-CABG-related nonfatal major bleeding observed in patients with prior cerebrovascular events who received prasugrel (HR, 1.54; 95% CI, 1.02-2.32; P=.04) shows that prasugrel should be avoided in these patients.42 Conversely, prasugrel was not associated with excess bleeding among patients with diabetes in TRITON (N=3100), suggesting that this particular group of high-risk patients may benefit from the use of this drug. In general, however, although the use of prasugrel was associated with a greater degree of platelet inhibition and greater suppression of clinical ischemic events, the threat of major, life-threatening bleeding was also elevated in most patient populations.
In the Prevention Regimen for Effectively Avoiding Second Strokes trial, a randomized head-to-head comparison, ER-dipyridamole failed to demonstrate noninferiority compared with clopidogrel in preventing recurrent strokes for a median of 2.4 years, and clopidogrel was better tolerated. The rate of recurrent stroke with twice-daily aspirin, 25 mg, plus ER-dipyridamole, 200 mg, was 9.0% compared with 8.8% with once-daily clopidogrel, 75 mg (P=.78). Major hemorrhagic events, including intracranial hemorrhage, were increased with ER-dipyridamole compared with clopidogrel. Furthermore, headaches leading to permanent discontinuation of treatment were more frequent in the ER-dipyridamole group (5.9%) compared with the clopidogrel cohort (0.9%).41
More recently, a reversible adenosine diphosphate receptor antagonist, AZD6140, has been evaluated for use in patients with atherosclerosis and acute coronary syndrome.44-46 In a study that compared clopidogrel (75 mg/d) to AZD6140 (50, 100, or 200 mg twice daily or 400 mg/d) in patients taking aspirin (75-100 mg/d), Husted et al45 found an increase in platelet inhibition and bleeding times in patients receiving AZD6140 compared with those receiving clopidogrel. The most common adverse events were minor bleeding and dyspnea, both of which occurred more frequently with the higher doses of AZD6140 than with either clopidogrel or AZD6140 at 50 mg.45
The Dose confIrmation Study assessing anti-Platelet Effects of AZD6140 vs. clopidogRel in non-ST-segment Elevation myocardial infarction (DISPERSE-2) trial compared AZD6140, given twice daily at 90 mg or 180 mg, with clopidogrel, administered as a 300-mg loading dose followed by 75 mg/d, in combination with aspirin.44,46 ADZ6140 produced statistically significantly greater platelet inhibition in a dose-dependent fashion (79%±22% for 90 mg twice daily; 95%±8% for 180 mg twice daily) compared with clopidogrel (64%±22%; P<.02), with no difference observed in the rate of protocol-defined major bleeding.46 However, an increase in minor bleeding was observed in patients receiving AZD6140, and discontinuation of use of the study drug due to bleeding episodes was more common than in patients receiving clopidogrel.44 Patients receiving AZD6140 also reported higher incidences of dyspnea, hypotension, and nausea,44 which could contribute to patient nonadherence and affect the overall benefit-to-risk profile. The experiences with prasugrel and AZD6140 highlight the need for caution in using new, relatively untested antiplatelet agents.
THE BENEFIT-TO-RISK BALANCE IN PATIENT SUBGROUPS
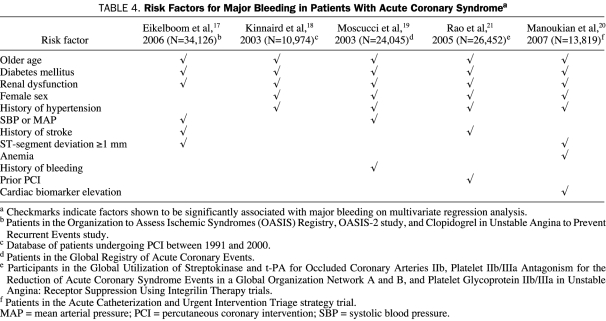
The risk of bleeding is not homogeneous across all patient groups, and some subgroup populations may have increased risk factors for major bleeding (Table 4), such as older age, diabetes mellitus, renal dysfunction, female sex, or a history of hypertension.17-21,27
TABLE 4.
Risk Factors for Major Bleeding in Patients With Acute Coronary Syndromea
The increased risk associated with age, female sex, and renal dysfunction may be because each of these patient subgroups appears to metabolize drugs differently than the general population.2,5 For women and elderly individuals, careful monitoring of antithrombotic therapy has been shown to maintain a positive benefit-to-risk ratio.2,5 The poor benefit-to-risk ratio with use of antiplatelet therapeutics for patients with renal dysfunction prompted the AHA to release a Science Advisory that all cardiovascular patients be screened for kidney disease.47 Bleeding complications due to platelet dysfunction and dosing errors can negate benefits from antithrombotic therapy in patients with renal dysfunction; these patients often receive insufficient doses of antiplatelet agents, probably because of concerns of bleeding.48 A further complication is that patients with renal dysfunction are at greater risk of restenosis after PCI.2,48 Creatinine clearance should be estimated and renally metabolized drugs adjusted accordingly in these patients.2
Procedural and therapeutic factors may also contribute to an increased risk of bleeding. In-hospital treatment with highly active anticoagulant or antiplatelet therapeutics (such as glycoprotein IIb/IIIa inhibitors, hirudin, thrombolytics, or bivalirudin) or performance of invasive procedures (including coronary angiography, intra-aortic balloon angioplasty, PCI, or CABG) also increases the incidence of bleeding.17-20,27
ANTIPLATELET EFFECTS AND BLEEDING RISK OF OTHER COMMONLY USED AGENTS
Some supplements or over-the-counter remedies that may affect platelet activity or interact with antiplatelets are occasionally recommended by physicians. Omega-3 fatty acids (fish oil) have shown benefit in reducing cardiovascular events, possibly by enhancing the stability of atherosclerotic plaques,49 and the Food and Drug Administration has announced the availability of a qualified health claim for the cardiovascular benefits of omega-3 fatty acids.50 A review of 3 large controlled trials (N=32,000) showed that docosahexaenoic acid and eicosapentaenoic acid omega-3 fatty acid supplements are associated with 19% to 45% reductions in cardiovascular events, suggesting that patients at risk might benefit from these supplements.51 Other widely used supplements, including ginkgo biloba, ginseng, green tea, papaya, St John's wort, coenzyme Q, and ginger, also affect antiplatelet activity and may interfere with antithrombotic activity.52
Nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase 2 (COX-2) inhibitors are analgesics widely used to treat arthritis pain but have also been shown to increase the risk of gastrointestinal bleeding and cardiovascular events.53,54 To minimize the risk of gastrointestinal bleeding, physicians should consider use of a proton pump inhibitor for patients who must take antiplatelets for cardioprotection and NSAIDs or COX-2 inhibitors for arthritis pain.55 Physicians may also consider using the “tweener” NSAIDs, which inhibit only COX-2 at low doses and are considered safer anti-inflammatory drugs with regard to bleeding.56 However, given the limited utility of COX-2 inhibitors because of cardiovascular events, cotherapy of NSAIDs with a gastroprotective agent may be the preferred option for patients who need cardioprotection and analgesic pain relief for arthritis.54
PATIENT ADHERENCE AND OUTCOME
Although considerable research has been done regarding the association between major bleeding and outcome, less attention has focused on the impact of bleeding on patient adherence. Recently, a case report was published of a patient who developed angina symptoms within 30 days of discharge after stent implantation and who admitted to stopping antiplatelet therapy (prasugrel and aspirin) because of repeated bleeding from cuts incurred while shaving.28 The platelet activation biomarkers in this patient were found to be higher than those previously observed on presentation with an acute vascular event,28 introducing the potential for hypercoagulation and related complications. In the CURE trial, a total of 21.1% of patients receiving clopidogrel plus aspirin permanently discontinued use of the study medication compared with 18.8% of patients receiving aspirin alone33; although this trial did not track patients' reasons for premature discontinuation of treatment, other studies29 have shown that adverse events are the most common reason patients stop taking their medication. There is increasing awareness that even nuisance bleeding complications are enough for some patients to stop therapy, especially since the benefits of antiplatelet therapy may not be readily apparent.28,57
The impact of nuisance bleeding is also evident in clinical trial settings. Analysis of COMMIT revealed that bleeding and other adverse events and elective PCI were the most common reasons for discontinuation of antiplatelet therapy.35 Similarly, patients receiving AZD6140 in the DISPERSE-2 trial were more likely to discontinue study treatment because of bleeding than patients receiving clopidogrel (2.4% in the 90-mg group, 1.5% in the 180-mg group, and 0.9% in the clopidogrel group).44 In the TRITON-TIMI 38 trial, the increase in the number of patients discontinuing prasugrel treatment because of bleeding compared with patients receiving clopidogrel was statistically significant (2.5% vs 1.4%; P<.001).42
Premature discontinuation of antiplatelet therapy is a concern because nonadherent patients face a substantially increased risk of an adverse outcome, ranging from a 1.5-fold increase for rehospitalization to a 9-fold increase in the risk of death.13,14,58 Furthermore, evidence shows that, after discontinuing antiplatelet therapy, platelet function rebounds to a level of activation higher than during the initial acute event.28 In fact, the phenomenon termed antiplatelet resistance (in which patients develop a second ischemic event despite use of antiplatelet therapy) is more likely to result from nonadherence than drug inefficacy because the incidence of resistance often mirrors the incidence of nonadherence.28,57,59,60 Factors identified as major predictors of nonadherence in patients with cardiovascular disease include having depression,61,62 having posttraumatic stress disorder,58 being unmarried,62 having low levels of educational achievement,14,62 and taking a large number of medications.62
Although bleeding is by far the most common adverse event with antiplatelet therapy, other less common adverse events may also affect adherence, including rash, headache, cramps, joint pain, nausea and vomiting, and constipation. Headache, which is particularly common with use of aspirin plus ER-dipyridamole in the secondary prevention of stroke or TIA, occurs in up to 39% of patients40,63,64 and is a common cause of treatment discontinuation.40 Headaches tend to peak 2 to 3 hours after administration, coincident with peak levels of dipyridamole when taken orally.65 However, the frequency of headache with this combination tends to diminish over time,63,65 so patients should be encouraged to continue use of their medication through this early period of high headache occurrence.
THE EFFECT OF PHYSICIAN PERCEPTIONS
Patient nonadherence is a major concern when assessing risks, but physician misconceptions of bleeding risks may contribute to patients discontinuing therapy. For example, patients may be erroneously advised to stop antiplatelet therapy before dental procedures. There may be slightly prolonged bleeding during dental procedures in patients undergoing antiplatelet therapy, but local hemostatic measures are sufficient to control it, and uncontrollable intraoperative or postoperative bleeding is unlikely.66,67 In fact, the AHA and American Dental Association recommend that patients taking clopidogrel and aspirin continue the dual regimen while undergoing dental procedures.68
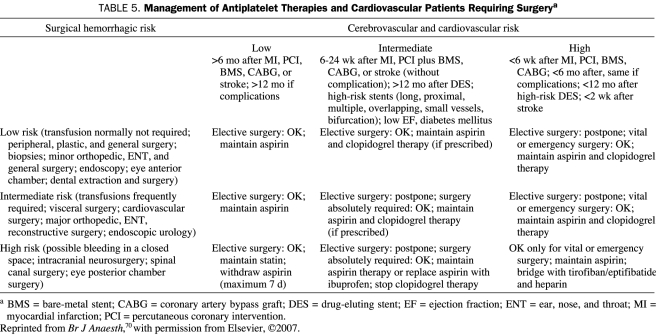
Other procedures for which physicians often withdraw antiplatelet therapy include colonoscopy, endoscopy, intraocular lens implantation, prostate biopsy, and vascular surgery.69 A meta-analysis has shown that continuation of low-dose aspirin therapy does not increase risk in these patients, except for transurethral prostatectomy,69 and recommends that patients continue to take aspirin. After PCI, the benefits of dual clopidogrel-aspirin therapy are such that it is strongly recommended that elective surgery be postponed rather than discontinue dual therapy.70 Table 5 proposes a management scheme for cardiovascular patients requiring surgery while taking antiplatelet agents.
TABLE 5.
Management of Antiplatelet Therapies and Cardiovascular Patients Requiring Surgerya
RECOMMENDATIONS FOR MAINTAINING PATIENT ADHERENCE
Studies indicate that use of antiplatelet therapy is lower in patients in primary care compared with those in specialist care.10 This suggests that primary care physicians should take a more active role in monitoring and treating their patients after hospital discharge. The following is a list of recommendations for primary care physicians with regard to monitoring and managing their patients receiving antiplatelet therapy after an acute event (Table 628,57,60-63,65,68,71-75):
Ensure that patients understand the need to take their medication and the type and severity of adverse events that they may encounter, including minor bleeding (gingival, minor cuts) and bruising. At each follow-up visit, always ask patients about adverse events they might be experiencing, including minor bleeding or bruising. Encourage patients to maintain adherence even if they encounter nuisance bleeding and to seek help promptly for bleeding or symptoms of greater concern (eg, prolonged epistaxis, melena, hematuria).28,57
Engage patients and their families in treatment, including lifestyle changes and therapeutic adherence. Encourage patients to learn more about their condition and to use any Internet-based tools for maintaining adherence. More knowledgeable patients can engage in more informed discussions with their physicians and are more likely to make rational decisions about their future health.71
Pay close attention to patients at risk of discontinuing treatment, including men, single individuals, those with a psychiatric comorbidity such as depression or posttraumatic stress disorder, those with low levels of education, and those taking multiple medications.61,62 Invest more time and effort in health education and promotion for these patients. Offer suggestions on how to maintain adherence with multiple medications, such as dosage boxes, which are readily available at pharmacies. If necessary, refer patients with depression or posttraumatic stress disorder to a psychiatrist or counselor.
Regularly question patients about nuisance bleeding and other adverse events (eg, headaches) and their effect on adherence. Encourage patients to continue with therapy despite these nuisance events; for example, headache occurrence with ER-dipyridamole is likely to decrease with persistent treatment.63,65
When nonadherence is suspected, consider performing a platelet function test. Although some physicians may use these assays to assess antiplatelet efficacy, objective assessment of platelet function can also be a good indicator of adherence and may be necessary in patients who are not completely honest about adherence during verbal questioning.60,72
Advise patients to maintain their antiplatelet therapy even when undergoing dental procedures.68 If in doubt about the risks of bleeding during oral surgery or other elective surgical procedures, consult with the patient's cardiologist before advising discontinuation of antiplatelet therapy. If antiplatelet therapy is discontinued, it should be resumed as soon as possible after the procedure.68
For patients who develop bleeding during dual antiplatelet therapy, consider reducing the aspirin dose rather than discontinuing use of either agent. Observational studies show that the incidence of bleeding during dual antiplatelet therapy is directly related to the aspirin dose and that a lower aspirin dose has a lower risk of bleeding but has similar efficacy.73
Engage other health care professionals in the management of patients. Nurse-led clinics and collaborative programs with pharmacists have been shown to enhance patient adherence with secondary prevention medications.74,75
TABLE 6.
Recommendations for Maintaining Patient Adherence
CONCLUSION
Adherence with secondary preventive therapy by both patients and physicians is a key determinant of patient outcome. There are many reasons for nonadherence, although adverse effects appear to be the main factors that affect patient adherence. With regard to antiplatelet therapy, perhaps the most important adverse event to address is the increased risk of bleeding. Although risk of bleeding is reported in clinical trials of antiplatelet therapy, the true clinical impact of bleeding is difficult to assess and compare between trials because of the use of various bleeding scales that differ in the criteria used to define bleeding and the categorizations used. In addition to patient concerns about bleeding, physician perceptions of bleeding risks and unfamiliarity with guidelines may contribute to nonadherence. Nuisance effects, such as minor bleeding, may be an underrecognized cause of nonadherence.
Nonadherence can be easily overcome through attention from physicians, patients, and families. Primary care physicians are often the ones who forge the closest relationships with patients through ongoing care and are therefore in a unique situation to identify not only the adverse events that may undermine adherence but also the socioeconomic and psychological factors that affect adherence. As Joseph B. Kirsner wrote in the Journal of the American Medical Association, “The patient-physician relationship is, in fact, a powerful therapeutic force, synergistic with medication.”76
Footnotes
Editorial support in preparation of the submitted manuscript was funded by the Bristol-Myers Squibb/Sanofi Pharmaceutical Partnership.
REFERENCES
- 1.Antithrombotic Trialists' Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients [published correction appears in BMJ. 2002;324(7330):141] BMJ 2002;324(7329):71-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) [published correction appears in J Am Coll Cardiol. 2008;51(9):974] J Am Coll Cardiol. 2007;50(7):e1-e157 [DOI] [PubMed] [Google Scholar]
- 3.Antman EM, Hand M, Armstrong PW, et al. 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2008;117(6):e162] Circulation 2008January15;117(2):296-329 Epub 2007 Dec 10 [DOI] [PubMed] [Google Scholar]
- 4.King SB, III, Smith SC, Jr, Hirshfeld JW, Jr, et al. 2005 Writing Committee 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2008;117(6):e161] Circulation 2007January;117(2):261-295 Epub 2007 Dec 13 [DOI] [PubMed] [Google Scholar]
- 5.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute [published correction appears in Circulation. 2006;113(22):e847] Circulation 2006;113(19):2363-2372 [DOI] [PubMed] [Google Scholar]
- 6.LaBresh KA, Ellrodt AG, Gliklich R, Liljestrand J, Peto R. Get with the guidelines for cardiovascular secondary prevention: pilot results. Arch Intern Med. 2004;164(2):203-209 [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Roe MT, Peterson ED, et al. CRUSADE Investigators Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA 2004;292(17):2096-2104 [DOI] [PubMed] [Google Scholar]
- 8.Mehta RH, Montoye CK, Gallogly M, et al. GAP Steering Committee of the American College of Cardiology Improving quality of care for acute myocardial infarction: the Guidelines Applied in Practice (GAP) Initiative. JAMA 2002;287(10):1269-1276 [DOI] [PubMed] [Google Scholar]
- 9.Mehta RH, Roe MT, Chen AY, et al. Recent trends in the care of patients with non-ST-segment elevation acute coronary syndromes: insights from the CRUSADE initiative. Arch Intern Med. 2006;166(18):2027-2034 [DOI] [PubMed] [Google Scholar]
- 10.Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Med. 2005December;2(12):e353 Epub 2005 Nov 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation 2006January17;113(2):203-212 Epub 2006 Jan 9 [DOI] [PubMed] [Google Scholar]
- 12.Glynn RJ, Buring JE, Manson JE, LaMotte F, Hennekens CH. Adherence to aspirin in the prevention of myocardial infarction: the Physicians' Health Study. Arch Intern Med. 1994;154(23):2649-2657 [DOI] [PubMed] [Google Scholar]
- 13.Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006November;27(22):2667-2674 Epub 2006 Oct 19 [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation 2006June20;113(24):2803-2809 Epub 2006 Jun 12 [DOI] [PubMed] [Google Scholar]
- 15.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293(17):2126-2130 [DOI] [PubMed] [Google Scholar]
- 16.Steinhubl SR, Kastrati A, Berger PB. Variation in the definitions of bleeding in clinical trials of patients with acute coronary syndromes and undergoing percutaneous coronary interventions and its impact on the apparent safety of antithrombotic drugs. Am Heart J. 2007;154(1):3-11 [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006August22;114(8):774-782 Epub 2006 Aug 14 [DOI] [PubMed] [Google Scholar]
- 18.Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92(8):930-935 [DOI] [PubMed] [Google Scholar]
- 19.Moscucci M, Fox KA, Cannon CP, et al. GRACE Investigators Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24(20):1815-1823 [DOI] [PubMed] [Google Scholar]
- 20.Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007March27;49(12):1362-1368 Epub 2007 Mar 9 [DOI] [PubMed] [Google Scholar]
- 21.Rao SV, O'Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005November;96(9):1200-1206 Epub 2005 Sep 12 [DOI] [PubMed] [Google Scholar]
- 22.Rao AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial—phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11(1):1-11 [DOI] [PubMed] [Google Scholar]
- 23.GUSTO Investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction [published correction appears in N Engl J Med. 1994;331(4):277] N Engl J Med. 1993;329(10):673-682 [DOI] [PubMed] [Google Scholar]
- 24.Serebruany VL, Atar D. Assessment of bleeding events in clinical trials—proposal of a new classification [published correction appears in Am J Cardiol. 2007;100(3):562] Am J Cardiol. 2007January15;99(2):288-290 Epub 2006 Nov 27 [DOI] [PubMed] [Google Scholar]
- 25.Rao SV, O'Grady K, Pieper KS, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol. 2006February21;47(4):809-816 Epub 2006 Jan 26 [DOI] [PubMed] [Google Scholar]
- 26.Cohen M, Alexander KP, Rao SV. Bleeding after antithrombotic therapy in patients with acute ischemic heart disease: is it the drugs or how we use them? J Thromb Thrombolysis 2008December;26(3):175-182 Epub 2007 Dec 15 [DOI] [PubMed] [Google Scholar]
- 27.Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary interventions: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994-2005. J Am Coll Cardiol Intv. 2008;1(2):202-209 [DOI] [PubMed] [Google Scholar]
- 28.Serebruany VL, Midei MG, Meilman H, Malinin AI, Lowry DR. Rebound platelet activation after termination of prasugrel and aspirin therapy due to confirmed non-compliance in patient enrolled in the JUMBO Trial. Int J Clin Pract. 2006;60(7):863-866 [DOI] [PubMed] [Google Scholar]
- 29.Hugtenburg JG, Blom AT, Kisoensingh SU. Initial phase of chronic medication use; patients' reasons for discontinuation. Br J Clin Pharmacol. 2006;61(3):352-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397 [DOI] [PubMed] [Google Scholar]
- 31.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention. Circulation 2006;113(10):e409-e449 [PubMed] [Google Scholar]
- 32.Helton TJ, Bavry AA, Kumbhani DJ, Duggal S, Roukoz H, Bhatt DL. Incremental effect of clopidogrel on important outcomes in patients with cardiovascular disease: a meta-analysis of randomized trials. Am J Cardiovasc Drugs 2007;7(4):289-297 [DOI] [PubMed] [Google Scholar]
- 33.The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation [published corrections appear in N Engl J Med. 2001;345(23):1716 and 2001;345(20):1506] N Engl J Med. 2001;345(7):494-502 [DOI] [PubMed] [Google Scholar]
- 34.Steinhubl SR, Berger PB, Mann JT, III, et al. CREDO Investigators Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial [published correction appears in JAMA. 2003;289(8):987] JAMA 2002;288(19):2411-2420 [DOI] [PubMed] [Google Scholar]
- 35.COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366(9497):1607-1621 [DOI] [PubMed] [Google Scholar]
- 36.Sabatine MS, Cannon CP, Gibson CM, et al. CLARITY-TIMI 28 Investigators Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005March24;352(12):1179-1189 Epub 2005 Mar 9 [DOI] [PubMed] [Google Scholar]
- 37.Bousser MG, Eschwege E, Haguenau M, et al. “AICLA” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke 1983;14(1):5-14 [DOI] [PubMed] [Google Scholar]
- 38.ESPS Group The European Stroke Prevention Study (ESPS): principal end-points. Lancet 1987;2(8572):1351-1354 [PubMed] [Google Scholar]
- 39.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study, 2: dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143(1-2):1-13 [DOI] [PubMed] [Google Scholar]
- 40.ESPRIT Study Group Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial [published correction appears in Lancet. 2007;369(9558):274] Lancet 2006;367(9523):1665-1673 [DOI] [PubMed] [Google Scholar]
- 41.Sacco RL, Diener HC, Yusuf S, et al. PRoFESS Study Group Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Eng J Med. 2008September18;359(12):1238-1251 Epub 2008 Aug 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiviott SD, Braunwald E, McCabe CH, et al. TRITON-TIMI 38 Investigators Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007November15;357(20):2001-2015 Epub 2007 Nov 4 [DOI] [PubMed] [Google Scholar]
- 43.Bhatt DL. Intensifying platelet inhibition—navigating between scylla and charybdis. N Engl J Med. 2007November15;357(20):2078-2081 Epub 2007 Nov 4 [DOI] [PubMed] [Google Scholar]
- 44.Cannon CP, Husted S, Harrington RA, et al. DISPERSE-2 Investigators Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial [published correction appears in J Am Coll Cardiol. 2007 Nov 27;50(22):2196] J Am Coll Cardiol. 2007November6;50(19):1844-1851 Epub 2007 Oct 23 [DOI] [PubMed] [Google Scholar]
- 45.Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006May;27(9):1038-1047 Epub 2006 Feb 13 [DOI] [PubMed] [Google Scholar]
- 46.Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007November;50(19):1852-1856 Epub 2007 Oct 23 [DOI] [PubMed] [Google Scholar]
- 47.Brosius FC, III, Hostetter TH, Kelepouris E, et al. Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease: a Science Advisory from the American Heart Association Kidney and Cardiovascular Disease Council; the Councils on High Blood Pressure Research, Cardiovascular Disease in the Young, and Epidemiology and Prevention; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: developed in collaboration with the National Kidney Foundation. Circulation 2006September5;114(10):1083-1087 Epub 2006 Aug 7 [DOI] [PubMed] [Google Scholar]
- 48.Gupta R, Birnbaum Y, Uretsky BF. The renal patient with coronary artery disease: current concepts and dilemmas [published correction appears in J Am Coll Cardiol. 2004;44(11):2283] J Am Coll Cardiol. 2004;44(7):1343-1353 [DOI] [PubMed] [Google Scholar]
- 49.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 2003;361(9356):477-485 [DOI] [PubMed] [Google Scholar]
- 50.US Food and Drug Administration FDA announces qualified health claims for omega-3 fatty acids [press release] September8, 2004. http://www.fda.gov/bbs/topics/news/2004/NEW01115.html Accessed December 16, 2008
- 51.Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection [published correction appears in Mayo Clin Proc. 2008;83(6):730] Mayo Clin Proc. 2008;83(3):324-332 [DOI] [PubMed] [Google Scholar]
- 52.Basila D, Yuan CS. Effects of dietary supplements on coagulation and platelet function. Thromb Res. 2005;117(1-2):49-53 [DOI] [PubMed] [Google Scholar]
- 53.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs [published correction appears in N Engl J Med. 1999;341(7):548] N Engl J Med. 1999;340(24):1888-1899 [DOI] [PubMed] [Google Scholar]
- 54.Bombardier C, Laine L, Reicin A, et al. VIGOR Study Group Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343(21):1520-1528 [DOI] [PubMed] [Google Scholar]
- 55.Cryer B. Reducing the risks of gastrointestinal bleeding with antiplatelet therapies [editorial]. N Engl J Med. 2005;352(3):287-289 [DOI] [PubMed] [Google Scholar]
- 56.Weideman RA, Kelly KC, Kazi S, et al. Risks of clinically significant upper gastrointestinal events with etodolac and naproxen: a historical cohort analysis. Gastroenterology 2004;127(5):1322-1328 [DOI] [PubMed] [Google Scholar]
- 57.Serebruany VL. The “clopidogrel resistance” trap [editorial]. Am J Cardiol. 2007September15;100(6):1044-1046 Epub 2007 Jul 6 [DOI] [PubMed] [Google Scholar]
- 58.Shemesh E, Yehuda R, Milo O, et al. Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction. Psychosom Med. 2004;66(4):521-526 [DOI] [PubMed] [Google Scholar]
- 59.Cotter G, Shemesh E, Zehavi M, et al. Lack of aspirin effect: aspirin resistance or resistance to taking aspirin? Am Heart J. 2004;147(2):293-300 [DOI] [PubMed] [Google Scholar]
- 60.Schwartz KA, Schwartz DE, Ghosheh K, Reeves MJ, Barber K, DeFranco A. Compliance as a critical consideration in patients who appear to be resistant to aspirin after healing of myocardial infarction. Am J Cardiol. 2005;95(8):973-975 [DOI] [PubMed] [Google Scholar]
- 61.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med. 2005;165(21):2508-2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kulkarni SP, Alexander KP, Lytle B, Heiss G, Peterson ED. Long-term adherence with cardiovascular drug regimens. Am Heart J. 2006;151(1):185-191 [DOI] [PubMed] [Google Scholar]
- 63.Lipton RB, Bigal ME, Kolodner KB, et al. Acetaminophen in the treatment of headaches associated with dipyridamole-aspirin combination. Neurology 2004;63(6):1099-1101 [DOI] [PubMed] [Google Scholar]
- 64.Redman AR, Ryan GJ. Analysis of trials evaluating combinations of acetylsalicylic acid and dipyridamole in the secondary prevention of stroke. Clin Ther. 2001;23(9):1391-1408 [DOI] [PubMed] [Google Scholar]
- 65.Theis JG, Deichsel G, Marshall S. Rapid development of tolerance to dipyridamole-associated headaches. Br J Clin Pharmacol. 1999;48(5):750-755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ardekian L, Gaspar R, Peled M, Brener B, Laufer D. Does low-dose aspirin therapy complicate oral surgical procedures? J Am Dent Assoc. 2000;131(3):331-335 [DOI] [PubMed] [Google Scholar]
- 67.Jeske AH, Suchko GD. Lack of a scientific basis for routine discontinuation of oral anticoagulation therapy before dental treatment [published correction appears in J Am Dent Assoc. 2004;135(1):28] J Am Dent Assoc. 2003;134(11):1492-1497 [DOI] [PubMed] [Google Scholar]
- 68.Grines CL, Bonow RO, Casey DE, Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a Science Advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Coll Cardiol. 2007;49(6):734-739 [DOI] [PubMed] [Google Scholar]
- 69.Burger W, Chemnitius JM, Kneissl GD, Rücker G. Low-dose aspirin for secondary cardiovascular prevention - cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation - review and meta-analysis. J Intern Med. 2005;257(5):399-414 [DOI] [PubMed] [Google Scholar]
- 70.Chassot PG, Delabays A, Spahn DR. Perioperative antiplatelet therapy: the case for continuing therapy in patients at risk of myocardial infarction. Br J Anaesth. 2007September;99(3):316-328 Epub 2007 Jul 23 [DOI] [PubMed] [Google Scholar]
- 71.Field K, Ziebland S, McPherson A, Lehman R. ‘Can I come off the tablets now?’ a qualitative analysis of heart failure patients' understanding of their medication. Fam Pract. 2006December;23(6):624-630 Epub 2006 Jul 11 [DOI] [PubMed] [Google Scholar]
- 72.von Pape KW, Strupp G, Bonzel T, Bohner J. Effect of compliance and dosage adaptation of long term aspirin on platelet function with PFA-100 in patients after myocardial infarction [letter]. Thromb Haemost. 2005;94(4):889-891 [DOI] [PubMed] [Google Scholar]
- 73.Peters RJ, Mehta SR, Fox KA, et al. Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Trial Investigators Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation 2003October7;108(14):1682-1687 Epub 2003 Sep 22 [DOI] [PubMed] [Google Scholar]
- 74.Campbell NC, Ritchie LD, Thain J, Deans HG, Rawles JM, Squair JL. Secondary prevention in coronary heart disease: a randomised trial of nurse led clinics in primary care. Heart 1998;80(5):447-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haggerty SA, Cerulli J, Zeolla MM, Cottrell JS, Weck MB, Faragon JJ. Community pharmacy Target Intervention Program to improve aspirin use in persons with diabetes. J Am Pharm Assoc (2003) 2005;45(1):17-22 [DOI] [PubMed] [Google Scholar]
- 76.Kirsner JB. The most powerful therapeutic force. JAMA 2002;287(15):1909-1910 [DOI] [PubMed] [Google Scholar]