Abstract
Contrast-induced acute kidney injury (AKI) (also known as contrast-induced nephropathy) is an abrupt deterioration in renal function that can be associated with use of iodinated contrast medium. Although the increase in serum creatinine concentration is transient in most cases, contrast-induced AKI may lead to increased morbidity and mortality rates in selected at-risk populations. This review summarizes the findings of a multidisciplinary panel composed of computed tomography radiologists, interventional radiologists, cardiologists, and nephrologists convened to address the specialty-specific issues associated with minimizing the incidence of contrast-induced AKI. As part of this initiative, the panel developed specialty-specific protocols for preventing contrast-induced AKI, taking into account, for example, the variations in patient risk profile, inpatient or outpatient status, and staffing resources that characterize various clinical settings. The 3 protocols, each reflecting a consensus of expert opinion, address the prevention of contrast-induced AKI in interventional radiology, diagnostic computed tomography radiology, and interventional cardiology settings. The protocols are presented in the context of a review of recent guidelines and published reports of trials that discuss contrast-induced AKI and its prevention. The panel reviewed materials retrieved by a PubMed search covering the period January 1990 through January 2008 and used combinations of key words associated with the prevention and treatment of contrast-induced AKI. In addition, the panel reviewed the reference lists of selected articles and the tables of contents posted on the Web sites of selected journals for relevant publications not retrieved in the PubMed searches.
ACC = American College of Cardiology; ACR = American College of Radiology; ACTIVE = Abdominal Computed Tomography: Iomeron-400 versus Visipaque-320 Enhancement; AHA = American Heart Association; AKI = acute kidney injury; CARE = Cardiac Angiography in Renally Impaired Patients; CIN = contrast-induced nephropathy; CKD = chronic kidney disease; CrCl = creatinine clearance; CT = computed tomography; eGFR = estimated glomerular filtration rate; ESUR = European Society of Urogenital Radiology; IMPACT = Isovue-370 and Visipaque-320 in renally IMpaired PAtients undergoing Computed Tomography; MDRD = Modification of Diet in Renal Disease; NAC = N-acetylcysteine; NEPHRIC = Nephrotoxicity in High-Risk Patients Study of Iso-Osmolar and Low-Osmolar Non-Ionic Contrast Media; NKF = National Kidney Foundation; PCI = percutaneous coronary intervention; PREDICT = Patients with REnal impairment and DIabetes undergoing Computed Tomography; RECOVER = Renal Toxicity Evaluation and Comparison Between Visipaque and Hexabrix in Patients With Renal Insufficiency Undergoing Coronary Angiography
Acute kidney injury (AKI) is a heterogeneous disorder with multiple etiologies, risk factors, and clinical presentations. Although patients with AKI are ultimately cared for by nephrologists, AKI occurs in various clinical settings and is associated with a specific etiology in each. The term contrast-induced AKI refers to the disorder as it occurs after exposure to iodinated contrast media, a disorder that has been more commonly known as contrast-induced nephropathy (CIN). The Acute Kidney Injury Network, recognizing the need for improving outcomes associated with the various forms of AKI, recently proposed using the following standardized diagnostic definition in all cases: an abrupt (within 48 hours) reduction in kidney function, evidenced by an increase in the serum creatinine concentration of at least 0.3 mg/dL (to convert to μmol/L, multiply by 88.4) or at least 50% from baseline or a reduction in urine output (documented oliguria of <0.5 mL/kg/h for >6 hours).1,2 The criterion of oliguria does not apply for many cases of contrast-induced AKI because patients are treated with intravenous fluids before and after the procedure with the goal of increasing periprocedural urine output. The most commonly used definition of CIN is an increase from the baseline serum creatinine concentration of at least 0.5 mg/dL or at least 25% within 48 to 72 hours after exposure to contrast media.3-5 Because the medical community is moving to adopt the concept and terminology of the American College of Radiology (ACR) in reference to studies of contrast-induced renal dysfunction and its prevention,6 this report will do the same. However, we recognize that the clinical effect of the slightly different definitions of CIN and AKI has yet to be clarified.
The incidence of contrast-induced AKI is low (2%) in the general population,4 but it is higher in certain at-risk groups of patients. Patients who have both chronic kidney disease (CKD) (defined as an estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2)7 and diabetes mellitus are at highest risk8-10; the incidence of contrast-induced AKI is as high as 50% for patients with multiple risk factors.11
Contrast-induced AKI has serious prognostic implications; it is linked to increases in length of hospital stay and to higher rates of in-hospital cardiovascular events, in-hospital mortality, and 1-year and 5-year mortality rates.5,9,12-14 Even relatively small changes in renal function after administration of contrast medium are associated with substantial increases in mortality rates14; this finding suggests that renal insufficiency is a sensitive marker of poor outcomes for patients at risk or perhaps that transient episodes of renal ischemia may produce secondary hemodynamic or vascular changes in other organs.15
The materials cited in this review include recent guidelines and published reports of clinical trials of contrast-induced AKI and its prevention. These materials were retrieved by a PubMed search covering the period January 1990 through January 2008. The search used combinations of the following key words: contrast agent, nephrotoxicity, hydration, N-acetylcysteine, sodium bicarbonate nephropathy, and acute kidney injury. In addition, we reviewed the reference lists of published articles and the tables of contents posted on the Web sites of selected journals for relevant publications not retrieved by the PubMed search.
EXISTING PRACTICE GUIDELINES
Several official organizations representing the disciplines of cardiology, nephrology, and radiology have recognized the importance of addressing the management of contrast-induced renal complications in their formal practice guidelines. The extent to which preventive measures for contrast-induced AKI are addressed varies across guidelines.
The most recent American Heart Association (AHA)/American College of Cardiology (ACC) guidelines for the management of patients with unstable angina or non-ST elevation myocardial infarction and for percutaneous coronary intervention (PCI) highlight the importance of using iso-osmolar contrast media as a preventive measure for patients with CKD who require coronary intervention: “In chronic kidney disease patients undergoing angiography, isosmolar contrast agents are indicated and are preferred.”16,17 This is a class I recommendation at level of evidence A and is based on the findings of clinical trials and meta-analyses.18,19 The guidelines advise that patients with cardiovascular disease should be screened for CKD according to the AHA/National Kidney Foundation (NKF) recommendations7 and that use of iso-osmolar contrast media should be guided by the results of such screening.
The latest ACR formal practice guidelines for using iodinated contrast media focus on determining which patients have an increased overall risk of adverse effects because such patients are likely to benefit from the use of low-osmolar rather than high-osmolar contrast media.20 Specific ACR recommendations (as they appear in the updated ACR Manual on Contrast Media) for preventing contrast-induced AKI include the following: initiating intravenous volume expansion with saline both before and after CM administration, administering the antioxidant N-acetylcysteine (NAC) to patients at risk, measuring the serum creatinine concentration of patients with suspected renal dysfunction, and using either low-osmolar or iso-osmolar contrast media for all patients with renal insufficiency.20
The NKF has issued clinical practice guidelines for evaluating and managing cardiovascular disease in patients undergoing dialysis: use of iso-osmolar contrast media and administration of NAC are appropriate because of their potential benefit in preserving renal function. The NKF guidelines caution against routine use of sodium bicarbonate and volume expansion for these patients.21
The European Society of Urogenital Radiology (ESUR) has issued guidelines for using contrast media. These guidelines incorporate risk factors for CIN, recommendations for identifying patients at risk, and strategies for reducing risk, such as using either low-osmolar or iso-osmolar contrast media, initiating intravenous volume expansion, and discontinuing administration of nephrotoxic drugs.22
THE NEED FOR SPECIALTY-SPECIFIC GUIDELINES
The existing guidelines often lack detail and do not cover all aspects of patient management. In particular, they often fail to address specialty-specific issues. Such issues may be associated with variation in risk-benefit ratios across different patient populations or with practical aspects of implementing risk assessment and prophylactic strategies for contrast-induced AKI in certain settings, such as a busy computed tomography (CT) unit with a large number of patients, limited space, and limited nursing resources or an interventional cardiology setting for patients undergoing elective PCI. Specialty-specific technical and procedural factors may also dictate management choices. In studies of peripheral vessels, particularly runoff studies, the choice of contrast media is dictated by patient tolerability because image quality is easily reduced by motion artifacts driven by patient discomfort. Local pain associated with injection of contrast media has been correlated with osmolality.23,24 Several studies that have compared the tolerability of the iso-osmolar contrast medium iodixanol with that of low-osmolar contrast medium report that iso-osmolar contrast medium is associated with a decrease in the intensity of discomfort (heat sensations) and pain.24-30
Overall, the key issue that becomes important to define for each specialty is the degree of risk that makes the effort needed to screen patients, identify those at risk, and then implement prevention strategies, a requirement despite any impracticality.
COMPONENTS OF AN EFFECTIVE PROTOCOL
Screening
Should all patients be screened for CKD, or should screening be reserved for a select group? Routinely performing serum creatinine assays for all patients may be costly, cumbersome, and inconvenient.31 In most cases, the clinical history provides enough information to allow determination of which patients are likely to have CKD and therefore the risk of contrast-induced AKI. In outpatient and emergency settings, a basic questionnaire that addresses the patient's history of renal disorders and additional risk factors may be simpler and more cost-effective than universal serum creatinine screening.31,32 Another approach, developed and validated for patients undergoing PCI, is to assess a patient's degree of risk by using a scoring system based on risk factors for contrast-induced AKI.33
What measurement should be used? Serum creatinine concentration alone is an insensitive indicator of kidney function. The commonly used cutoff, a serum creatinine concentration of 1.5 mg/dL or higher, fails to detect 40% of patients at risk of contrast-induced AKI.34 The GFR is thought to provide the best overall index of renal function, but measuring it may be impractical. Instead, estimates of renal function, either the eGFR or the calculated creatinine clearance (CrCl) rate, are determined by empirically derived formulas based on the serum creatinine concentration. The eGFR is calculated by using the Modification of Diet in Renal Disease (MDRD) formula; the CrCl rate, by using the Cockcroft-Gault formula.35,36 Although both methods have limitations,37 estimating the GFR from the serum creatinine concentration is recommended because it provides a more sensitive and specific measure of renal function than does the serum creatinine concentration alone. The 4-variable MDRD formula, which uses the serum creatinine concentration and the patient's age, with adjustments for sex and race, is preferable to the Cockcroft-Gault formula,37 particularly for patients with diabetes.38,39
Should the eGFR be assessed immediately before the procedure? If not, how recent should the assessment be? For many inpatients, a current serum creatinine measurement should be available. In outpatient settings, should the referring physician be expected to provide results of a recent assay? For high-risk patients, should the procedure be deferred until results of a recent assessment of kidney function are available? Point-of-care testing, which can provide the serum creatinine concentration within minutes, is likely to be useful in such situations.40 With regard to timing the serum creatinine assay, it is important to establish the baseline serum creatinine concentration before intravenous volume expansion is initiated (discussed subsequently). Otherwise, estimates of renal function may be misleading because of the decrease in the serum creatinine concentration that is induced by increased extracellular fluid volume.
In an emergency situation, the importance of a procedure using contrast medium and the risk of delaying that procedure must be balanced against the risk of kidney injury. Because specialties that use contrast-enhanced procedures differ in the extent to which inpatient or outpatient populations and emergency or nonemergency procedures are represented, the issues raised by the preceding questions are probably best addressed by specialty-specific, rather than general, protocols.
Determining Which Patients Are At Risk
The risk of contrast-induced AKI is considered increased and clinically important when the eGFR is lower than 60 mL/min/1.73 m2.36 However, this threshold probably includes too many patients to allow a focus on those who are truly at high risk. For example, patients with an eGFR of 30 mL/min/1.73 m2 have a 30% to 40% risk of contrast-induced AKI and a 2% to 8% risk of requiring dialysis.41 Furthermore, because an eGFR cutoff of less than 60 mL/min/1.73 m2 places a large number of patients in the at-risk group, the practicality and implementation of guidelines may be reduced. Also, a moderate reduction in eGFR is common among elderly patients, for whom eGFR levels of 50 to 59 mL/min/1.73 m2 may not have the same clinical importance as similar values in younger patients with underlying specific forms of CKD.42 Therefore, using a lower eGFR threshold (<30, 45, or 50 mL/min/1.73 m2) to define contrast-induced AKI risk may be more appropriate in some situations.
Minimizing Risk
Volume Expansion. Extracellular volume expansion plays a well-established role in reducing the risk of contrast-induced AKI, although few trials have directly addressed the ideal protocol.43 Intravenous volume expansion before and after administration of contrast medium appears to be more effective than either bolus volume expansion during the procedure44 or removal of restrictions on oral fluid intake.45 Isotonic saline has been found to be better than 0.45% saline and is given intravenously before and after administration of contrast medium for a total of 24 hours.46 The findings of several recent trials47-51 and a meta-analysis52 indicate that volume expansion with bicarbonate is more effective than volume expansion with saline. However, these collective findings await confirmation in light of other recent reports that argue against a clear benefit for bicarbonate volume expansion in preventing contrast-induced AKI.53,54
Pharmacological Prophylaxis. Prophylactic administration of NAC was first reported to be beneficial for patients with CKD who were undergoing CT with a low-osmolar contrast medium; oral NAC plus volume expansion with 0.45% saline was associated with a significantly lower rate of contrast-induced AKI (2%) than placebo plus 0.45% saline (21%; P=.01).55 Since this early report, the numerous clinical studies and meta-analyses performed have yielded conflicting results; however, some have found substantial efficacy for NAC, as illustrated by a recent meta-analysis,56 and most have found no benefit.57
Another antioxidant that has been investigated for preventing contrast-induced AKI is ascorbic acid. In a multicenter, blinded, placebo-controlled trial (N=231), oral ascorbic acid (over-the-counter vitamin C) at a dose of 3 g administered the night before the procedure and a dose of 2 g administered twice after the procedure (on the same day) reduced the occurrence of contrast-induced AKI to 9%, compared with 20% with placebo.58
Contrast Medium. Volume, dose, and type of contrast medium also may alter the risk of contrast-induced AKI. Generally, a volume of contrast medium of no more than 100 mL is preferable for patients with an eGFR lower than 60 mL/min/1.73 m2,59 and even small (about 30 mL) volumes of contrast medium may cause AKI in patients at very high risk.11 A volume limit of 5 mL/kg body weight normalized to the serum creatinine concentration has also been proposed as a threshold for contrast-induced AKI in patients with CKD (serum creatinine concentration >1.8 mg/dL).60 A retrospective study has confirmed the validity of this threshold dose as a predictor of AKI that requires dialysis and of in-hospital mortality.61 Similarly, the ratio of contrast medium volume to CrCl has recently been suggested to be a more accurate predictor of AKI than other factors; a ratio higher than 3.7 is associated with increased risk. This ratio could be applied prospectively to determine the maximum contrast medium volume that can be administered without substantially increasing the risk of AKI.62
Central to concerns about contrast-induced AKI and its prevention is the role played by the type of contrast medium used, specifically, the contribution of contrast medium osmolality to comparative nephrotoxicity. Although there is general agreement that high-osmolar contrast media pose a greater risk of AKI than low-osmolar contrast media in patients at risk,8,63 does a further reduction in contrast medium osmolality minimize the risk of contrast-induced AKI even more? Randomized controlled trials in the interventional cardiology setting have shown that iodixanol has no advantage over iopromide (a low-osmolar agent) for patients with normal renal function.64 However, in higher-risk populations, iodixanol has been shown to reduce the incidence of nephrotoxicity significantly more than iohexol (the Nephrotoxicity in High-Risk Patients Study of Iso-Osmolar and Low-Osmolar Non-Ionic Contrast Media [NEPHRIC]: 3% vs 26%; P=.002)65 and ioxaglate (the Renal Toxicity Evaluation and Comparison Between Visipaque and Hexabrix in Patients With Renal Insufficiency Undergoing Coronary Angiography [RECOVER] study: 8% vs 17%; P=.02).18 Two trials compared iodixanol with iopamidol for patients with CKD who were undergoing coronary angiography or PCI (the Cardiac Angiography in Renally Impaired Patients [CARE] study)66 or various CT procedures (the Isovue-370 and Visipaque-320 in renally IMpaired PAtients undergoing Computed Tomography [IMPACT] study).67 These studies found low incidences of contrast-induced AKI with no significant difference in incidence between the iso-osmolar and low-osmolar groups. A third trial, the Patients with REnal impairment and DIabetes undergoing Computed Tomography [PREDICT] study, which also compared iopamidol and iodixanol in the CT setting but included patients with both CKD and diabetes, found similarly low incidences of contrast-induced AKI for the 2 agents (5.6% vs 4.9%).68 In a recent trial (the Abdominal Computed Tomography: Iomeron-400 versus Visipaque-320 Enhancement [ACTIVE] study) that compared iodixanol and the low-osmolar contrast medium iomeprol in patients undergoing CT, iodixanol was found to be associated with a significantly higher incidence of contrast-induced AKI (6.9%) than iomeprol (0%; P=.03).69
The inconsistency of these findings may be explained in part by important differences in study design, such as timing and standardization of postprocedural serum creatinine assays. In the NEPHRIC and RECOVER trials, patients underwent at least 2 follow-up serum creatinine assays after receiving contrast medium; the timing of each assessment was mandated for all patients. The IMPACT, CARE, PREDICT, and ACTIVE trials required a single serum creatinine assay after administration of the contrast medium dose, but the timing of this assessment varied, in some cases widely, across the studies. Another factor that may contribute to the inconsistency of results is the differing risk profiles of the study populations; the proportion of high-risk patients with CKD and diabetes ranged from 0% to 100% across the 6 trials.
FOLLOW-UP PROCEDURES
Procedures for ensuring follow-up serum creatinine assays at an appropriate time point must be in place. Timing and setting may vary across specialties, but follow-up is an essential part of an effective protocol. Systems are also required for further follow-up of patients with evidence of contrast-induced AKI.
MULTIMODALITY PREVENTION APPROACHES
If the previously mentioned interventions have minimal effects on various pathophysiological elements of contrast-induced AKI, including vasoconstriction, ischemic injury, oxidative stress, and inflammation, then multimodality approaches using multiple interventions for the same patient may yield benefit whereas singular approaches may not. The findings of clinical trials suggest that this concept may hold true for patients at risk of contrast-induced AKI. Therefore, using iso-osmolar contrast agents and volume expansion, maintaining high urinary flow rates to reduce contrast medium dwell time in the renal tubules, initiating urinary alkalinization with bicarbonate, and using antioxidants may provide synergistic protection against kidney injury.6,70
SPECIALTY-SPECIFIC PROTOCOLS
With these collective considerations, the following specialty-specific protocols for minimizing the risk of contrast-induced AKI have been developed by a multidisciplinary Advisory Board on Contrast-Induced Nephropathy, based on expert opinion. Board members had specific research interests, publications, and professional activities in the specialties of radiology, cardiology, and nephrology. The materials presented and reviewed were determined at the independent discretion of each panel member.
Interventional Radiology
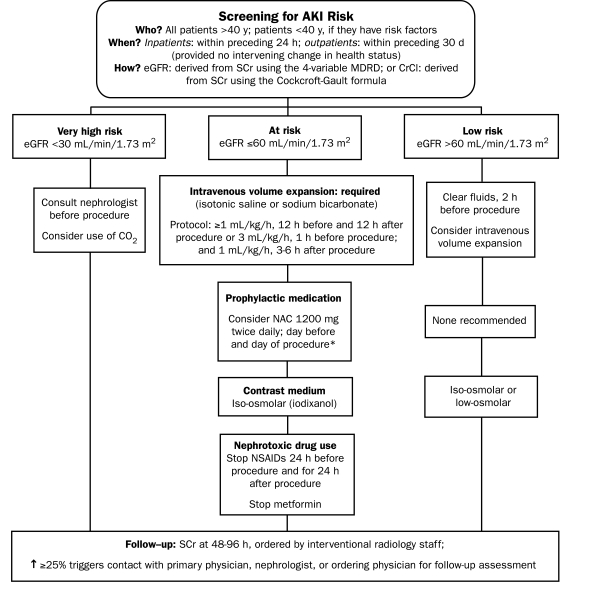
In this specialty, it is impractical and unnecessary to perform serum creatinine assays for all patients. A review of the patient's clinical history and an interview with the patient will generally be sufficient for determining which patients are aged 40 years or younger and have no risk factors. For outpatients taking stable medication regimens and exhibiting no change in health status, measuring the serum creatinine concentration within 30 days of the procedure is acceptable. For inpatients, the serum creatinine concentration and the eGFR should be assessed within 24 hours of the procedure (Figure 1).
FIGURE 1.
Protocol for interventional radiology. AKI = acute kidney injury; CO2 = carbon dioxide; CrCl = creatinine clearance; eGFR = estimated glomerular filtration rate; MDRD = Modification of Diet in Renal Disease; NSAIDs = nonsteroidal anti-inflammatory drugs.
*Do not measure the baseline serum creatinine (SCr) concentration after administration of N-acetylcysteine (NAC); do not cancel or delay the procedure if NAC is not administered.
Patients with an eGFR lower than 30 mL/min/1.73 m2 are at very high risk of contrast-induced AKI; for these patients, all recommended prophylactic strategies should be used, as guided by a consultant nephrologist, and alternatives to the administration of iodinated contrast medium should be considered. Intravenous volume expansion is considered a requirement for patients with an eGFR lower than 60 mL/min/1.73 m2 and is considered good practice for other patients. For patients determined to be at increased risk of contrast-induced AKI, iso-osmolar contrast medium should be used, as recommended by the AHA/ACC and NKF.16,17,21 Either iso-osmolar contrast medium or low-osmolar contrast medium may be used for patients with an eGFR higher than 60 mL/min/1.73 m2, although for these low-risk patients with mild or no renal impairment, other issues such as comparative cost should probably factor into the selection of contrast medium (Figure 1).
Prophylactic NAC may be administered, with the proviso that the baseline serum creatinine concentration should be determined before the medication is administered. Metformin use should be discontinued by diabetic patients with preexisting renal impairment (but not by those considered at no or at low risk of contrast-induced AKI) because lactic acid toxicity may be caused by critical levels of metformin accumulation in response to contrast-induced deterioration in these patients whose renal function is already compromised. According to both ESUR and ACR recommendations, diabetic patients with an elevated serum creatinine concentration should discontinue the use of metformin before the contrast medium is administered and should not resume it for 48 hours after the procedure. The decision to resume metformin use should be based on the results of follow-up serum creatinine assays. The ESUR and ACR differ in their recommendations about when metformin use should be discontinued (either 48 hours before administration of contrast medium or just before its administration) and about whether metformin use should be interrupted for diabetic patients with normal renal function.20,22
Follow-up serum creatinine assays should be performed 48 to 96 hours after the administration of a contrast agent. Interventional radiology staff members are responsible for ordering this laboratory test and for ensuring that systems are in place to alert others involved in the patient's care if contrast-induced AKI is detected (Figure 1).
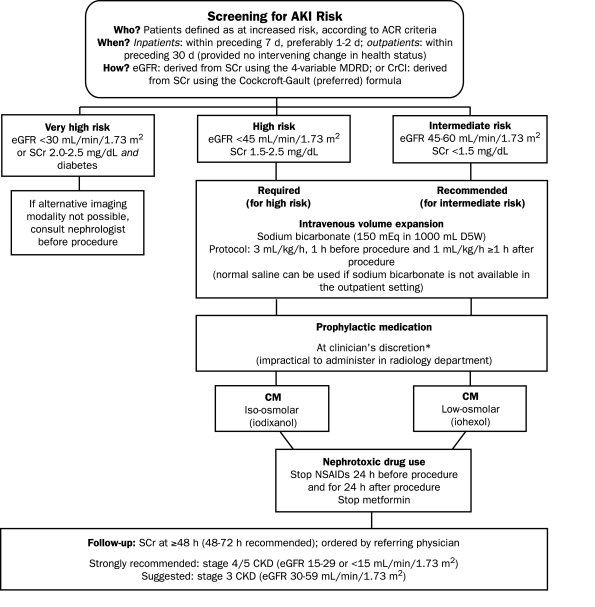
Diagnostic CT
In urgent cases, screening and preventive measures may not be possible. Screening is recommended when ACR criteria indicate that the patient is at increased risk of adverse events.20 Preventive measures should be focused on patients with stage 4 or 5 CKD, for whom the risk of contrast-induced AKI is sufficiently high to warrant the extra cost and effort involved. For patients at very high risk (eGFR <30 mL/min/1.73 m2 and diabetes mellitus), a nephrology consultation is necessary, and iodinated contrast medium should be used only if there is no alternative (Figure 2).
FIGURE 2.
Protocol for diagnostic computed tomography risk defined according to American College of Radiology (ACR) criteria.20 AKI = acute kidney injury; CKD = chronic kidney disease; CM = contrast medium; CrCl = creatinine clearance; eGFR = estimated glomerular filtration rate; MDRD = Modification of Diet in Renal Disease; NSAIDs = nonsteroidal anti-inflammatory drugs.
*Do not measure the baseline serum creatinine (SCr) concentration after administration of N-acetylcysteine (NAC); do not cancel or delay the procedure if NAC is not administered.
Intravenous volume expansion is required for patients with an eGFR lower than 45 mL/min/1.73 m2 and is recommended for those at intermediate risk of AKI (eGFR 45-60 mL/min/1.73 m2). Volume expansion is sufficiently important as a preventive measure to justify the burden it imposes on radiology departments. A protocol recommending rapid volume expansion with bicarbonate is most practical. When patients with an eGFR lower than 45 mL/min/1.73 m2 undergo diagnostic CT, whether or not they have diabetes, the iso-osmolar contrast medium iodixanol should be used. If an iodinated contrast medium is essential, iodixanol is indicated for patients with an eGFR lower than 30 mL/min/1.73 m2. The referring physician should perform a follow-up assessment 48 to 72 hours after the procedure (Figure 2).
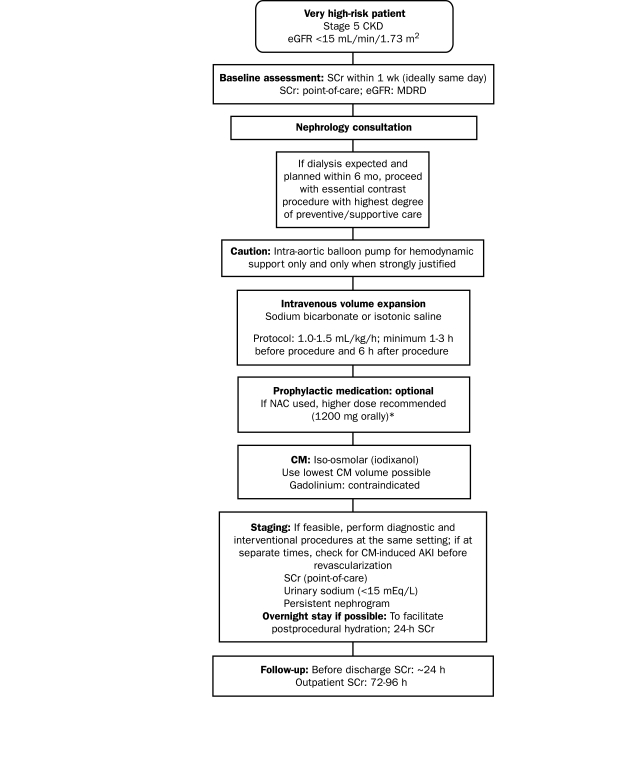
Interventional Cardiology
The interventional cardiology protocol focuses on patients at very high risk and those undergoing dialysis (Figure 3). It is not feasible to recommend that none of these patients should undergo contrast-enhanced procedures because their risk of AKI is too high. Of note, patients with CKD are often denied the benefit of indicated contrast-enhanced coronary procedures because of concerns about nephrotoxicity. The findings of a large registry study showed that, although patients with CKD would probably be eligible for coronary angiography according to standard eligibility criteria, only 25.2% of such patients underwent the procedure compared with 46.8% of patients who did not have CKD. Thus, a large proportion of patients with CKD were denied the benefit of a reduction in the risk of death.71
FIGURE 3.
Protocol for interventional cardiology. AKI = acute kidney injury; CKD = chronic kidney disease; CM = contrast medium; eGFR = estimated glomerular filtration rate; MDRD = Modification of Diet in Renal Disease.
*Do not measure the baseline serum creatinine (SCr) concentration after administration of N-acetylcysteine (NAC); do not cancel or delay the procedure if NAC is not administered.
Because of their advanced disease, patients already undergoing maintenance hemodialysis for end-stage renal disease require no specific attention to volume expansion or other approaches for preservation of renal function. Also, these patients do not require emergent dialysis treatment after exposure to contrast medium unless they have incipient congestive heart failure and the volume expansion induced by the contrast material itself results in acute cardiac decompensation.
A nephrology consultation is mandatory for the assessment of potential renal damage; the findings should be factored into any decision made. If dialysis is expected within 6 months and the incidence of contrast-induced AKI would likely precipitate the need for dialysis, the contrast-enhanced coronary procedure should be conducted with the highest level of preventive and supportive care. Because the use of intra-aortic balloon pumps is associated with the incidence of AKI in patients with CKD,72 these pumps should be used for hemodynamic support only; their use in this high-risk patient population requires strong justification (Figure 3).
A recent (within 1 week, ideally on the same day) assessment of the serum creatinine concentration is required for determining baseline eGFR. Intravenous volume expansion should be performed as detailed in Figure 3. An overnight stay in the hospital is recommended to facilitate postprocedural volume expansion and to enable postprocedural (24-hour) serum creatinine assays. In accordance with NKF and AHA/ACC guidelines, iodixanol is the indicated contrast medium for these high-risk patients.16,17,21 The lowest possible volume of contrast medium should be used, and therapeutic intervention, if required, should be performed immediately after diagnostic angiography so that the risk incurred by sequential contrast-enhanced procedures can be avoided. Patients are often referred for therapeutic intervention after undergoing coronary angiography at another institution; such patients should be evaluated for AKI before the interventional procedure is performed. In these cases, a persistent nephrogram density73 or a urinary sodium concentration lower than 15 mEq/L indicates that the patient may already be experiencing contrast-induced AKI. Point-of-care serum creatinine assays would also be useful in this situation (Figure 3).
CONCLUSION
Contrast-induced AKI is an important clinical entity faced by practitioners across a wide range of specialties. Patient management guidelines must consider risk factors for contrast-induced AKI and the most up-to-date information about preventive strategies. The specialty-specific, multimodality protocols outlined here are intended to promote best care while taking into account the practicalities that may limit screening and preventive measures in various situations.
Footnotes
Dr Goldfarb received honoraria from GE Healthcare. Dr McDermott received income for attending the GE multidisciplinary protocols meeting. Dr Gay is on the speakers bureau for GE Healthcare, is a member of the GE CT Advisory Board and the Carestream Health Advisory Board, and is a consultant for Carestream Health.
The multidisciplinary Advisory Board on Contrast-Induced Nephropathy was supported by an unrestricted educational grant from GE Healthcare and met in Dallas, TX, on September 28 and 29, 2007.
Editorial support and research assistance over the course of the preparation of the submitted manuscript were provided, under the direction of the authors, by Joan Sobel, PhD, and Lisa Cowen, PhD, at PAREXEL Inc and funded by GE Healthcare Limited.
REFERENCES
- 1.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11(2):R31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molitoris BA, Levin A, Warnock DG, et al. Acute Kidney Injury Network Working Group Improving outcomes of acute kidney injury: report of an initiative. Nat Clin Pract Nephrol. 2007;3(8):439-442 [DOI] [PubMed] [Google Scholar]
- 3.Morcos SK. Contrast medium-induced nephrotoxicity. In: Dawson P, Cosgrove DO, Grainger RG, eds. Textbook of Contrast Media Oxford, England: Isis Medical Media Ltd; 1999:135-148 [Google Scholar]
- 4.Nikolsky E, Aymong ED, Dangas G, Mehran R. Radiocontrast nephropathy: identifying the high-risk patient and the implications of exacerbating renal function. Rev Cardiovasc Med. 2003;4(suppl 1):S7-S14 [PubMed] [Google Scholar]
- 5.McCullough PA, Adam A, Becker CR, et al. CIN Consensus Working Panel Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol. 2006September16;98(6A):5K-13K Epub 2006 Feb 10 [DOI] [PubMed] [Google Scholar]
- 6.McCullough PA. Multimodality prevention of contrast-induced acute kidney injury. Am J Kidney Dis. 2008;51(2):169-172 [DOI] [PubMed] [Google Scholar]
- 7.Brosius FC, III, Hostetter TH, Kelepouris E, et al. Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease: a science advisory from the American Heart Association Kidney and Cardiovascular Disease Council; the Councils on High Blood Pressure Research, Cardiovascular Disease in the Young, and Epidemiology and Prevention; and the Quality of Care and Outcomes Research Interdisciplinary Working Group developed in collaboration with the National Kidney Foundation. Circulation 2006September5;114(10):1083-1087 Epub 2006 Aug 7 [DOI] [PubMed] [Google Scholar]
- 8.Rudnick MR, Goldfarb S, Wexler L, et al. Iohexol Cooperative Study Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. Kidney Int. 1995;47(1):254-261 [DOI] [PubMed] [Google Scholar]
- 9.Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002;105(19):2259-2264 [DOI] [PubMed] [Google Scholar]
- 10.McCullough PA, Adam A, Becker CR, et al. CIN Consensus Working Panel Risk prediction of contrast-induced nephropathy. Am J Cardiol. 2006September18;98(6A):27K-36K Epub 2006 Feb 23 [DOI] [PubMed] [Google Scholar]
- 11.Manske CL, Sprafka JM, Strony JT, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89(5):615-620 [DOI] [PubMed] [Google Scholar]
- 12.McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368-375 [DOI] [PubMed] [Google Scholar]
- 13.Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36(5):1542-1548 [DOI] [PubMed] [Google Scholar]
- 14.Weisbord SD, Chen H, Stone RA, et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol. 2006October;17(10):2871-2877 Epub 2006 Aug 23 [DOI] [PubMed] [Google Scholar]
- 15.Hassoun HT, Grigoryev DN, Lie ML, et al. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol. 2007July;293(1):F30-F40 Epub 2007 Feb 27 [DOI] [PubMed] [Google Scholar]
- 16.King SB, III, Smith SC, Jr, Hirshfeld JW, Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee [published correction appears in Circulation. 2008;117(6):e161] Circulation 2008January15;117(2):261-295 Epub 2007 Dec 13 [DOI] [PubMed] [Google Scholar]
- 17.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons; endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):652-726 [DOI] [PubMed] [Google Scholar]
- 18.Jo SH, Youn TJ, Koo BK, et al. Renal toxicity evaluation and comparison between visipaque (iodixanol) and hexabrix (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: the RECOVER study: a randomized controlled trial. J Am Coll Cardiol. 2006September5;48(5):924-930 Epub 2006 Aug 17 [DOI] [PubMed] [Google Scholar]
- 19.McCullough PA, Bertrand ME, Brinker JA, Stacul F. A meta-analysis of the renal safety of isosmolar iodixanol compared to low-osmolar contrast media. J Am Coll Cardiol. 2006August16;48(4):692-699 Epub 2006 Jul 24 [DOI] [PubMed] [Google Scholar]
- 20.Segal A, Ellis JH, Baumgartner BR, et al. Manual on Contrast Media: Version 6; 2008. ACR Am Coll Radiol. http://www.acr.org/SecondaryMainMenuCategories/quality_safety/contrast_manual.aspx Accessed December 23, 2008
- 21.National Kidney Foundation DOQI http://www.kidney.org/professionals/KDOQI/guidelines_cvd/guide2.htm. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Accessed December 23, 2008. [PubMed]
- 22.European Society of Urogenital Radiology http://www.esur.org/ESUR_Guidelines_NEW.6.0.html. ESUR Guidelines on Contrast Media, Version 6.0. Accessed December 23, 2008.
- 23.Holder JC, Dalrymple GV. Pain and aortofemoral arteriography: the importance of chemical structure and osmolality of contrast agents. Invest Radiol. 1981;16(6):508-512 [PubMed] [Google Scholar]
- 24.Manke C, Marcus C, Page A, Puey J, Batakis O, Fog A. Pain in femoral arteriography: a double-blind, randomized, clinical study comparing safety and efficacy of the iso-osmolar iodixanol 270 mgI/ml and the low-osmolar iomeprol 300 mgI/ml in 9 European centers. Acta Radiol. 2003;44(6):590-596 [DOI] [PubMed] [Google Scholar]
- 25.Kløw NE, Levorstad K, Berg KJ, et al. Iodixanol in cardioangiography in patients with coronary artery disease: tolerability, cardiac and renal effects. Acta Radiol. 1993;34(1):72-77 [PubMed] [Google Scholar]
- 26.Tveit K, Bolz KD, Bolstad B, et al. Iodixanol in cardioangiography: a double-blind parallel comparison between iodixanol 320 mg I/ml and ioxaglate 320 mg I/ml. Acta Radiol. 1994;35(6):614-618 [PubMed] [Google Scholar]
- 27.Palmers Y, De Greef D, Grynne BH, Smits J, Put E. A double-blind study comparing safety, tolerability and efficacy of iodixanol 320 mgI/ml and ioxaglate 320 mgI/ml in cerebral arteriography. Eur J Radiol. 1993;17(3):203-209 [DOI] [PubMed] [Google Scholar]
- 28.Pugh ND, Sissons GR, Ruttley MS, Berg KJ, Nossen JO, Eide H. Iodixanol in femoral arteriography (phase III): a comparative double-blind parallel trial between iodixanol and iopromide. Clin Radiol. 1993;47(2):96-99 [DOI] [PubMed] [Google Scholar]
- 29.Verow P, Nossen JO, Sheppick A, Kjaersgaard P. A comparison of iodixanol with iopamidol in aorto-femoral angiography. Br J Radiol. 1995;68(813):973-978 [DOI] [PubMed] [Google Scholar]
- 30.Justesen P, Downes M, Grynne BH, Lang H, Rasch W, Seim E. Injection-associated pain in femoral arteriography: a European multicenter study comparing safety, tolerability, and efficacy of iodixanol and iopromide. Cardiovasc Intervent Radiol. 1997;20(4):251-256 [DOI] [PubMed] [Google Scholar]
- 31.Thomsen HS. How to avoid CIN: guidelines from the European Society of Urogenital Radiology. Nephrol Dial Transplant. 2005;20(suppl 1):i18-i22 [DOI] [PubMed] [Google Scholar]
- 32.Choyke PL, Cady J, DePollar SL, Austin H. Determination of serum creatinine prior to iodinated contrast media: is it necessary in all patients? Tech Urol. 1998;4(2):65-69 [PubMed] [Google Scholar]
- 33.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393-1399 [DOI] [PubMed] [Google Scholar]
- 34.Band RA, Gaieski DF, Mills AM, et al. Discordance between serum creatinine and creatinine clearance for identification of ED patients with abdominal pain at risk for contrast-induced nephropathy. Am J Emerg Med. 2007;25(3):268-272 [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461-470 [DOI] [PubMed] [Google Scholar]
- 36.Lameire N, Adam A, Becker CR, et al. CIN Consensus Working Panel Baseline renal function screening. Am J Cardiol. 2006September18;98(6A):21K-26K Epub 2006 Feb 20 [DOI] [PubMed] [Google Scholar]
- 37.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005March;16(3):763-773 Epub 2005 Jan 19 [DOI] [PubMed] [Google Scholar]
- 38.Rigalleau V, Lasseur C, Perlemoine C, et al. Estimation of glomerular filtration rate in diabetic subjects: Cockcroft formula or Modification of Diet in Renal Disease study equation? Diabetes Care 2005;28(4):838-843 [DOI] [PubMed] [Google Scholar]
- 39.Beauvieux MC, Le Moigne F, Lasseur C, et al. New predictive equations improve monitoring of kidney function in patients with diabetes. Diabetes Care 2007August;30(8):1988-1994 Epub 2007 May 29 [DOI] [PubMed] [Google Scholar]
- 40.Firestone D, Wos A, Killeen JP, et al. Can urine dipstick be used as a surrogate for serum creatinine in emergency department patients who undergo contrast studies? J Emerg Med. 2007August;33(2):119-122 Epub 2007 Jun 13 [DOI] [PubMed] [Google Scholar]
- 41.McCullough PA, Sandberg KR. Epidemiology of contrast-induced nephropathy. Rev Cardiovasc Med. 2003;4(suppl 5):S3-S9 [PubMed] [Google Scholar]
- 42.O'Hare AM, Bertenthal D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol. 2006March;17(3):846-853 Epub 2006 Feb 1 [DOI] [PubMed] [Google Scholar]
- 43.Stacul F, Adam A, Becker C, et al. CIN Consensus Working Panel Strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol. 2006September18;98(6A):59K-77K Epub 2006 Mar 20 [DOI] [PubMed] [Google Scholar]
- 44.Bader BD, Berger ED, Heede MB, et al. What is the best hydration regimen to prevent contrast media-induced nephrotoxicity? Clin Nephrol. 2004;62(1):1-7 [DOI] [PubMed] [Google Scholar]
- 45.Trivedi HS, Moore H, Nasr S, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93(1):C29-C34 [DOI] [PubMed] [Google Scholar]
- 46.Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162(3):329-336 [DOI] [PubMed] [Google Scholar]
- 47.Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA 2004;291(19):2328-2334 [DOI] [PubMed] [Google Scholar]
- 48.Masuda M, Yamada T, Mine T, et al. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol. 2007September1;100(5):781-786 Epub 2007 Jun 13 [DOI] [PubMed] [Google Scholar]
- 49.Briguori C, Airoldi F, D'Andrea D, et al. Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation 2007March13;115(10):1211-1217 Epub 2007 Feb 19 [DOI] [PubMed] [Google Scholar]
- 50.Recio-Mayoral A, Chaparro M, Prado B, et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO study. J Am Coll Cardiol. 2007March27;49(12):1283-1288 Epub 2007 Mar 12 [DOI] [PubMed] [Google Scholar]
- 51.Ozcan EE, Guneri S, Akdeniz B, et al. Sodium bicarbonate, N-acetylcysteine, and saline for prevention of radiocontrast-induced nephropathy: a comparison of 3 regimens for protecting contrast-induced nephropathy in patients undergoing coronary procedures: a single-center prospective controlled trial. Am Heart J. 2007;154(3):539-544 [DOI] [PubMed] [Google Scholar]
- 52.Hogan SE, L'Allier P, Chetcuti S, et al. Current role of sodium bicarbonate-based preprocedural hydration for the prevention of contrast-induced acute kidney injury: a meta-analysis. Am Heart J. 2008;156(3):414-421 [DOI] [PubMed] [Google Scholar]
- 53.From AM, Bartholmai BJ, Williams AW, Cha SS, Pflueger A, McDonald FS. Sodium bicarbonate is associated with an increased incidence of contrast nephropathy: a retrospective cohort study of 7977 patients at Mayo Clinic. Clin J Am Soc Nephrol. 2008January;3(1):10-18 Epub 2007 Dec 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maioli M, Toso A, Leoncini M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52(8):599-604 [DOI] [PubMed] [Google Scholar]
- 55.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343(3):180-184 [DOI] [PubMed] [Google Scholar]
- 56.Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy [published correction appears in Ann Intern Med. 2008;149(3):219] Ann Intern Med. 2008;148(4):284-294 [DOI] [PubMed] [Google Scholar]
- 57.Fishbane S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol. 2008January;3(1):281-287 Epub 2007 Nov 14 [DOI] [PubMed] [Google Scholar]
- 58.Spargias K, Alexopoulos E, Kyrzopoulos S, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 2004November2;110(18):2837-2842 Epub 2004 Oct 18 [DOI] [PubMed] [Google Scholar]
- 59.Davidson CJ, Hlatky M, Morris KG, et al. Cardiovascular and renal toxicity of a nonionic radiographic contrast agent after cardiac catheterization: a prospective trial. Ann Intern Med. 1989;110(2):119-124 [DOI] [PubMed] [Google Scholar]
- 60.Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989;86(6, pt 1):649-652 [DOI] [PubMed] [Google Scholar]
- 61.Freeman RV, O'Donnell M, Share D, et al. Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol. 2002;90(10):1068-1073 [DOI] [PubMed] [Google Scholar]
- 62.Laskey WK, Jenkins C, Selzer F, et al. NHLBI Dynamic Registry Investigators Volume-to-creatinine clearance ratio: pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007August;50(7):584-590 Epub 2007 Jul 30 [DOI] [PubMed] [Google Scholar]
- 63.Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology 1993;188(1):171-178 [DOI] [PubMed] [Google Scholar]
- 64.Feldkamp T, Baumgart D, Elsner M, et al. Nephrotoxicity of iso-osmolar versus low-osmolar contrast media is equal in low risk patients. Clin Nephrol. 2006;66(5):322-330 [DOI] [PubMed] [Google Scholar]
- 65.Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ, NEPHRIC Study Investigators Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348(6):491-499 [DOI] [PubMed] [Google Scholar]
- 66.Solomon RJ, Natarajan MK, Doucet S, et al. Investigators of the CARE Study Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation 2007June26;115(25):3189-3196 Epub 2007 Jun 11 [DOI] [PubMed] [Google Scholar]
- 67.Barrett BJ, Katzberg RW, Thomsen HS, et al. Contrast-induced nephropathy in patients with chronic kidney disease undergoing computed tomography: a double-blind comparison of iodixanol and iopamidol. Invest Radiol. 2006;41(11):815-821 [DOI] [PubMed] [Google Scholar]
- 68.Kuhn MJ, Chen N, Sahani DV, et al. The PREDICT study: a randomized double-blind comparison of contrast-induced nephropathy after low- or isoosmolar contrast agent exposure. AJR Am J Roentgenol. 2008;191(1):151-157 [DOI] [PubMed] [Google Scholar]
- 69.Thomsen HS, Morcos SK, Erley CM, et al. Investigators in the Abdominal Computed Tomography: IOMERON 400 Versus VISIPAQUE 320 Enhancement (ACTIVE) Study The ACTIVE Trial: comparison of the effects on renal function of iomeprol-400 and idoixanol-320 in patients with chronic kidney disease undergoing abdominal computed tomography. Invest Radiol. 2008;43(3):170-178 [DOI] [PubMed] [Google Scholar]
- 70.McCullough PA. Contrast-induced acute kidney injury [published correction appears in J Am Coll Cardiol. 2008;51(22):2197] J Am Coll Cardiol. 2008;51(15):1419-1428 [DOI] [PubMed] [Google Scholar]
- 71.Chertow GM, Normand SL, McNeil BJ. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15(9):2462-2468 [DOI] [PubMed] [Google Scholar]
- 72.Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95(1):13-19 [DOI] [PubMed] [Google Scholar]
- 73.Love L, Johnson MS, Bresler ME, Nelson JE, Olson MC, Flisak ME. The persistent computed tomography nephrogram: its significance in the diagnosis of contrast-associated nephrotoxicity. Br J Radiol. 1994;67(802):951-957 [DOI] [PubMed] [Google Scholar]