Abstract
Purpose: Very few studies have examined cognitive decline in caregivers versus noncaregivers, and only 1 study has examined mediators of such decline. We evaluated the relationship between caregiver status and decline on the digit symbol test (DST; a measure of processing speed, attention, cognitive–motor translation, and visual scanning) and whether this relationship was mediated by depressed mood. Design and Methods: Caregivers for spouses with Alzheimer's disease (n = 122) were compared with demographically similar noncaregiver spouses (n = 117) at study entry (Time 1 = T1), T2 (1 year later), and T3 (2 years after T1). Results: Caregivers had lower DST scores and higher Hamilton depression scores at T1, T2, and T3 than noncaregivers (all p < .05). Hierarchical linear modeling revealed that although caregivers started well below noncaregivers, they experienced a more rapid rate of decline than noncaregivers (p = .047). Caregivers declined 4.5 times faster than noncaregivers. Greater depressed mood at T1 (p < .01) and T2 (p < .01) predicted DST decline and mediated DST decline in caregivers vs. noncaregivers. Implications: Depressed mood in caregivers relative to noncaregivers may influence their greater risk for DST decline. This is important because the DST predicts problem solving and everyday functions necessary for independent living and the potential well-being of their care recipients.
Keywords: Stress, Caregiving, Depression, Cognition, Processing speed, Attention
Chronic stressors are usually defined as long-term unrelenting demands placed on an organism. Many caregivers of family members with Alzheimer's disease (AD) are exposed to such stressors via the progressive cognitive, functional, and behavioral problems of their care recipients. In response, caregivers have been shown to have higher levels of depression, burden, poor health habits, physiological dysregulation, psychiatric or medical morbidity, and mortality than noncaregivers (Haley, Levine, Brown, & Bartolucci, 1987; Pinquart & Sorensen, 2003; Schulz & Beach, 1999; Vitaliano, Zhang, & Scanlan, 2003).
These responses represent various stages of caregiver adaptation that has been conceptualized as a dynamic process between the caregiver and the care recipient (e.g., Aneshensel, Pearlin, Mullan, Zarit, & Whitlatch, 1995). In this view, care recipient behavior, cognitions, and affect elicit negative caregiver outcomes (e.g., depression) across time (see Gaugler, Davey, Pearlin, & Zarit, 2000; Whitlatch, Feinberg, & Sebesta, 1997). The continuous exposure to stressors in the absence of rest results in excessive chronic secretion of hormones (e.g., cortisol and epinephrine; de Kloet & Derijk, 2004; Selye, 1979), exhaustion, depletion of reserves, and progressive physical and mental problems.
In this article, we focus on one such problem, depression, because of its importance as a caregiver outcome (Covinsky et al., 2003; Gallagher-Thompson et al., 2006; Wisniewski et al., 2003) and its relationship to cognitive processing. It is well known that stress and depression influence several regions of the brain (Kim & Diamond, 2002; Roozendaal, McReynolds, & McGaugh, 2004) and cognitive dysfunction (Bremner, 1999; Levy, Dachir, Arbel, & Kadar, 1994; Lupien et al., 1994; Newcomer, Craft, Hershey, Askins, & Bardgett, 1994). In fact, both depressed mood (Chepenik, Cornew, & Farah, 2007; Roose, Devanand, & Hamilton, 2007) and clinical depression (Lee, Potter, Wagner, Welsh-Bohmer, & Steffens, 2007; Naismith, Longley, Scott, & Hickie, 2007; Steffens et al., 2006) are associated with cognitive decline. Depression and cognitive processes may be related (Baune, Suslow, Arolt, & Berger, 2007) because they are each associated with elevations in stress hormones (Erickson, Drevets & Schulkin, 2003; B. K. Lee et al., 2007), the strongest physiological correlates of caregiver status (Vitaliano et al., 2003).
Given this research, it is not surprising that the cognitive status of caregivers is receiving greater attention. To our knowledge, the first study to examine such relationships in caregivers versus noncaregivers showed that spouse caregivers of persons with AD (n = 44) had lower scores on a measure of processing speed and attention, the digit symbol test (DST; Wechsler, 1997), than did demographically similar spouse noncaregivers (n = 77; Caswell et al., 2003). A second study by Lee, Kawachi, and Grodstein (2004) telephone administered cognitive tests to 13,740 Nurses' Health Study participants aged 70–79 years. They extended the DST finding to other cognitive measures by observing significant deficits in immediate–delayed recall, verbal fluency, and digit span backward in spouse caregivers compared with spouse noncaregivers. A third study by de Vugt and colleagues (2006) replicated Caswell and colleagues and found that spouse caregivers had lower DST scores as well as delayed recall scores and greater Stroop interference scores than noncaregivers. Since these studies, two longitudinal analyses have been reported. Vitaliano and colleagues (2005) found that caregivers experienced a small but significant drop in vocabulary relative to demographically similar noncaregivers despite beginning with similar scores. Finally, Mackenzie, Smith, Hasher, Leach, and Behl (2007) showed that caregivers of palliative family members exhibited significant impairments in attention (monitoring performance and regulating attentional resources) compared with healthy normative samples.
Although these studies suggest relationships between caregiver status and adverse cognitive function, they remain few. Because the DST has been shown to be particularly sensitive to caregiver status cross-sectionally (de Vugt et al., 2006), in this study we attempted to extend previous research by examining this measure longitudinally. The DST is a good screening tool because it measures functions that are hallmarks of future cognitive impairment such as processing speed, attention, cognitive–motor translation, and visual scanning (Lezak, 1995). Although nonspecific, the DST is highly sensitive to neurocognitive processes (Park et al., 2002) and stress and age in noncaregiver samples (Salthouse, 1996), and it has high test–retest reliability (Dikmen, Heaton, Grant, & Temkin, 1999).
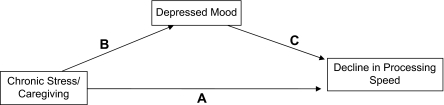
From the previous relationships, we hypothesized that the spouse caregivers will show DST decline, but this will not be observed in demographically and physically similar noncaregiver spouses (Research Hypothesis 1 [H1]). If Research Hypothesis 1 is supported, our longitudinal design will also allow us to assess the temporal mediation of DST decline in caregivers versus noncaregivers. Depression is one such mediator, as it is associated with DST decline in older adults (Kizilbash, Vanderploeg, & Curtiss, 2002). We hypothesized that if a relationship is observed between caregiver status and DST decline, it will be mediated by depression (H2) because caregivers will have higher depression levels than noncaregivers and higher depression scores will be associated with poorer DST scores. These relationships are represented in Figure 1 by Pathway B (caregiver status predicting depression), Pathway C (depression predicting DST), and Pathway A (caregiver status predicting DST decline). This model is an abbreviated form of a more comprehensive model of chronic stress and health that includes pathways from caregiver stressors, vulnerabilities, resources, distress, health habits, and physiological dysregulation to health outcomes. This model has been shown to predict caregiver health (Vitaliano et al., 2002).
Figure 1.
Mediation model of caregiver stress to digit symbol test decline via depressed mood.
Because demographic and health-related variables have been shown to be related to cognitive decline, caregiver status, or depressed mood, or all, we assessed these factors and controlled them if necessary. These included age (Park, 1996), education (Alley, Suthers, & Crimmins, 2007), morbidities in particular stroke (Vermeer et al., 2003), obesity (Taylor & MacQueen, 2007), health habits (e.g., alcohol; Solfrizzi et al., 2007), and medications (Elias, Wolf, D'Agostino, Cobb, & White, 1993).
The results of these research hypotheses are potentially important for three reasons. First, cognitive problems among caregivers have implications for society given that caregivers provide more than 80% of long-term care services with a cost of $196 billion. This figure greatly exceeds national spending for home health or nursing home care (Arno, Levine, & Memmott, 1999). Total direct costs of care for persons with AD living at home are 20.8% lower than for comparable persons living in an institutional setting (Zhu et al., 2006). Second, the processes assessed by the DST are critical to everyday living and problem solving (Willis, Jay, Diehl, & Marsiske, 1992). The caregiving role demands organizational and problem-solving skills to maintain the household and deliver care. If caregiver cognitive functions are compromised, this could influence their well-being and that of their care recipients. Third, these hypotheses provide further tests of caregiver models of adaptation using a novel and important indicator.
Methods
Design and Participants
Caregiver couples were recruited from the general community in western Washington State via printed or electronic media, physicians’ offices, the University of Washington Alzheimer's Disease registry, and the Alzheimer's Association. Criteria for care recipient inclusion were living with one's spouse, aged 55 years or older, and Diagnostic and Statistical Manual (DSM)-IV diagnosis of dementia of the Alzheimer's type or possible/probable primary degenerative dementia (McKhann et al., 1984). Caregivers had to function independently and be the primary caregiver for their spouse care recipient. Demographically similar noncaregivers were recruited from senior centers, retirement organizations, and the media. Noncaregivers and their spouses had to be aged 55 years or older, functioning independently, and not providing care for anyone on a regular basis. During recruitment, we asked all prospective caregivers and noncaregivers if they were physically mobile and able to perform activities of independent maintenance (e.g., grooming, house cleaning) and higher functioning (e.g., managing finances). These criteria were then verified during the initial assessment (Ware et al., 1995).
The demographic similarities between noncaregivers and caregivers were accomplished by matching a noncaregiver to a caregiver within a 5-year age interval and by similar education (e.g., high school, some college), income (e.g., $10,000), and gender or ethnic groups. The University of Washington Institutional Review Board approved the study and informed consent was obtained.
We assessed caregivers and noncaregivers at study entry (Time 1 = T1), 1 year after T1 (T2), and 2 years after T1 (T3). All assessments were face-to-face interviews that occurred in our university offices at T1 and T2. At T3, 87% of the interviews occurred in our offices and 13% occurred in the participants' homes. At T1, we sampled 130 spouse caregivers and their spouses (AD care recipients) and 125 noncaregiver spouses (and their AD-free spouses). In 2 years, 3 caregivers and 1 noncaregiver died, 4 caregivers and 4 noncaregivers moved, 1 caregiver and 1 noncaregiver reported being too ill, and 2 noncaregivers refused to continue. This left 122 caregivers and 117 noncaregivers. Our high 2-year retention rate may have resulted from strategies we learned after having performed three previous longitudinal studies of caregivers and noncaregivers. These included regular contact with caregivers or noncaregivers by calling them twice and sending one greeting card during each time interval, and by obtaining next of kin or friend contacts in case communications were lost between the couples in the study and our research staff.
Between study entry and Time 2, all AD care recipients were still living at home, but by T3, we observed that 17% of the spouses of caregivers and 7% of the spouses of noncaregivers entered nursing homes (p < .05). The location of the AD care recipient (home vs. nursing home) and the location of the T3 interview were therefore examined in subsequent analyses.
Measures
Psychosocial or Health Habit Measures.—
The Hamilton Depression Rating Scale (Hamilton, 1960 [24 items]) was used to assess depressive symptoms (e.g., mood, guilt) present for at least 2 days, from 0 (absent) to 4 (severe). It includes atypical symptoms such as hypersomnia, increased appetite and weight gain, diurnal variation of mood, paranoid thoughts, obsessive-compulsive symptoms, and depersonalization. The mean alpha for Times 1–3 was .85 and the mean intraclass correlation (ICR) was .54 (p < .01).
Clinical depression was assessed by the Structured Clinical Interview for the DSM-IV (Williams et al., 1992). The same trained interviewer (Lisa W. Caswell) performed all face-to-face ratings.
Sleep quality was assessed using 13 items (Vitaliano et al., 1999) each with options almost never = 0, sometimes = 1, often = 2, and almost always = 3. The mean alpha was .81 and ICR was .77 (p < .01).
Alcohol use was assessed using a screen for lifetime drinking (CAGE, Ewing, 1984). Greater than two positive answers suggested covert drinking (Ewing, 1984). The mean ICR was .94 (p < .001). We also recorded bottles or cans of beer and shots of liquor consumed per week.
Physical illnesses or medications were assessed using medical records or self-reports. Medical records were obtained 5 years after T1 and coded for at least 5 previous years, a period that overlapped with each participant's T1 assessment. The coder was blind to caregiver status. To examine the quality of the medical records, we obtained date or nature of diagnosis, treatment, prognosis, and medications (Hanken, 1989). In 60% of records, blood pressure was recorded for 4 or more years, and in 35% for 1 year. In 88% of records, treatment and codes of the International Classification of Diseases, 10th revision; diagnostic tests; or dates; or all; were listed. Eighteen percent of the medical records did not list an illness, but the self-reports did; and 22% of medical records did not list a medication, but the self-reports did. If a medication was listed, but not a diagnostic code, we recorded the illness usually treated by the medication; if neither a medication nor an illness was listed in the records, but it was self-reported, we included it only if it was self-reported again at T2 or T3, or both. Self-reports were used for the participants (6%) who did not have medical records.
Obesity was defined as greater than or equal to the 90th percentile of body mass index (weight in kg/height in m2) on the age and gender norms of the Northwest Lipids Laboratory (mean ICR = .96, p < .001).
Cognitive Measure.—
The DST (Wechsler, 1997) was used to assess processing speed, complex attention psychomotor speed, cognitive–motor translation, and concentration. A paired array of digits and abstract symbols is shown and participants must write in the correct digit from the first array next to a corresponding array of symbols. A raw score is the number of correct pairs from 0 to 93. Test–retest reliability has been shown to be .89 (Dikmen et al., 1999), and in the current study, the mean ICR was .89, p < .01.
Care recipient mental status.
To verify that spouses of caregivers were cognitively impaired and spouses of noncaregivers were not, their spouses' mental status (orientation, memory, etc.) was assessed using the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975).
Hours per day of care reported by caregivers and noncaregivers were also assessed to verify that spouses of caregivers were providing care and spouses of noncaregivers were not.
Statistical Analysis
Digit symbol test decline was modeled using hierarchical linear modeling (HLM; Raudenbush & Bryk, 2002). It allows one to model change in each individual's DST scores (referred to as Level 1 equations, within-subjects model) and then to explain variations in these changes using mediators and covariates (Level 2 equations, between-subjects model). Because Level 1 equations model intra-individual changes, DST scores are predicted against time (T1 = 0; T2 = 1; T3 = 2). The slopes (β1) represent the rates of DST change per year. The intercepts (β0) represent each participant's T1 DST score.
At Level 2, the intercept (β0) and slope (β1) become dependent variables predicted by inter-individual variables (e.g., caregiver status, depressed mood). Potential confounders are included (e.g., gender, age, education, health) as covariates. The theoretical model is as follows:
Level 1:
where Yij is a participant's (j = 1, …, n) DST score at time i (i = 0, 1, 2), β0j is the intercept and β1j is the slope for participant j. Rij is the error term.
Level 2:
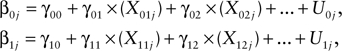 |
where ,00 is the mean intercept across all caregivers and noncaregivers; ,10 is the mean slope across all caregivers and noncaregivers; X01j and X02j are values of Level 2 predictors for β0j for participant j; X11j and X12j are values of Level 2 predictors for β1j for participant j; ,01 is the regression coefficient of X01 predicting β0. Similarly, ,02 is the regression coefficient of X02 predicting β0; ,11 and ,12 are regression coefficients of X11 and X12 predicting β1, respectively. Finally, U0j and U1j represent error terms in the theoretical model. Given our hypotheses, we used X1 to denote caregiver status and X2 to denote depression for participant j.
We first examined the , parameters that define the mean growth curve for the combined samples (,00 = mean intercept and ,10 = mean slope). We then focused on the relationships of caregiver status and depression with DST entry scores (β0) and slopes (β1). We first estimated fixed effects to see if caregiver status (,01) and depression (,02) were related to DST at study entry (i.e., β0). We then assessed whether these variables were associated with individual slopes (β1). If caregiver status was significant and negative, we interpreted the coefficient as follows: With no other variables in the model, caregivers were decreasing K DST points faster per year than noncaregivers, where K is a positive integer.
Hierarchical linear modeling was also used to test the potential mediation effects of depression on the association of caregiver status with DST decline. The mediation effects were tested following a general approach (Baron & Kenny, 1986). To do this, we first tested to see if caregiver status was related to DST decline. If it was, we then tested to see if T1 depression was also related to DST decline. If T1 depression was related to DST decline, we tested to see if the effect of caregiver status was insignificant when T1 depression was included in the model with caregiver status. The same procedure was also used to test for T2 depression as a mediator.
To control for variables at Level 2 that were either related to both caregiver status and DST decline (confounders) or only related to DST decline, we examined gender, age, education, stroke history, obesity, alcohol intake, location of the T3 interview, and nursing home placement by T3. We also tested to see if these variables acted as suppressor variables as they were theoretically relevant to caregiver status or DST decline, or both. The number of variables controlled in the HLM model was relatively small compared with the sample size. A log-likelihood deviance statistic was used to assess the goodness of fit of models.
Results
Univariate Results
The groups did not differ in gender, race, age, education, income, years married, illnesses, medication use, and alcohol intake (Table 1). Caregivers had higher Hamilton scores than noncaregivers at T1, t(235.6) = –3.49, p = .001; T2, t(236.9) = –4.62, p = .001; and T3, t(195.1) = −5.44, p = .001. Caregivers reported more sleep problems at all times and had a higher rate of obesity than noncaregivers (only T1 is shown because the percentages remain the same across time). Caregivers also had lower DST scores than noncaregivers at T1, t(237) = 2.28, p = .02; T2, t(237) = 2.49, p = .01; and T3, t(237) = 2.94, p = .004. Finally, care recipients had lower MMSE scores than spouses of noncaregivers, and spouse caregivers spent more hours per day providing care for their spouses than did spouses of noncaregivers.
Table 1.
Caregivers Versus Noncaregivers: Demographic, Health, Psychosocial, and Cognitive Measures
| Variables | Caregivers (n = 122) | Noncaregivers (n = 117) |
| Demographic/health factors | ||
| % Women | 62 | 64 |
| % Caucasian | 94 | 92 |
| Age (years, M ± SD) | 71.7 ± 8.9 | 70.2 ± 7.2 |
| Education (years, M ± SD) | 15.2 ± 2.6 | 15.2 ± 2.6 |
| Income ($, M ± SD)a | 52.0 ± 31.0 | 50.7 ± 26.5 |
| No. of years married (M ± SD) | 42.1 ± 15.3 | 40.5 ± 13.7 |
| % Hormone replacement | 36b | 36b |
| % Antihypertensive meds | 38 | 31 |
| % Sleep meds | 3 | 2 |
| % CHD | 18 | 17 |
| % Hypertension | 39 | 33 |
| % Diabetes | 8 | 6 |
| % Stroke | 3 | 3 |
| Psychosocial measures | ||
| % Current depression | 0 | 0 |
| % History of depression | 6 | 7 |
| Hamilton depression T1 (M ± SD) | 2.43 ± 3.1 | 1.1 ± 2.8*** |
| Hamilton depression T2 (M ± SD) | 2.51 ± 2.8 | .85 ± 2.7*** |
| Hamilton depression T3 (M ± SD) | 2.62 ± 3.1 | .86 ± 1.8*** |
| Sleep problems T1 (M ± SD) | 27.4 ± 6.5 | 24.1 ± 5.1*** |
| Sleep problems T2 (M ± SD) | 27.0 ± 6.2 | 24.1 ± 5.3*** |
| Sleep problems T3 (M ± SD) | 26.6 ± 6.3 | 24.5 ± 5.3*** |
| Health habits (M ± SD) | ||
| CAGE alcohol score | 0.30 ± 0.74 | 0.35 ± 0.96 |
| No. of cans/bottles beer/week (sqrt) | 0.20 ± 0.51 | 0.22 ± 0.60 |
| No. of shots liquor/week (sqrt) | 0.36 ± 0.91 | 0.35 ± 0.87 |
| Physiological measures | ||
| % Obese | 30 | 16* |
| Cognitive measures (M ± SD) | ||
| DST T1 | 46.0 ± 10.8 | 49.3 ± 11.8*** |
| DST T2 | 45.7 ± 11.6 | 49.5 ± 11.6*** |
| DST T3 | 45.0 ± 11.5 | 49.5 ± 12.0*** |
| Validity variables (M ± SD) | ||
| MMSE T1 | 17.0 ± 6.6 | 28.3 ± 1.8*** |
| MMSE T2 | 14.8 ± 8.1 | 28.2 ± 2.3*** |
| MMSE T3 | 13.5 ± 8.4 | 28.5 ± 2.2*** |
| Hours care per day T1 | 7.0 ± 8.2 | 1.0 ± 3.6*** |
| Hours care T2 | 9.0 ± 8.6 | 1.2 ± 3.6*** |
| Hours care T3 | 7.0 ± 8.1 | 1.3 ± 3.6*** |
Notes: CAGE = cut down, annoyed, guilty, eye-opener; CHD = chronic heart disease; MMSE = Mini-Mental State Examination; DST = digit symbol test; Sqrt = square root.
In 1,000’s.
In women.
*p < .05. **p < .01. ***p < .001.
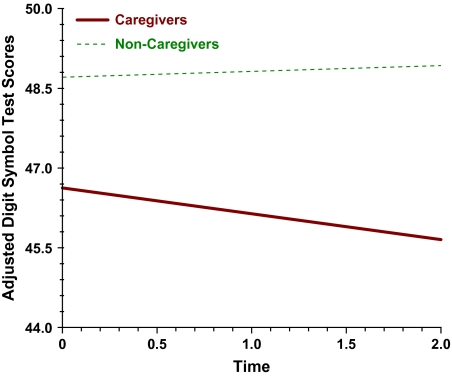
We observed that at study entry caregivers had been already caring for their spouses for a median of 44.1 months and that by this time they had a mean DST score that was 3.30 points lower than demographically similar noncaregivers, effect size = .29. Despite the initially lower DST mean of almost 0.3 SD, over the next 2 years caregivers still showed further DST decline relative to noncaregivers, who did not decline. After 2 years, the mean for caregivers was 45.0 versus 49.5 for noncaregivers, effect size = .38.
HLM Results
Results for HLM Analyses of DST.—
We modeled change in DST for caregivers and noncaregivers. The mean initial DST score (,00) was 47.65 for the combined samples of caregivers and noncaregivers. This is presented in Model 0 of Table 2. This value is based on the regression model and may not be equal to the baseline DST mean. The mean linear slope (,10) was −0.19 (Model 0; Table 2). We then added caregiver status to predict β0 (i.e., the initial mean difference between caregivers and noncaregivers) and it was significant, ,01 = 3.65, df = 236, p = .01, suggesting that the mean T1 DST score for caregivers was 3.65 points lower than that for noncaregivers. When covariates were added, the mean T1 DST score for caregivers was still significantly greater than that for noncaregivers, ,01 = 2.29, df = 231, p = .047. These covariates represented additional risk factors for low baseline DST scores, namely, older age, low education level, male gender, obesity, and having a stroke history.
Table 2.
Fixed Effects in Level 2 Hierarchical Linear Modeling Equations
| Model 0a | Model 1b | Model 2ac | Model 2bd | |
| Intercept: Mean (,00) | 47.65***,e | 43.40*** | 43.19*** | 43.20*** |
| Slope: Mean (,10) | −0.19f | −0.81 | −0.55 | −0.44 |
| CG status (,11) | 0.64** | 0.34 | 0.27 | |
| T1 Ham D (,12) | −0.19*** | |||
| T2 Ham D (,12) | −0.20*** | |||
| Deviance statistic (−2 log likelihood, no. of estimated parameters = 4) | 4,683.48 | 4,571.24 | 4,568.04 | 4,568.88 |
Notes: CG = caregiver; Ham D = Hamilton depression.
Model 0: unconditional linear growth model, with no predictors at Level 2.
Model 1: CG status predicting digit symbol test decline.
Model 2a: CG status predicting digit symbol test decline, with Hamilton T1 as a mediator.
Model 2b: CG status predicting digit symbol test decline, with Hamilton T2 as a mediator.
For all models, covariates for intercept equation: age, education, gender, obesity, and stroke history.
Covariates for slope equation: age, gender, obesity, and stroke history.
*p < .10. **p < .05. ***p < .01.
Table 2 presents the equations used in the Level 2 models. Given our hypotheses, Models 1, 2a, and 2b focus on differences in caregiver–noncaregiver slopes rather than differences in intercepts. The row labeled “intercept” (,00) is the intercept of the Level 2 equation predicting intercepts in Level 1 equations, which are the estimated initial DST scores at study entry. The row labeled “slope” (,10) is the intercept of the Level 2 equation that predicts slopes in Level 1 equations. It is the average rate of decline in DST (,10) in the combined sample after controlling for other predictors in the equation. The average rate of decline in DST was significant when there were no other variables in the equation, but it was not significant with covariates in the model. This was expected from H1, which specified caregiver decline and not decline in noncaregivers.
In contrast to the result for the combined samples, Table 2 also shows that hypothesized predictors (caregiver status, depressed mood) were significant in predicting the variability of rate of decline across participants. In fact, individual slopes (β1, rate of DST decline) were influenced by caregiver status (H1), ,11 = 0.68, df = 236, p = .03 and the mean DST slope (,10) was −1.21, p = .02, suggesting that caregivers declined more than noncaregivers in DST (caregivers were coded 1, noncaregivers coded 2). On average, caregivers decreased by 0.53 DST points (i.e., −1.21 + 0.68 = −0.53) in each time interval (e.g., T1 to T2, T2 to T3) and −1.06 points by the end of T3. Noncaregivers increased on DST by 0.15 (i.e., −1.21 + 2 × 0.68 = 0.15) after 1 year and 0.30 points after 2 years. For empirical or theoretical reasons, we then predicted β0 and β1 after controlling for gender, age at T1, education, obesity, history of stroke (we did not include alcohol intake, the location of the T3 interview, and nursing home placement of one’s spouse at T3 because these were nonsignificant). After adding the covariates, caregiver status was still a significant predictor of DST decline (β1): ,11 = 0.64, df = 232, p = .048 (Model 1; Table 2). To compare the relative rate of decline in caregivers versus noncaregivers, we first took the difference in the slopes for caregivers versus noncaregivers, which was 1.36 (−1.06 vs. +0.30), and then took the ratio of these values. Hence, over 2 years, caregivers declined by a rate that was at least 4.5 times faster than noncaregivers or 1.36 versus 0.30 points (see Figure 2). This is notable when one considers that caregivers began with a mean DST score that was one third of a standard deviation below noncaregivers.
Figure 2.
Digit symbol decline in caregivers versus noncaregivers.
Mediation Results
Tests of Mediation.—
Because DST decline was related to caregiver status, we tested for mediation of this relationship by T1 depression (H2). When T1 depression was included in the model, it predicted DST decline, ,12 = −0.19, p = .007, and caregiver status became nonsignificant, decreasing from ,11 = 0.64 to ,11 = 0.34, p = .28 (Model 2a; Table 2). We then tested whether T2 depression was a mediator. Similar to T1 depression, it was significant (Model 2b). The fact that (a) caregiver status was significantly associated with decline before depression at T1 or T2 was in the model, (b) caregiver status became nonsignificant when depression scores were in the model, and (c) depression scores significantly predicted DST decline in the presence of caregiver status and covariates suggests that depression may mediate the relationship between caregiver status and DST decline. The result for T1 depression could be interpreted using its standard deviation (3.0). Individuals who were 1 SD above the depression mean had a 1.08 point (i.e., −0.18 × 2 × 3.0 = −1.08) faster decline each year or 2.16 points by T3 compared with individuals 1 SD below the mean. Depression was also a mediator because it was related to DST decline—21.5% of caregivers and only 7% of noncaregivers were 1 SD (3.00) above the grand mean (1.79) for T1 depression (i.e., scores of 5 or greater).
The log-likelihood deviance statistic showed decreased values for Models 2a and 2b, compared with Models 0 and 1, indicating better fit. Because sleep problems are associated with depression and are included as a symptom of depression in most measures, we also assessed whether they mediated the relationship between caregiver status and DST decline. Unlike depression, sleep problems at T1 or T2 were not a mediator; however, when they were included in the same equation as depression, they did decrease the ability of depression to mediate the relationship of caregiver status with DST decline.
Discussion
We observed that at study entry caregivers had lower mean DST scores than demographically similar noncaregivers. Over the next 2 years, caregivers still showed further DST decline relative to noncaregivers, who did not decline. Because caregivers and noncaregivers were similar on variables known to be associated with cognition or these variables were controlled (e.g., demographics, stroke, obesity), caregiver status may have influenced this decline. In accordance with our selection criteria, caregivers and noncaregivers differed on both the mental or functional impairment of their spouses and the hours of care they provided for their spouses. It is also possible that with the cognitive decline and communication deficits of the care recipient and with increasing social isolation, the caregiver's environment becomes less intellectually stimulating, contributing to and exacerbating further decline. These factors as well as the demands placed on caregivers and the loss of their spouses may contribute to depression, which in turn may mediate the relationship between caregiver status and DST decline.
Indeed, higher depressed mood in caregivers at T1 and T2 mediated the difference in DST decline between the two groups. As in other studies, caregivers reported more depression than noncaregivers—but this was primarily mild depression (Pinquart & Sorensen, 2003). This result is consistent with reviews showing that mild depression is more common in community sampled caregivers than is clinical depression, especially when caregivers were not actively seeking an intervention, as in the current study (Neundorfer, 1991; Wright, Clipp, & George, 1993). In fact, even at the levels of caregiver depression observed here, depression was variable enough to show relationships with processing speed decline in the absence of clinical depression. This is important because depressed mood is much more prevalent among caregivers than is clinical depression. Also, this decline was probably not just a function of fatigue because although caregivers also reported greater sleep problems than noncaregivers, these did not mediate the relationship between caregiver status and DST decline.
Caregiver cognitive decline has important implications. Cognitive decline may interfere with a caregiver's ability to provide care and may create an unsafe environment if judgment is impaired (Willis et al., 1992). If caregivers experience decline, they may be less capable of maintaining a care recipient at home rather than in a nursing home or alternate facility. Monitoring complex medication regimens for oneself and one's spouse is difficult (Park, Morrell, Frieske, & Kincaid, 1992), and caregivers typically oversee medication management, ensuring that correct drugs and dosages are taken, skills requiring cognitive ability (Katzman et al., 1983). Even without frank “clinical” impairment, older persons may experience difficulty performing complex activities of daily living, and they may have a greater risk for functional dependence as community-dwelling adults (Cahn-Weiner, Malloy, Boyle, Marran, & Salloway, 2000; Carlson et al., 1999; Grigsby, Kaye, Baxter, Shetterly, & Hamman, 1998) or as caregivers (Boucher, Renvall, & Jackson, 1996).
This research has limitations that if addressed could greatly improve its generalizability. First, we assessed only one cognitive measure. Future research should include measures of memory, concentration, and executive function. However, the DST is strongly related to overall cognitive impairment and is used as a screening measure (Lezak, 1995). More robust examination of the domains of cognitive function would elucidate its relationship to specific caregiving activities, such as financial management, medication management, safety awareness, and household maintenance. Second, we do not know if DST declines in caregivers are temporary or if they could be remedied by decreases in depression. Interventions could speak to this important issue. Third, by the end of this study only 20% of the caregivers scored in the borderline to moderately impaired DST range (Wechsler, 1997). This may be partially explained by the lack of clinical depression and high level of educational attainment of these caregivers. We would expect the full implications of cognitive decline to unfold over the years of the caregiver's trajectory. Fourth, caregivers have shared their lives with persons who have developed AD, including the same risk factors such as diet (Davis, Murphy, Neuhaus, Gee, & Quiroga, 2000) and alcohol consumption (Demers, Bisson, & Palluy, 1999), and they may be at higher risk for health and cognitive problems independent of caregiving. In fact, the shared environment of a couple may increase the risk of a second spouse having poor health if the first spouse has a disabling condition (Pinquart & Sorensen, 2003; Wilson, 2001, 2002). A final limitation of this study is that we did not assess factors that elicit chronic stress (e.g., patient problem behaviors) that have been shown to be important to adaptation (Gaugler et al., 2000). Future studies should specify the degree to which cognitive decline is influenced by stressors and precaregiving lifestyle. Both are important. Doubly prospective studies could examine persons before exposure to caregiving and before cognitive problems to assess the temporal importance of risk factors.
Despite these limitations, we believe this study has advantages. Because older adults are at risk for cognitive impairment (Jorm, Korten, & Henderson, 1987; Park, 1996) and illness may compromise cognitive function (Desmond, Tatemichi, Paik, & Stern, 1993; Elias et al., 1993; Haan, Shemanski, Jagust, Manolio, & Kuller, 1999; Strachan, Deary, Ewing, & Frier, 1997), we compared older adult caregivers with older adult noncaregivers. This allowed us to isolate the influence of caregiver status and depression on DST scores. Our design avoided the limitations of cross-sectional stress studies that are subject to age cohort differences and survivor effects and examined or controlled for variables known to be related to depression and cognition.
To our knowledge this is the first study to examine caregiver DST decline and its mediators. By assessing predictors of decline at all times, we were able to perform mediation tests that were not only statistical but also temporal. In caregivers, T1 depression and T2 depression predicted DST decline and also mediated the relationship of caregiver status and DST decline. We observed that relationships between caregiver status, depression, and DST decline were not influenced by demographic variables, illnesses, or health habits. The relationship between depression and poorer DST performance has implications for other cognitive domains because the influence of mild depression on immediate memory is also mediated by attention (Adams, Stanczak, Leutzinger, Waters, & Brown, 2001). Moreover, executive cognitive dysfunction, combined with depression, is associated with poorer overall functioning, controlling for comorbid conditions (Sanders, Lyness, Eberly, King, & Caine, 2006).
The current results support pathways among caregiver status, depression, and processing speed or attentional decline. They thus have implications not only for laboratory or clinical research but also for interventions and public policy. Future research should examine whether caregiver cognitive decline is related to everyday problem solving and the ability to care for oneself and one's care recipient. Because education and income are important to cognitive function and its maintenance, studies should focus on disadvantaged groups. The caregiving career is marked by many important transitional events and experiences, and caregiver responses are heterogeneous. The current findings form a basis for inclusion of cognitive status in caregiving research, with the need for future studies to explicate the complexity of the relationships among caregiver cognition and characteristics of the caregiving situation.
Promising work in the area of cognitive enhancement (Cassilhas et al., 2007; McDougall, 2002; Willis et al., 2006) could be an important inclusion in the armament of potential interventions to prevent cognitive decline and support caregivers as they enact their roles over the many years of the AD trajectory. Importantly, Mackenzie and colleagues (2007) observed that some caregiver deficits in attentional regulation may not be permanent and may be reversed after the caregivers are widowed and are no longer caregivers.
Caregivers make substantial contributions to long-term care (Arno et al., 1999), and any decrease in their ability to provide support has implications for the burden on the formal health care system at a time when demand is increasing. With advances in chronic disease management and medical technology, coupled with greater constraints on health care reimbursement, the role of family caregivers is becoming more complex and demanding, often including tasks and activities previously managed by paid health care providers (Schulz & Martire, 2004; Vitaliano, et al., 2007).
Funding
This research was supported by National Institute of Mental Health grant R01MH57663, Clinical Nutrition Research Unit grant DK38516, and National Institutes of Health Clinical Research Center grant M01-RR000.
References
- Adams RA, Stanczak DE, Leutzinger MR, Waters MD, Brown T. The impact of psychological disturbances on immediate memory. Archives of Clinical Neuropsychology. 2001;16:605–618. [PubMed] [Google Scholar]
- Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans: Results from the AHEAD sample. Research on Aging. 2007;29:73–94. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneshensel CS, Pearlin LI, Mullan JT, Zarit SH, Whitlatch CJ. San Diego, CA: Academic Press; 1995. Profiles in caregiving: The unexpected career. [Google Scholar]
- Arno PS, Levine C, Memmott MM. The economic value of informal caregiving. Health Affairs. 1999;18(2):182–188. doi: 10.1377/hlthaff.18.2.182. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Baune BT, Suslow T, Arolt V, Berger K. The relationship between psychological dimensions of depressive symptoms and cognitive functioning in the elderly: The MEMO Study. Journal of Psychiatric Research. 2007;41:247–254. doi: 10.1016/j.jpsychires.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Boucher L, Renvall MJ, Jackson JE. Cognitively impaired spouses as primary caregivers for demented elderly people. Journal of the American Geriatric Society. 1996;44:828–831. doi: 10.1111/j.1532-5415.1996.tb03742.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Does stress damage the brain? Biological Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Malloy PF, Boyle PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. Clinical Neuropsychologist. 2000;14:187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1999;54:S262–S270. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, et al. The impact of resistance exercise on the cognitive function of the elderly. Medicine and Science in Sports and Exercise. 2007;39:1401–1407. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- Caswell LW, Vitaliano PP, Croyle KL, Scanlan JM, Zhang J, Daruwala A. Negative associations of chronic stress and cognitive performance in older adult spouse caregivers. Experimental Aging Research. 2003;29:303–318. doi: 10.1080/03610730303721. [DOI] [PubMed] [Google Scholar]
- Chepenik LG, Cornew LA, Farah MJ. The influence of sad mood on cognition. Emotion. 2007;7:802–811. doi: 10.1037/1528-3542.7.4.802. [DOI] [PubMed] [Google Scholar]
- Covinsky KE, Newcomer R, Fox P, Wood J, Sands L, Dane K, et al. Patient and caregiver characteristics associated with depression in caregivers of patients with dementia. Journal of General Internal Medicine. 2003;18:1006–1014. doi: 10.1111/j.1525-1497.2003.30103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Murphy SP, Neuhaus JM, Gee L, Quiroga SS. Living arrangements affect dietary quality for U.S. adults aged 50 years and older: NHANES III 1988–1994. Journal of Nutrition. 2000;130:2256–2264. doi: 10.1093/jn/130.9.2256. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Derijk R. Signaling pathways in brain involved in predisposition and pathogeneiss of stress-related disease: Genetic and kinetic factors affecting the MR/GR balance. Annals of the New York Academy of Sciences. 2004;1032:14–34. doi: 10.1196/annals.1314.003. [DOI] [PubMed] [Google Scholar]
- Demers A, Bisson J, Palluy J. Wives' convergence with their husbands’ alcohol use: Social conditions as mediators. Journal of Studies on Alcohol. 1999;60:368–377. doi: 10.15288/jsa.1999.60.368. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Tatemichi TK, Paik M, Stern Y. Risk factors for cerebrovascular disease as correlates of cognitive function in a stroke-free cohort. Archives of Neurology. 1993;50:162–166. doi: 10.1001/archneur.1993.00540020040015. [DOI] [PubMed] [Google Scholar]
- de Vugt ME, Jolles J, van Osch L, Stevens F, Aalten P, Lousberg R, et al. Cognitive functioning in spousal caregivers of dementia patients: Findings from the prospective MAASBED study. Age and Ageing. 2006;35:160–166. doi: 10.1093/ageing/afj044. [DOI] [PubMed] [Google Scholar]
- Dikmen SS, Heaton RK, Grant I, Temkin NR. Test-retest reliability and practice effects of expanded Halstead-Reitan Neuropsychological Test Battery. Journal of the International Neuropsychological Society. 1999;5:346–356. [PubMed] [Google Scholar]
- Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: The Framingham Study. American Journal of Epidemiology. 1993;138:353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience and Biobehavioral Reviews. 2003;27:233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Ewing JA. Detecting alcoholism. The CAGE questionnaire. Journal of the American Medical Association. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Shurgot GR, Rider K, Gray HL, McKibbin CL, Kraemer HC, et al. Ethnicity, stress, and cortisol function in Hispanic and non-Hispanic White women: A preliminary study of family dementia caregivers and noncaregivers. American Journal of Geriatric Psychiatry. 2006;14:334–342. doi: 10.1097/01.JGP.0000206485.73618.87. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Davey A, Pearlin LI, Zarit SH. Modeling caregiver adaptation over time: The longitudinal impact of behavior problems. Psychology and Aging. 2000;15:437–450. doi: 10.1037//0882-7974.15.3.437. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Kaye K, Baxter J, Shetterly SM, Hamman RF. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. Journal of the American Geriatric Society. 1998;46:590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. Journal of the American Medical Association. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Haley WE, Levine E, Brown SL, Bartolucci A. Stress, appraisal, coping, and social supports as predictors of adaptational outcome among dementia caregivers. Psychology and Aging. 1987;2:323–330. doi: 10.1037//0882-7974.2.4.323. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurological and Neurosurgical Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanken MA. University of Washington; 1989. A study of physician performance in a physician oriented in-patient clinical record system. Unpublished doctoral dissertation. [Google Scholar]
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: A quantitative integration of the literature. Acta Psychiatrica Scandinavica. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. American Journal of Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kizilbash AH, Vanderploeg RD, Curtiss G. The effects of depression and anxiety on memory performance. Archives of Clinical Neuropsychology. 2002;17:57–67. [PubMed] [Google Scholar]
- Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, et al. Associations of salivary cortisol with cognitive function in the Baltimore Memory Study. Archives of General Psychiatry. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. International Psychogeriatrics. 2007;19:125–135. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- Lee S, Kawachi I, Grodstein F. Does caregiving stress affect cognitive function in older women? Journal of Nervous and Mental Disease. 2004;192:51–57. doi: 10.1097/01.nmd.0000106000.02232.30. [DOI] [PubMed] [Google Scholar]
- Levy A, Dachir S, Arbel I, Kadar T. Aging, stress, and cognitive function. Annals of the New York Academy of Sciences. 1994;717:79–88. doi: 10.1111/j.1749-6632.1994.tb12075.x. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. Journal of Neurosciences. 1994;14(5 Pt. 1):2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie CS, Smith MC, Hasher L, Leach L, Behl P. Cognitive functioning under stress: Evidence from informal caregivers of palliative patients. Journal of Palliative Medicine. 2007;10:749–758. doi: 10.1089/jpm.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ. Memory improvement in octogenarians. Applied Nursing Research. 2002;15:2–10. doi: 10.1053/apnr.2002.29518. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Naismith SL, Longley WA, Scott EM, Hickie IB. Disability in major depression related to self-rated and objectively-measured cognitive deficits: A preliminary study. BMC Psychiatry. 2007;7:32. doi: 10.1186/1471-244X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neundorfer MM. Coping and health outcomes in spouse caregivers of persons with dementia. Nursing Research. 1991;40:260–265. [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. Journal of Neuroscience. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC. In: Aging, health, and behavior: The interplay between basic and applied science. Resnick RJ, Rozensky RH, editors. Washington, DC: American Psychological Association; 1996. Health Psychology Through the Lifespan: Practice and Research Opportunities (pp. 59-75) [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidon NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Morrell RW, Frieske D, Kincaid D. Medication adherence behaviors in older adults: Effects of external cognitive supports. Psychology and Aging. 1992;7:252–256. doi: 10.1037//0882-7974.7.2.252. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. 2nd ed. Thousand Oaks, CA: Sage; 2002. Hierarchical linear models: Applications and data analysis methods. [Google Scholar]
- Roose SP, Devanand DP, Hamilton R. Cognitive impairment associated with depression in the elderly. Journal of Clinical Psychiatry. 2007;68:1601–1612. doi: 10.4088/jcp.v68n1020. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. Journal of Neuroscience. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sanders ML, Lyness JM, Eberly S, King DA, Caine ED. Cerebrovascular risk factors, executive dysfunction, and depression in older primary care patients. American Journal of Geriatric Psychiatry. 2006;14:145–152. doi: 10.1097/01.JGP.0000192482.27931.1e. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. Journal of the American Medical Association. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Schulz R, Martire LM. Family caregiving of persons with dementia: Prevalence, health effects, and support strategies. American Journal of Geriatric Psychiatry. 2004;12:240–249. [PubMed] [Google Scholar]
- Selye H. Stress and the reduction of distress. Journal of the South Carolina Medical Association. 1979;75:562–566. [PubMed] [Google Scholar]
- Solfrizzi V, D'Introno A, Colacicco AM, Capurso C, Gagliardi G, Santamato A, et al. Lifestyle-related factors, alcohol consumption, and mild cognitive impairment. Journal of the American Geriatric Society. 2007;55:1679–1681. doi: 10.1111/j.1532-5415.2007.01313.x. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Otey E, Alexopoulos GS, Butters MA, Cuthbert B, Ganguli M, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Archives of General Psychiatry. 2006;63:130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- Strachan MW, Deary IJ, Ewing FM, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- Taylor VH, MacQueen GM. Cognitive dysfunction associated with metabolic syndrome. Obesity Reviews. 2007;8:409–418. doi: 10.1111/j.1467-789X.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. New England Journal of Medicine. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Echeverria D, Yi J, Phillips P, Young HM, Siegler IC. Psychophysiological mediators of caregiver stress and differential cognitive decline. Psychology and Aging. 2005;20:402–411. doi: 10.1037/0882-7974.20.3.402. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Echeverria D, Shelkey M, Zhang J, Scanlan JM. A Cognitive Psychophysiological Model to Predict Functional Decline in Chronically Stressed Older Adults. Journal of Clinical Psychology in Medical Settings. 2007;14:177–190. [Google Scholar]
- Vitaliano PP, Scanlan JM, Moe K, Siegler IC, Prinz PN, Ochs HD. Stress, sleep problems, and immune function in persons with cancer histories. Cancer Research, Therapy, and Control. 1999;10:167–182. [Google Scholar]
- Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary disease. Psychosomatic Medicine. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one's physical health? A meta-analysis. Psychological Bulletin. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Medical Care. 1995;33(Suppl. 4):AS264–AS279. [PubMed] [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Whitlatch CJ, Feinberg LF, Sebesta DS. Depression and health in family caregivers: Adaptation over time. Journal of Aging and Health. 1997;9:222–243. doi: 10.1177/089826439700900205. [DOI] [PubMed] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Willis SL, Jay GM, Diehl M, Marsiske M. Longitudinal change and prediction of everyday task competence in the elderly. Research on Aging. 1992;14:68–91. doi: 10.1177/0164027592141004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Mann Koepke K, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E. Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. the ACTIVE Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE. Socioeconomic status and the prevalence of health problems among married couples in late midlife. American Journal of Public Health. 2001;91:131–135. doi: 10.2105/ajph.91.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE. The health capital of families: An investigation of the inter-spousal correlation in health status. Social Science and Medicine. 2002;55:1157–1172. doi: 10.1016/s0277-9536(01)00253-2. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Belle SH, Coon DW, Marcus SM, Ory MG, Burgio LD, et al. The Resources for Enhancing Alzheimer's Caregiver Health (REACH): Project design and baseline characteristics. Psychology and Aging. 2003;18:375–384. doi: 10.1037/0882-7974.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LK, Clipp EC, George LK. Health consequences of caregiver stress. Medicine, Exercise, Nutrition and Health. 1993;2:181–195. [Google Scholar]
- Zhu CW, Scarmeas N, Torgan R, Albert M, Brandt J, Blacker D, et al. Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology. 2006;67:998–1005. doi: 10.1212/01.wnl.0000230160.13272.1b. [DOI] [PubMed] [Google Scholar]