Abstract
Background
Left ventricular (LV) remodeling after myocardial infarction (MI) commonly causes infarct expansion (IE). This study sought to interrupt IE through microinjections of a biocompatible composite material into the post-MI myocardium.
Methods
MI was created in 21 pigs (coronary ligation). Radiopaque markers (2-mm diameter) were placed for IE (fluoroscopy). Pigs were randomized for microinjections (25 injections; 2- × 2-cm array; 200 µL/injection) at 7 days post-MI of a fibrin-alginate composite (Fib-Alg; fibrinogen, fibronectin, factor XIII, gelatin-grafted alginate, thrombin; n = 11) or saline (n = 10).
Results
At 7 days after injection (14 days post-MI), LV posterior wall thickness was higher in the Fib-Alg group than in the saline group (1.07 ± 0.11 vs 0.69 ± 0.07 cm, respectively, p = 0.002). At 28 days post-MI, the area within the markers (IE) increased from baseline (1 cm2) in the saline (1.71 ± 0.13 cm2, p = 0.010) and Fib-Alg groups (1.44 ± 0.23 cm2, p < 0.001). However, the change in IE at 21 and 28 days post-MI was reduced in the Fib-Alg group (p=0.043 and p=0.019). Total collagen content within the MI region was similar in the saline and Fib-Alg groups (12.8 ± 1.7 and 11.6 ± 1.5 µg/mg, respectively, p = NS). However, extractable collagen, indicative of solubility, was lower in the Fib-Alg group than the saline group (59.1 ± 3.5 vs 71.0 ± 6.1 µg/mL, p = 0.020).
Conclusions
Targeted myocardial microinjection of the biocomposite attenuated the post-MI decrease in LV wall thickness and infarct expansion. Thus, intraoperative microinjections of biocompatible material may provide a novel approach for interrupting post-MI LV remodeling.
Sequelae after a myocardial infarction (MI) include left ventricular (LV) dysfunction and remodeling of the LV in terms of progressive dilation as well as ultrastructural changes that culminate in the formation of a fibrotic scar [1– 6]. The infarct region is often either hypokinetic or dyskinetic, and the formation of the fibrotic scar alters myocardial material properties, such as stiffness [1, 2]. In addition, the presence of a “noncontractile” region results in heterogeneous alterations in stress and strain patterns, which can contribute to further thinning and expansion of the MI region [2]. More important, MI expansion is considered to be a key component in the progressive LV dilation that can occur after MI [1].
Strategies that limit infarct expansion through modification of the material properties of the MI region may provide a means to prevent or at least attenuate LV dilation. Several nonpharmacologic approaches for attenuation and or reversal of LV remodeling have been introduced, including passive restraint of the LV [3–5] and modification of MI scar constituents [7–9]. For example, embedding of a polymer mesh around the MI region [5] and implantation of an alginate scaffold seeded with macrophages reduced LV dilation after MI [7]. However, the effects of implantation of only a biocompatible composite on regional and global LV remodeling after MI remained unknown. Accordingly, the goal of this study was to determine the effects of modifying the MI scar through the injection of a biocompatible fibrin-alginate blended material on LV remodeling after MI.
Material and Methods
Induction of MI
Animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC, 1996) and the protocol was approved by the Institutional Animal Care and Use Committee. Permanent coronary ligation was performed in 21 mature Yorkshire pigs (25 kg, Hambone Farms, Orangeburg, SC) [10].
Briefly, after baseline echocardiographic measurements, the pigs were anesthetized and 4 stainless steel markers (1.6-mm diameter, VNUS Medical Systems, Sunnyvale, CA) were sutured onto the myocardium, centrally located between obtuse marginal 1(OM1) and OM2, 2 cm below the circumflex artery. The markers were placed in a quadrilateral array with an intermarker distance of 1 cm. Two additional markers, placed 1 cm apart, were sutured onto the thoracic wall as internal calibrators. MI was induced by ligation of OM1 and OM2 (4-0 Prolene, Ethicon, Somerville, NJ).
At 7 days after MI, LV echocardiographic measurements were repeated. Orthogonal fluoroscopic images of the radiopaque markers were recorded to determine the end-diastolic marker area, as previously described [2, 10]. The pigs were then assigned, in alternating fashion, to undergo myocardial injections with the fibrin-alginate composite (Fib-Alg group) or saline.
Injection of Biocomposite Material
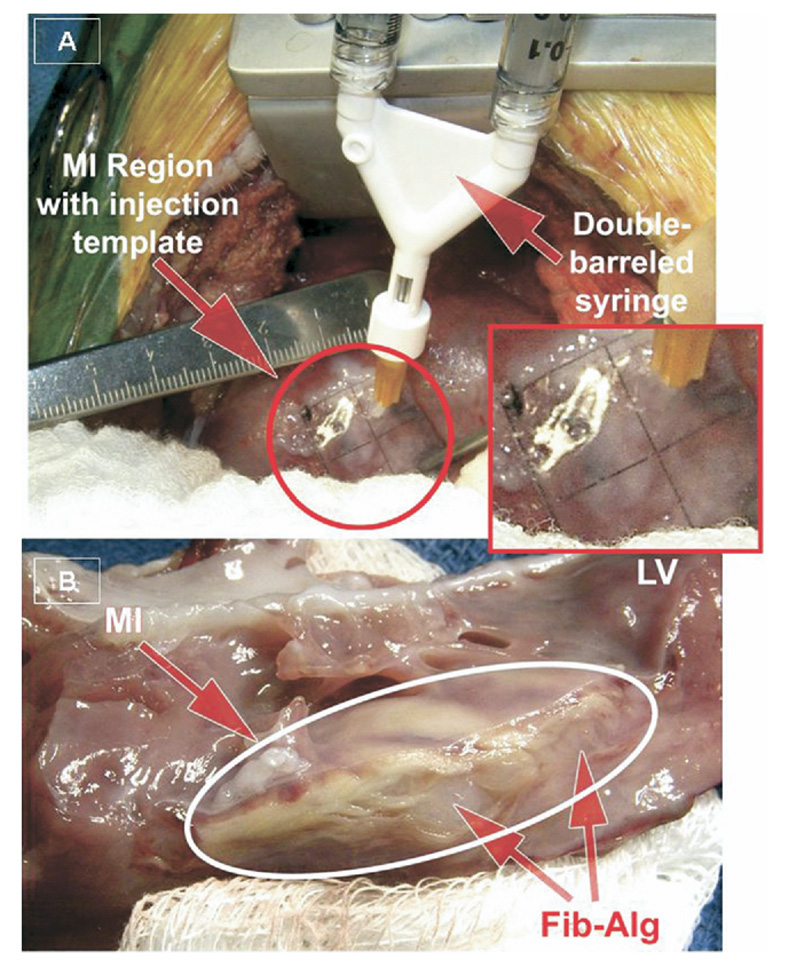
The initial thoracotomy site was reopened and the MI region visualized. The radiopaque markers were used a frame of reference to suture an injection guide of plastic laminate onto the MI region. The injection guide (2 × 2 cm, Fig 1A) was a grid with perforations at 0.5-cm intervals for a total of 25 predefined injection sites. The biocomposite injection comprised a dual component mixture of gels. The first component (8845-005) was a mixture of Sealer Protein Concentrate (Baxter Tisseel kit, a mixture of fibrinogen, fibronectin, factor XIII, plasminogen), dissolved in an aprotinin solution and to which gelatin-grafted alginate (8845-003) was added. The second component consisted of thrombin and 40mM calcium chloride solution (Baxter Tisseel). A double-barreled injection device (FibriJet, SA3670, Micromedics, MN; 26-gauge needle) was used for simultaneous injection of both materials (100 µL/ each) at an injection depth of 0.5 cm for each injection site. After injection of the fibrin-alginate composite into the myocardium, no retrograde flow of the viscous composite was noted through the injection site, suggesting that the fibrin-alginate composite polymerized rapidly and compartmentalized to the site of injection. Saline injections were performed using an identical syringe system and injection protocol.
Fig 1.
(A) Intraoperative injection of the fibrin-alginate (Fib-Alg) composite was performed using a 2- × 2-cm template with injection sites arrayed at 0.5-cm intervals within the region of myocardial infarction (MI). (B) At necropsy, the Fib-Alg could be visualized as amorphous densities within the MI region. (LV = left ventricle.)
Myocardial Function at 28 Days After MI
LV echocardiography and radiopaque marker measurements were repeated at days 14, 21 and 28 after MI [2]. After the final set of serial measurements, the pigs were instrumented for hemodynamic measurements of cardiac output and pressures from the LV, aorta, and pulmonary artery [10]. In conjunction with the echocardiographic determinations of LV geometry, the peak LV pressure was used to determine regional wall stress as previously described [10]. A sternotomy was performed and a vascular ligature placed around the inferior vena cava to perform transient caval occlusion. Piezoelectric crystals (2 mm, Sonometrics, London, Ontario, Canada) were placed within the area demarcated by the quadrilateral marker array (end-diastolic distance between crystals ranged from 8.9 to 10.1 mm). Hemodynamics and crystal array dimensions were recorded (1 kHz) to determine myocardial stiffness (rKm) of the MI region using the log transformation of the end-diastolic stress-strain relationship [σ = Ae(rKm∈) + B], where σis regional end-diastolic wall stress, ∈ is the regional strain, and A and B are constants [10, 11].
For the purposes of obtaining reference control values, 5 pigs matched for age and weight were identically instrumented for LV myocardial function measurements.
Myocardial Sampling, Histology, and Biochemistry
The heart was excised and a short-axis section of the LV (1-cm thick) was dissected through the central portion of the marker array to determine MI size by tetrazolium staining and measurement of the areas of the MI and non-MI regions by digital planimetry [12]. The fibrin-alginate composite could be visualized as amorphous densities within the MI region (Fig 1B). The remaining LV was divided into MI, border (2-cm peri- MI), and remote regions. Sections were flash frozen for biochemistry analysis or fixed in formalin for histology.
LV sections (5 µm) were stained with hematoxylin and eosin, picrosirius red (for collagen), or immunostained with the lectin Griffonia simplicifolia (GSA-B4, Sigma, St Louis, MO) for capillary density. The relative area of collagen staining and capillary density were determined using computer-assisted morphometry (Axioskop-2/AxioCam, Zeiss, Thornwood, NY) in a minimum of 5 random high power fields from each myocardial region of each pig.
LV myocardial samples (0.25 g) were lyophilized and underwent hydrochloric acid digestion for hydroxyproline measurements to determine total collagen content [13]. In parallel samples, the myocardium was homogenized and centrifuged and the supernatant subjected to biochemical measurement of soluble collagen using the picrosirius method [14]. Relative matrix metalloproteinase (MMP)-2 and MMP-9 levels were determined by substrate zymography [2].
Data Analysis
Data were collected in a blinded fashion and remained coded until the end of the study. Serial measurements of LV geometry and function and infarct expansion were compared using a repeated measures two-way analysis of variance (ANOVA) model. Single point measurements were compared between treatment and control groups using a one-way ANOVA. If the ANOVA revealed significant differences, post hoc pair-wise comparisons were performed using unpaired t tests corrected for number of comparisons. The change in infarct expansion from 7-day post-MI values was compared using a t test. For biochemical and morpho-metric measurements, comparisons with reference control values were performed using a two-way ANOVA in which the treatment effects were group and region. Linear regression analyses were performed to determine the relationship between MI size and LV end-diastolic volumes at 28 days after MI, and the slopes were compared between groups using ANOVA of regression coefficients [15]. All statistical analyses were performed using Stata software (StataCorp, College Station, TX). Results are presented as mean ± standard error of the mean. Values of p < 0.05 were considered to be statistically significant.
Results
Mortality and Final Sample Sizes
All 21 pigs survived initial instrumentation and MI induction. During the 7-day post-MI injection procedure, intraoperative refractory ventricular fibrillation developed in 2 of 11 pigs in the Fib-Alg group. Refractory ventricular tachycardia developed in an additional pig in the Fib-Alg group on post-MI day 14. Ventricular tachycardia developed in 1 pig in the saline group on post-MI day 28, but it but was successfully cardioverted. Thus, 9 pigs in the saline group and 8 pigs in the Fib-Alg group completed the 28-day post-MI protocol. Mortality rate after MI was not different between groups (χ2, p > 0.31).
Serial Measurements
Serial LV echocardiographic and infarct expansion measurements are presented in Table 1. At 14 days after MI, LV posterior wall thickness was higher in the Fib-Alg group than in the saline group. The increase in the area enclosed by the radiopaque markers (ie, infarct expansion) was blunted in the Fib-Alg group at 28 days after MI. As a change from 7-day post-MI values, infarct expansion was lower in the Fib-Alg group compared with the saline group.
Table 1.
Serial Changes in Echocardiographically Derived Variables and Infarct Expansion After Myocardial Infarction: Effects of Saline or Fibrin-Alginate Injection at 7 Days After Myocardial Infarction
| Time Postmyocardial Infarction (days) | p Values from Analysis of Variance | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | 7 | 14 | 21 | 28 | Within Group (Time) | Between Group | Interaction |
| Heart rate, beats/min | ||||||||
| Saline | 119 ± 6 | 117 ± 8 | 116 ± 4 | 118 ± 2 | 129 ± 24 | NS | … | … |
| Fibrin-alginate | 130 ± 9 | 123 ± 4 | 135 ± 6 | 123 ± 4 | 121 ± 7 | |||
| Posterior wall thickness at end-systole, cm | ||||||||
| Saline | 1.10 ± 0.05 | 0.73 ± 0.08a | 0.69 ± 0.07a | 0.73 ± 0.09a | 0.67 ± 0.08a | 0.004 | 0.001 | 0.228 |
| Fibrin-alginate | 1.13 ± 0.04 | 0.80 ± 0.06a | 1.07 ± 0.11bc | 0.90 ± 0.12 | 0.86 ± 0.11a | |||
| Septal wall thickness at end-systole, cm | ||||||||
| Saline | 1.10 ± 0.03 | 1.05 ± 0.03 | 1.09 ± 0.05 | 1.18 ± 0.05b | 1.23 ± 0.07ab | 0.001 | 0.625 | 0.914 |
| Fibrin-alginate | 1.04 ± 0.03 | 1.05 ± 0.04 | 1.10 ± 0.03 | 1.19 ± 0.04ab | 1.20 ± 0.04ab | |||
| End-diastolic volume, mL | ||||||||
| Saline | 47.5 ± 2.1 | 55.3 ± 1.9a | 59.4 ± 1.5a | 69.9 ± 2.2ab | 75.6 ± 3.2ab | <0.001 | 0.274 | 0.494 |
| Fibrin-alginate | 45.3 ± 1.0 | 55.5 ± 1.6a | 62.1 ± 2.7a | 64.9 ± 2.9ab | 71.7 ± 3.9ab | |||
| Ejection fraction (%) | ||||||||
| Saline | 67.0 ± 0.9 | 53.2 ± 1.1a | 49.8 ± 1.6a | 47.0 ± 1.9ab | 45.6 ± 3.2ab | <0.001 | 0.20 | 0.983 |
| Fibrin-alginate | 68.1 ± 1.0 | 54.7 ± 1.7a | 51.2 ± 1.2a | 48.2 ± 3.8ab | 49.0 ± 2.6a | |||
| LV mass/body weight, g/kg | ||||||||
| Saline | 4.4 ± 0.4 | 4.2 ± 0.3 | 3.8 ± 0.3 | 3.8 ± 0.3 | 3.5 ± 0.2 | NS | … | … |
| Fibrin-alginate | 3.7 ± 0.6 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.1 ± 0.4 | 3.4 ± 0.5 | |||
| End-diastolic marker area, infarct expansion, cm2 |
||||||||
| Saline | 1.00 ± … | 1.21 ± 0.05 | 1.11 ± 0.07 | 1.42 ± 0.08a | 1.71 ± 0.13ab | 0.018 | 0.322 | 0.320 |
| Fibrin-alginate | 1.00 ± … | 1.25 ± 0.10 | 1.22 ± 0.16 | 1.18 ± 0.17 | 1.44 ± 0.23a | |||
| Change in marker area from 7 days post-MI, mm2 |
||||||||
| Saline | … | 0 | −9.2 ± 9.0 | 21.4 ± 9.0b | 50.6 ± 14.0b | 0.072 | 0.018 | 0.056 |
| Fibrin-alginate | … | 0 | −2.5 13.0 | −22.4 ± 18.0* | 0.5 23.0c | |||
p < 0.05 vs baseline.
p < 0.05 vs 7 days post-MI.
p < 0.05 vs. saline.
Values presented as mean ±standard error of the mean.
Saline: n = 9; Fibrin-alginate: n = 8.
LV = left ventricular; MI = myocardial infarction; NS = not significant.
Day-28 Post-MI Studies
During the placement of the sonomicrometry crystals, refractory ventricular fibrillation developed in 2 pigs in the Fib-Alg group and 1 pig in the saline group; thus, hemodynamics were recorded for 8 pigs in the saline group and 6 pigs in the Fib-Alg group (Table 2). The cardiac index was reduced from reference controls in the saline group but was similar to control values in the Fib-Alg group. MI size was 23.2% ± 1.4% in the saline group and 28.4% ± 2.1% Fib-Alg groups, and did not differ between the MI groups (p = 0.12). However, the slope of the relationship between LV end-diastolic volume and MI size was lower in the Fib-Alg group than in the saline group (p = 0.037, Fig 2).
Table 2.
Hemodynamic Variables at 28 Days After Myocardial Infarction: Effects of Saline or Fibrin-Alginate Injection at 7 Days After Myocardial Infarction
| Variable | Control | Saline | Fibrin-Alginate | p Valuea |
|---|---|---|---|---|
| LV peak pressure, mm Hg | 113 ± 2 | 110 ± 2 | 107 ± 2 | NS |
| LV end-diastolic pressure, mm Hg | 10 ± 1 | 10 ± 1 | 11 ± 1 | NS |
| Peak dP/dt, mm Hg/s | 1372 ± 121 | 1668 ± 212 | 1814 ± 161 | NS |
| Mean aortic pressure, mm Hg | 94 ± 1 | 93 ± 3 | 88 ± 4 | NS |
| Mean PA pressure, mm Hg | 22 ± 2 | 21 ± 1 | 26 ± 4 | NS |
| Cardiac index, mL/min/kg | 109 ± 4 | 87 ± 4b | 98 ± 5 | 0.007 |
| Wall stress in remote region, g/cm2 | 306.4 ± 17.2 | 306.4 ± 29.7 | 257.7 ± 22.9 | NS |
| Wall stress in MI region, g/cm2 | 277.8 ± 22.9 | 487.7 ± 54.6b | 431.7 ± 30.0b | 0.016 |
| Regional MI stiffness constant | 3.71 ± 0.57 | 10.38 ± 1.85b | 13.73 ± 2.08b | 0.006 |
| Sample size | 5 | 8 | 6 |
Calculated by analysis of variance.
p < 0.05 vs control.
Values presented as mean ± standard error of the mean.
LV = left ventricular; MI = myocardial infarction; NS = not significant; PA = pulmonary artery.
Fig 2.
There were significant relationships between myocardial infarction (MI) size and left ventricular end-diastolic volume at 28 days after MI in the saline (circles; y = 3.06x + 40.74; r2 = 0.50, p = 0.03) and fibrin-alginate (Fib-Alg; squares) groups (y = 1.62x + 41.69; r2 = 0.44, p = 0.04).
Regional radial wall stress remote from the MI region was similar to control values in the MI groups (Table 2). Within the MI region, regional wall stress was higher than control values in both MI groups. Regional myocardial stiffness was increased in both post-MI groups compared with control values. The difference in regional myocardial stiffness between the saline and Fib-Alg groups was not statistically significant (p = 0.19).
LV Histology and Biochemistry
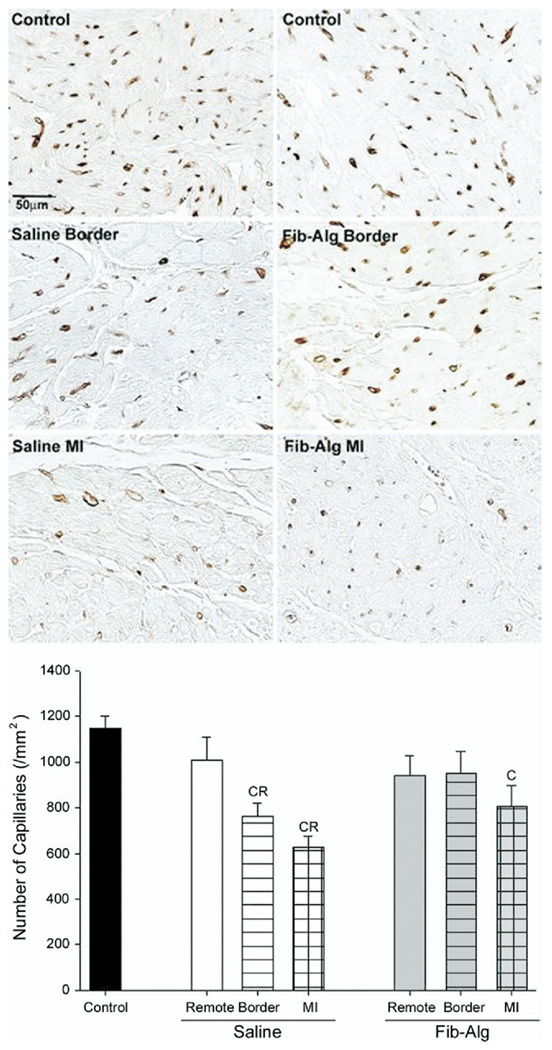
Sections of the MI region stained with hematoxylin and eosin showed increased cellularity and remnants of the fibrin-alginate injectate (Fig 3). Higher power images revealed the presence of giant cells in sections from the Fib-Alg group. Capillary density (Fig 4) within the MI region was reduced in both post-MI groups compared with reference controls. In the saline group, capillary density was reduced within the border and MI regions compared with the remote region. In contrast, capillary density was similar to controls within the border region and not different from the remote region in the Fib-Alg group. Compared with the saline group, morphometrically determined collagen content (Table 3) was increased in the border region and reduced within the MI region of the Fib-Alg group. Hydroxyproline content increased in a region-dependent manner from the remote to MI regions, with no significant differences between MI groups (Table 3). However, soluble collagen content within the MI region of the Fib-Alg group was lower than in the saline group.
Fig 3.
Representative low-power (top panels) and high-power (bottom panels) photomicrographs of hematoxylin and eosin–stained left ventricular sections at 28 days after either saline or fibrin-alginate (Fib-Alg) injection. Increased cellularity, which was focally distributed around amorphous densities, likely that of injected composite material (arrows with green borders), could be readily appreciated in sections from the Fib-Alg group. The edges of the amorphous bodies corresponding to the sites of the fibrin-alginate injections were well defined, suggesting that localized diffusion was minimal. High-power photomicrographs were imaged from the areas represented by the insets. In the Fib-Alg group, the presence of giant cells (arrows with red borders) was noted. Scale bars are 100 µm.
Fig 4.
(A) Representative photomicrographs of immunostaining for lectin-positive capillaries from control left ventricular (top panels) and from the border and myocardial infarction (MI) regions (middle and bottom panels, respectively) after either saline or fibrin-alginate (Fib-Alg) injection within the MI region. Scale bar for all photomicrographs is 50 µm. (B). Summary of capillary density measurements. Compared with respective remote region values, the number of capillaries in the MI region was lower in the saline group, but remained similar in the Fib-Alg group. (C = p < 0.05 vs reference controls; R = p < 0.05 vs treatment-matched remote region). Data are presented as the mean and standard error of the mean.
Table 3.
Collagen Content in Left Ventricular Regions at 28 Days After Myocardial Infarction: Effects of Saline or Fibrin-Alginate Injection at 7 Days After Myocardial Infarction
| Saline | Fibrin- Alginate |
Within Group (Region) |
Between Group | Interaction | |
|---|---|---|---|---|---|
| Fibrillar collagen content (morphometry, %) | |||||
| Remote | 8.3 ± 1.0 | 8.6 ± 1.1 | <0.001 | <0.001 | 0.017 |
| Border | 17.4 ± 1.6a | 25.0 ± 3.6ab | |||
| MI | 64.2 ± 2.6ac | 56.5 ± 4.0abc | |||
| Hydroxyproline (µg/mg of dry tissue, weight) | |||||
| Remote | 3.5 ± 0.4 | 3.3 ± 0.3 | <0.001 | 0.485 | 0.915 |
| Border | 5.9 ± 1.5 | 5.1 ± 0.7 | |||
| MI | 12.8 ± 1.7ac | 11.6 ± 1.5ac | |||
| Soluble collagen (µg/mL) | |||||
| Remote | 48.4 ± 2.9 | 45.4 ± 2.7 | <0.001 | 0.020 | 0.237 |
| Border | 52.2 ± 2.8 | 53.5 ± 4.0 | |||
| MI | 71.0 ± 6.1ac | 59.1 ± 3.5ab | |||
| Sample size (n) | 9 | 8 |
p < 0.05 vs remote.
p < 0.05 vs saline.
p< 0.05 vs border.
Values presented as mean ± standard error of the mean.
MI = myocardial infarction.
Compared with controls, MMP-9 and MMP-2 levels were increased within the MI region in both groups (Fig 5). MMP-2 levels within the MI region, particularly that of active MMP-2 (64 kDa), were lower in the Fib-Alg group than in the saline group.
Fig 5.
Zymographic bands for matrix metalloproteinase (MMP)-9 and MMP-2 were detected in all myocardial samples for the myocardial infarct (MI), border, and remote regions at 92 kDa (MMP-9) and at 64 to 72 kDa, consistent with that for the latent and active forms of MMP-2. (Top) MMP-9 levels were increased from reference control values in the border and MI regions of both MI groups. (Bottom) Within the MI region, levels of activated MMP-2 were lower in the fibrin-alginate (Fib-Alg) group than the saline group. (C = p < 0.05 vs reference controls; R = p < 0.05 vs treatment-matched remote region; B = p < 0.05 vs treatment-matched border region; *p < 0.05 vs same region of the saline group). Data are presented as the mean and standard error of the mean.
Comment
It is estimated that more than 1 million patients annually within the United States incur LV injury due to MI. Although new pharmacologic or interventional therapies have improved the outcome of an acute MI [1, 16], long-term sequelae remain a significant clinical problem. Specifically, LV remodeling after MI leads to thinning and expansion of the infarcted region [1, 2]. The goal of the present study was to determine the effects of MI scar modification through the intramyocardial injection of a biocompatible fibrin-alginate blended material on biocomposite material on infarct expansion.
This study had two main findings. First, targeted myocardial microinjection of a biocomposite reduced infarct expansion. Second, the amount of soluble collagen within the MI region of pigs injected with the fibrin-alginate biocomposite was reduced. Thus, the fibrin-alginate injection was associated with a reduction in the amount of collagen vulnerable to degradation and was a likely mechanism for the attenuation infarct expansion.
Infarct Expansion, Restraint, and Tissue Engineering
A number of cellular and extracellular events occur after MI and contribute to short- and long-term changes in LV geometry and function [1, 2, 4–7, 14]. Early after MI, myocyte loss occurs concomitantly with an infiltration of inflammatory cell types and thinning of the MI region. Interference of the early post-MI healing response is associated with adverse LV remodeling, evidenced by an increased incidence of LV rupture [17]. Accordingly, intramyocardial injections of fibrin-alginate were performed at 7 days after MI to avoid the confounding influences surrounding the acute phase of an MI. An important finding was that the similar MI sizes in the saline and Fib-Alg groups suggest that the initial response to MI was complete and that injection of the fibrin-alginate composite did not exacerbate adverse post-MI remodeling.
Infarct expansion, as measured as a function of marker area, occurred in both MI groups, and the magnitude of the change in infarct expansion during the first 7 days after MI was consistent with past reports [2]. After the intramyocardial injection of either saline or the fibrin-alginate biocomposite, infarct expansion appeared to plateau between days 7 and 14 after MI in the saline group and between days 7 and 21 in the Fib-Alg group. Although these findings remain speculative, they suggest that acute changes in the volume/thickness of the MI region could potentially have altered regional stress-strain patterns and provided a transient attenuation of infarct expansion. Nevertheless, the lower value for the slope of the relationship between MI size and end-diastolic volume in the Fib-Alg group indicates that for a given MI size, the end-diastolic volume was lower, suggesting that the attenuation of infarct expansion in the Fib-Alg group might have revealed significant differences in post-MI LV dilation if the pigs had been studied for longer post-MI durations.
Strategies to prevent adverse LV remodeling after MI through a physical intervention were demonstrated through restraint of the post-MI myocardium [4, 5]. For example, Kelley and colleagues [5] demonstrated that suturing a polypropylene mesh around an anteroapical MI modulated wall stress patterns and attenuated infarct expansion. A polyester mesh deployed around both ventricles reduced post-MI LV dilation and provided beneficial effects in terms of LV pump performance [3, 4]. However, potential limitations to implementing epicardial restraints include the requirement for surgical implantation, and the long-term effects remain unclear.
Tissue engineering approaches have been designed to repair lost or damaged tissue through the use of cellular transplantation and biomaterial scaffolds [7, 8, 18–23]. Li and colleagues [22] demonstrated that fetal myocytes that were seeded in vitro into a biodegradable gelatin mesh remained viable after the engineered construct was implanted onto the surface of a myocardial scar [22] In a rat MI model, Leor and colleagues [7] reported that implantation of an alginate scaffold containing seeded myocytes preserved LV pump function after MI [7]. However, the effects of implantation of the scaffold alone were not examined. Therefore, the present study builds on these past reports by demonstrating that post-MI intramyocardial implantation of the fibrin-alginate complex, without seeded cells or growth factors, influenced changes in LV geometry, pump function, and infarct expansion.
Composite Injection and the Matrix
Material properties of the myocardium are determined by various factors, including the content, composition, and structure of the extracellular matrix (ECM) [24]. For example, studies have demonstrated an association between myocardial collagen content and myocardial stiffness properties [25, 26]. In the present study, total collagen was increased in the border and MI regions, consistent with a post-MI fibrotic response [1, 2]. However, the relatively lower levels of soluble collagen content within the MI region in the Fib-Alg group suggests that the collagen was less vulnerable to degradation, which would favor infarct stiffening and tethering of the border region. Indeed, relative myocardial stiffness appeared higher in the Fib-Alg group, and fibrillar collagen content was increased within the border region in the Fib-Alg group. Thus, the potentially increased inflammatory response observed in the Fib-Alg group did not increase collagen degradation, but rather facilitated maturation of collagen within the MI and border regions. However, this issue remains speculative and studies examining the effects of fibrin-alginate on the confluence and continuity of the collagen matrix in the setting of MI are warranted.
The MMPs are enzymes that contribute to proteolysis and remodeling of the ECM, and a causal role for several MMP types in LV remodeling after MI has been described [2, 6, 10, 17, 27]. The levels of MMP-2 and MMP-9, which degrade a number of ECM components, are increased after MI [2, 6, 17]. Consistent with past findings, zymographic levels of both MMP-2 and MMP-9 were increased within the MI region of both the saline and Fib-Alg groups [2, 6, 14]. However, active MMP-2 levels were lower in the Fib-Alg group compared with the saline group, suggesting that a net reduction in proteolytic activity in the Fib-Alg group likely contributed to the reduction in soluble collagen content. Nevertheless, it must be recognized that ECM composition is determined through the interactions of a number of complex systems, including the balance between ECM deposition (synthesis) and degradation mediated by the stoichiometric balance between the MMPs and the endogenous tissue inhibitors of the metalloproteinases (TIMPs) [27]. Whether and to what degree fibrin-alginate injection after MI may have altered levels of the TIMPs remains unclear. Therefore, future studies to examine wider portfolio of MMPs and TIMPs in the context of post-MI fibrin-alginate injection would be appropriate.
Future Directions and Conclusion
The present study demonstrated that intramyocardial implantation of a fibrin-alginate biocompatible blend after MI attenuated infarct expansion. Nevertheless, several unexplored issues remain that would be appropriate for future studies. First, we used a single blend of the fibrin-alginate complex, which comprised a number of components, including alginate, factor XIII, aprotinin, and thrombin; therefore, the contributory role for each of these individual constituents in the attenuation of infarct expansion could not be determined. Moreover, whether and to what degree inclusion of components of the coagulation cascade is necessary to effect attenuation of infarct expansion remain to be ascertained. For example, thrombin, if leaked within the LV, could lead to the formation of intraventricular thrombi. Thus, future studies would be required to characterize—and optimize—the effects of varying the blend composition on infarct expansion.
Second, whether the implanted biocomposite matrix served as scaffolding for endogenous cell types was not examined. A future study that characterizes the cell types within the biocomposite matrix would be appropriate.
Third, the introduction of foreign materials into actively remodeling myocardium could form an arrhythmogenic substrate. Although not statistically significant, post-MI mortality was numerically higher in the Fib-Alg group; therefore, examination of whether introduction of the fibrin-alginate composite altered post-MI electrical activation patterns is warranted.
Finally, this study examined the effects of just the biocomposite on LV remodeling after MI. Whether impregnation of the biocomposite with growth factors, TIMPs, or different cell types provide additive/synergistic effects on infarct expansion remain to be determined.
The present proof-of-concept study demonstrated that fibrin-alginate injection modified the acceleration of infarct expansion by altering the collagen composition within the MI and border regions. Thus, intraoperative microinjections of a biocompatible material may provide a novel approach for interrupting post-MI LV remodeling.
Acknowledgments
This study was supported in part by National Heart, Lung, and Blood Institute Grants HL-45024, HL-97012, PO1–48788, a VA Merit Award (FGS), and a grant from Abbott Vascular.
Footnotes
Presented at the Poster Session of the Forty-fourth Annual Meeting of The Society of Thoracic Surgeons, Fort Lauderdale, FL, Jan 28–30, 2008.
Drs Basu, Spinale, and Michal and Mr Sheehy disclose that they have a financial relationship with Abbott Vascular.
References
- 1.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee R, Brinsa TA, Dowdy KB, et al. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003;107:618–625. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 3.Blom AS, Pilla JJ, Gorman RC, 3rd, et al. Infarct size reduction and attenuation of global left ventricular remodeling with the CorCap cardiac support device following acute myocardial infarction in sheep. Heart Fail Rev. 2005;10:125–139. doi: 10.1007/s10741-005-4640-2. [DOI] [PubMed] [Google Scholar]
- 4.Blom AS, Mukherjee R, Pilla JJ, et al. Cardiac support device modifies left ventricular geometry and myocardial structure after myocardial infarction. Circulation. 2005;112:1274–1283. doi: 10.1161/CIRCULATIONAHA.104.499202. [DOI] [PubMed] [Google Scholar]
- 5.Kelley ST, Malekan R, Gorman JH, 3rd, et al. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation. 1999;99:135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- 6.Wilson EM, Moainie SL, Baskin JM, et al. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 7.Leor J, Rozen L, Zuloff-Shani A, et al. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006;114:I94–I100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- 8.Li RK, Weisel RD, Mickle DA, et al. Autologous porcine heart cell transplantation improved heart function after a myocardial infarction. J Thorac Cardiovasc Surg. 2000;119:62–68. doi: 10.1016/s0022-5223(00)70218-2. [DOI] [PubMed] [Google Scholar]
- 9.Yau TM, Li G, Weisel RD, et al. Vascular endothelial growth factor transgene expression in cell-transplanted hearts. J Thorac Cardiovasc Surg. 2004;127:1180–1187. doi: 10.1016/j.jtcvs.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 10.Yarbrough WM, Mukherjee R, Brinsa TA, et al. Matrix metalloproteinase inhibition modifies left ventricular remodeling after myocardial infarction in pigs. J Thorac Cardiovasc Surg. 2003;125:602–610. doi: 10.1067/mtc.2003.197. [DOI] [PubMed] [Google Scholar]
- 11.Mirsky I, Pasipoularides A. Clinical assessment of diastolic function. Prog Cardiovasc Dis. 1990;32:291–318. doi: 10.1016/0033-0620(90)90018-w. [DOI] [PubMed] [Google Scholar]
- 12.Yarbrough WM, Mukherjee R, Escobar GP, et al. Pharmacologic inhibition of intracellular caspases after myocardial infarction attenuates left ventricular remodeling: a potentially novel pathway. J Thorac Cardiovasc Surg. 2003;126:1892–1899. doi: 10.1016/j.jtcvs.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Baicu CF, Stroud JD, Livesay VA, et al. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284:H122–H132. doi: 10.1152/ajpheart.00233.2002. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee R, Mingoia JT, Bruce JA, et al. Selective spatiotemporal induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 transcription after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291:H2216–H2228. doi: 10.1152/ajpheart.01343.2005. [DOI] [PubMed] [Google Scholar]
- 15.Steel R, Torrie J. principles and procedures of statistics: a biometrical approach. New York, NY: McGraw Hill; 1980. Regression analysis; pp. 258–261. [Google Scholar]
- 16.McGovern PG, Jacobs DR, Jr, Shahar E, et al. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: the Minnesota heart survey. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Ducharme A, Frantz S, Aikawa M, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou CH, Cheng WT, Kuo TF, et al. Fibrin glue mixed with gelatin/hyaluronic acid/chondroitin-6-sulfate tri-copolymer for articular cartilage tissue engineering: the results of real-time polymerase chain reaction. J Biomed Mater Res A. 2007;82:757–767. doi: 10.1002/jbm.a.31186. [DOI] [PubMed] [Google Scholar]
- 19.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Leor J, Aboulafia-Etzion S, Dar A, et al. Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation. 2000;102:III56–III61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 21.Leor J, Barbash IM. Cell transplantation and genetic engineering: new approaches to cardiac pathology. Expert Opin Biol Ther. 2003;3:1023–1039. doi: 10.1517/14712598.3.7.1023. [DOI] [PubMed] [Google Scholar]
- 22.Li RK, Jia ZQ, Weisel RD, et al. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100:II63–II69. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- 23.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 25.Carroll EP, Janicki JS, Pick R, Weber KT. Myocardial stiffness and reparative fibrosis following coronary embolisation in the rat. Cardiovasc Res. 1989;23:655–661. doi: 10.1093/cvr/23.8.655. [DOI] [PubMed] [Google Scholar]
- 26.Weber KT, Janicki JS, Shroff SG, et al. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988;62:757–765. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 27.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]