Abstract
Background
Estimates of chronic kidney disease (CKD) in the United States, using the continuous National Health and Nutrition Examination Survey (NHANES) dataset 1999–2004, indicate that 13.1% of the population (26.3 million people based on the 2000 census) has CKD stages 1–4.
Study Design
We performed sensitivity analyses to highlight assumptions underlying these estimates and to illustrate their robustness to varying assumptions.
Setting & Participants
NHANES 1999–2004 was a nationally representative cross-sectional continuous survey of the civilian, non-institutionalized US population. Our sample included participants aged ≥ 20 years.
Reference Test
Estimated glomerular filtration rate (GFR) < 60 mL/min/1.73m2 defined from the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation; albuminuria defined as persistence of urinary albumin-to-creatinine ratio > 30 mg/g.
Index Tests
[EF1]. We compared the prevalence estimates using the MDRD Study equation with 2 other GFR estimating equations (equation #5 by Rule and colleagues from the Mayo Clinic Donors study; Cockcroft-Gault equation adjusted for body surface area and corrected for the bias in the MDRD Study sample), and sex-specific cut points to define albuminuria.
Results
We found CKD stages 1–4 prevalence estimates ranging from 11.7% to 24.9%, a more than 2-fold difference, resulting in population estimates between 25.8 million and 54.0 million people using 2006 population estimates. Considering only stages 3 and 4, which are not affected by the choice of cut points to define albuminuria, prevalence estimates ranged from 6.3% to 18.6%, resulting in population estimates of 13.7 million to 40.3 million people, a nearly 3-fold difference.
Limitations
NHANES 1999–2004 is a cross-sectional survey, and allows for GFR and albumin-creatinine ratio estimates at one point in time. NHANES does not account for seniors in long-term care facilities.
Conclusions
While CKD prevalence is high regardless of varying modeling assumptions, different assumptions yield large differences in prevalence estimates.
Keywords: Index key words: Chronic kidney disease, GFR estimating equations, NHANES, prevalence estimates
Chronic kidney disease (CKD) is receiving increased attention in the United States and internationally due to efforts by the National Kidney Foundation (NKF; Kidney Disease Outcomes and Quality Initiative [KDOQI], Kidney Early Evaluation Program [KEEP]), and the National Institute of Diabetes, Digestive, and Kidney Diseases (National Kidney Disease Education Program [NKDEP]). Recent US CKD prevalence estimates, using the continuous National Health and Nutrition Examination Survey (NHANES) dataset from 1999–2004, indicate that 13.1% of the civilian, non-institutionalized population has CKD stage 1–4, corresponding to 26.3 million people based on the 2000 census estimates.1
In 2002, the NKF recommended a 5-stage CKD classification system, published as K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification.2 The system is based on evidence of reduced glomerular filtration rate (GFR) and evidence of kidney damage. To estimate national CKD prevalence, GFR has been estimated by various versions of the Modification of Diet in Renal Disease (MDRD) Study equation.3 The MDRD Study equation is useful for estimating GFR because of its relative ease of calculation based on serum creatinine, age, sex, and race.2 It is clinically useful as a measure of GFR up to 60 mL/min/1.73m2 because it was developed in a population with known CKD (GFR in mL/min/1.73m2 may be converted to mL/s/1.73m2 by multiplying by 0.01667).2 The equation was updated in 2007 for use with serum creatinine measurements standardized to an assay traceable to isotope dilution mass spectroscopy (IDMS).4
Kidney damage is determined by “pathologic abnormalities or markers of damage, including abnormalities in blood or urine tests or imaging studies.”2 To assess kidney damage, proteinuria is assessed using an untimed (spot) urine specimen by calculating the urinary albumin-creatinine ratio (ACR), measured in mg/g.2;5 Non-sex-specific cut points classifying micro- and macroalbuminuria based on ACR are recommended by the American Diabetes Association.6 However, as creatinine excretion is higher in normal men than normal women, sex-specific cut points have also been proposed.7;8
NHANES has historically provided a rich source of data for estimating national CKD prevalence, as its estimates are generalizable to the US non-institutionalized civilian population. Past estimates of CKD prevalence based on different definitions, different GFR estimating equations, and different NHANES datasets have varied (Table 1).1;9–12
Table 1.
Estimates of CKD Prevalence in the United States.
| Study | Dataset | Prevalence Estimate |
Note | |
|---|---|---|---|---|
| Percent | No. of People, Millions | |||
| Jones, 19989 | NHANES III | 9.7 men; 1.8 women | 10, aged > 12 years | CKD defined as SCr ≥ 1.5 mg/dL |
| Coresh, 200110 | NHANES III | 3.0 | 5.6, adults | CKD defined as SCr ≥ 1.6 mg/dL, men; ≥ 1.4 mg/dL, women |
| Coresh, 200311 | NHANES III | 10.8 | 19.2 | Stages 1–4, 2000 MDRD Study equation, sex-specific ACR cut points, albuminuria persistence estimates, KDOQI CKD classification. |
| Coresh, 200512 | NHANES 1999–2000 | 9.4, non-sex-specific ACR cut points; 11.7, sex-specific ACR cut points. | 18.3 | Stages 1–4, 2000 MDRD Study equation, albuminuria persistence estimates, KDOQI CKD classification. |
| Coresh, 20071 | NHANES 1999–2004 | 13.07 | 26.3 | Stage 1–4, 2007 MDRD Study equation, non-sex-specific ACR cut points, KDOQI CKD classification. |
Note: Serum creatinine in mg/dL may be converted to μmol/L by multiplying by 88.4.
Abbreviations: ACR, albumin-creatinine ratio; CKD: chronic kidney disease; KDOQI, Kidney Disease Outcomes Quality Initiative; MDRD, Modification of Diet in Renal Disease; NHANES, National Health and Nutrition Examination Survey; SCr, serum creatinine.
The current analysis aims to highlight the assumptions underlying CKD prevalence estimates using NHANES data, and to illustrate how sensitive these estimates are to varying assumptions. We address 3 GFR estimating equations: the 4-variable MDRD Study equation re-expressed for use with standardized serum creatinine values4; equation #5 put forth by Rule and colleagues in 2004, developed from the Mayo Clinic donor study13; and the Cockcroft-Gault equation adjusted for body surface area and corrected for the bias in the MDRD Study sample.14 The Mayo equation was developed in a patient population with known CKD (n = 320) and a population of healthy individuals being considered for kidney donation (n = 580). Additionally, we assess use of sex-specific cut points for ACR compared with non-sex-specific cut points.
Methods
Prevalence of CKD stages 1–4 was assessed using data from the continuous NHANES 1999–2004. From 1999 to 2004, the National Center for Health Statistics (NCHS) used NHANES to continually monitor the health of the US population. NHANES uses a complex multistage probability sampling design to assess the health of a nationally representative sample of the civilian, non-institutionalized US population. NCHS releases data from NHANES in 2-year intervals. This analysis used data releases from 1999–2000, 2001–2002, and 2003–2004. The NHANES 1999–2004 procedures have been well detailed.15
NHANES 1999–2004 included an in-home interview and an examination in a mobile examination center (MEC). Some participants were sampled to participate only in the in-home interview, and others to participate in both the in-home interview and the MEC examination. NCHS provides separate weights to account for the complex multi-stage sampling scheme of both the in-home interviewed and the MEC-examined participants to facilitate generalization to the survey’s target population, the civilian, non-institutionalized US population. Our analyses were limited to NHANES participants who were sampled to participate in both the in-home and the MEC portions of the survey. The MEC-examined sample size for NHANES 1999–2004 was 29,402. We further limited the analysis to participants aged ≥ 20 years at the time of examination, for a final sample size of 14,213.
Prevalence of various CKD stages was assessed using a combination of measures of albuminuria and eGFR in accordance with the KDOQI Guidelines.2 Albuminuria was assessed using ACR and is expressed in mg/g. Both sex-specific and non-sex-specific cut points were explored to define levels of albuminuria. The non-sex-specific ACR cut points define microalbuminuria as 30–299 mg/g and macroalbuminuria as ≥ 300 mg/g.6 The sex-specific cut points define microalbuminuria in men as ACR 17–250 mg/g and macroalbuminuria as > 250 mg/g, and microalbuminuria in women as ACR 25–355 mg/g, and macroalbuminuria as > 355 mg/g.7
Estimated GFR was calculated using 3 previously published equations. The first was the MDRD Study equation re-expressed for use with IDMS-traceable serum creatinine values4: eGFR(ml/min/1.73m2 =175.0(SCr)−1.154×age−0.203 ×0.742[if female]×1.212[if African American]
SCr is standardized serum creatinine in mg/dL and age is expressed in years. Serum creatinine values were standardized to IDMS-traceable values following NKDEP and NHANES recommendations.16;17
The second was the Mayo equation, proposed by Rule et al13:
Because the Mayo equation was developed using serum creatinine measurements not standardized to an IDMS-traceable gold standard, NHANES serum creatinine values were first standardized to the Mayo Clinic laboratory using a previously published conversion formula (SCrMayo=[(SCrNHANES/0.9948)+0.1967].18 If the serum creatinine was less than 0.8 mg/dL after standardization to the Mayo Clinic laboratory, 0.8 (71) was the value used, according to recommendations by Rule (serum creatinine in mg/dL may be converted to μmol/L by multiplying by 88.4).
The third equation was the Cockcroft-Gault equation adjusted for body surface area and corrected for the bias in the MDRD Study sample14:
BSA, body surface area, is estimated using the formula by Mosteller.19 The original Cockcroft-Gault equation estimates creatinine clearance, not GFR; however, the correction re-expresses the equation to estimate GFR.14
Patients who indicated on the kidney and urology questionnaire that they received dialysis in the past 12 months, or said during the oral health questionnaire that they had been told by a doctor that they have kidney disease requiring renal dialysis (n = 46, 0.3%) were assumed to be in stage 5 CKD. Patients who indicated receiving dialysis were included in the eGFR estimation using their measured serum creatinine value. To assess any differences introduced by the handling of these possible dialysis patients, they were also assumed to have eGFR < 15 mL/min/1.73 m2 regardless of their measured serum creatinine.
The KDOQI workgroup recommends that patients who test positive for albuminuria undergo repeat testing within 3 months to confirm its presence.2 NHANES 1999–2004 participants have urinary albumin and creatinine measured only once; therefore, patients with persistently elevated ACR cannot be identified with certainty. Data from NHANES III, in which a subset of patients had repeat measurements of urinary albumin and creatinine, were analyzed by Coresh and colleagues.11 They found persistent microalbuminuria in 53.9% of patients with eGFR ≥ 90 mL/min/1.73m2, and in 72.7% of patients with eGFR between 60 and 89 mL/min/1.73m2, using sex-specific ACR cut points. Using non-sex-specific ACR cut points, they found persistent microalbuminuria in 50.9% of patients with eGFR ≥ 90 mL/min/1.73m2, and in 75.0% of patients with eGFR between 60 and 89 mL/min/1.37m2. All (100%) patients with macroalbuminuria are assumed to have persistent albuminuria upon repeat measurement. These estimates of persistence were used in the estimation of prevalence to classify CKD stage (Table 2).
Table 2.
CKD Classification Algorithms
| CKD Stage | Description | eGFR (mL/min/1.73 m2) | Prevalence Estimation* |
|---|---|---|---|
| 1 | Kidney damage, normal or higher GFR | ≥ 90 | P[macroalbuminuria | eGFR≥90]*P[eGFR≥90] + (0.539)* P[microalbuminuria | eGFR≥90]* P[eGFR≥90] |
| 2 | Kidney damage, mild GFR decrease | 60–89 | P[macroalbuminuria | eGFR 60–89]*P[eGFR 60–89] + (0.727)* P[microalbuminuria | eGFR 60–89]* P[eGFR 60–89] |
| 3 | Moderate GFR decrease | 30–59 | P[eGFR 30–59] |
| 4 | Severe GFR decrease | 15–29 | P[eGFR 15–29] |
| 5 | Kidney failure (ESRD) | < 15 (or dialysis) | P[eGFR < 15 or dialysis] |
Note: eGFR in mL/min/1.73 m2 may be converted to mL/s/1.73 m2 by multiplying by 0.01667.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; GFR, glomerular filtration rate.
P[x | y] can be interpreted as the probability of x given y. Constants for estimating persistence of microalbuminuria assume sex-specific ACR cut points; when using the non-sex-specific cut points, replace 0.539 with 0.509 and 0.727 with 0.750.
All analyses were conducted using SAS v9.1.3 (SAS Institute, Cary, NC). Sampling weights were incorporated into all analyses to obtain unbiased estimates of population percentages accounting for the complex sampling design of the continuous NHANES surveys using SUDAAN (SAS callable version 9.0.1, RTI Inc., Cary, NC). Prevalence estimates were calculated on the NHANES 1999–2004 population as a whole using combined sampling weights following the procedures recommended by NHANES.20 Standard errors were calculated using the Taylor series (linearization) method.
In the primary analysis, patients with missing eGFR or ACR were included when possible. For example, patients with eGFR < 60 mL/min/1.73 m2 were classified in the corresponding CKD stage category regardless of whether ACR was known. Patients with albuminuria and unknown eGFR were classified conservatively as stage 1 CKD. As a sensitivity analysis, additional models were developed to assess the effect of including or excluding pregnant women from the GFR estimation and pregnant and menstruating women from the ACR calculation, and the effect of including or excluding patients with missing eGFR or ACR. In total, 6 models were developed according to various criteria (Table 3). Comparisons were also made using the sex-specific and non-sex-specific ACR cut points. Results for the primary model are presented here. Results for other models are presented in figures S1 and S2 (provided as online supplementary material available with this article at www.ajkd.org).
Table 3.
Models Used to Estimate CKD Prevalence.
| Include or Exclude Pregnant or Menstruating Women in Estimations |
|||
|---|---|---|---|
| Missing eGFR or ACR | Include in eGFR and ACR | Include pregnant women in eGFR; exclude pregnant or menstruating women from ACR | Exclude pregnant women from eGFR; exclude pregnant or menstruating women from ACR |
| Excluded | Model 1 | Model 2 | Model 3 |
| Included when possible | Model 4 | Model 5 (primary model) | Model 6 |
Note: Each model used each of the 3 GFR estimating equations and sex-specific and non-sex-specific ACR cut points.
Abbreviations: ACR, albumin-creatinine ratio; eGFR, estimated glomerular filtration rate.
Results
Age, sex, and race data were present for all 14,213 participants included in the analysis. Serum creatinine values were missing for 939 (6.6%), and urinary albumin or creatinine values for 424 (3.0%) participants. Thus, GFR was estimated using the MDRD Study equation or the Mayo equation for 13,274 (93.4%) participants. Body surface area values were missing for 502 (3.5%) participants. Thus, GFR was estimated using the Cockcroft-Gault equation for 12,895 (90.7%) participants.
Table 4 displays prevalence estimates of eGFR levels comparing the MDRD Study equation to the Mayo equation and the Cockcroft-Gault equation. Participants who indicated receiving dialysis were classified in 2 ways, by estimated GFR and by assuming a GFR less than 15 mL/min/1.73 m2. The Mayo equation estimated higher prevalence of eGFR values for CKD stages 4 and 5 and lower prevalence for stages 2 and 3. The Cockcroft-Gault equation estimated 2- and 3-fold higher prevalence of eGFR values for stages 2 and 3.
Table 4.
Prevalence of Estimated GFR Categories: MDRD Study vs. Mayo vs. Cockcroft-Gault Estimating Equations
| Estimating Equation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDRD Study* |
Mayo* |
Cockcroft-Gault* |
|||||||||
| eGFR | N | Prevalence % |
95% CI | N | Prevalence % |
95% CI | Prevalence Ratio*† |
N | Prevalence % |
95% CI | Prevalence Ratio*‡ |
| ≥ 90 | 5882 (5880) | 40.63 (40.61) | 38.74–42.55 (38.72–42.53) | 7226 (7224) | 59.05 (59.03) | 57.31–60.78 (57.29–60.75) | 1.45 (1.45) | 4120 (4119) | 31.82 (31.80) | 30.19–33.49 (30.17–33.47) | 0.78 (0.78) |
| 60–89 | 5954 (5952) | 51.17 (51.15) | 49.58–52.75 (49.56–52.73) | 4755 (4753) | 34.65 (34.63) | 33.06–36.28 (33.04–36.26) | 0.68 (0.68) | 5522 (5520) | 50.08 (50.05) | 48.61–51.54 (48.60–51.51) | 0.98 (0.98) |
| 30–59 | 1317 (1308) | 7.68 (7.64) | 7.03–8.39 (6.98–8.35) | 1119 (1111) | 5.57 (5.53) | 5.11–6.06 (5.07–6.02) | 0.72 (0.72) | 2942 (2935) | 16.77 (16.73) | 15.92–17.66 (15.88–17.62) | 2.17 (2.17) |
| 15–29 | 80 (77) | 0.35 (0.34) | 0.26–0.46 (0.25–0.45) | 123 (120) | 0.51 (0.50) | 0.40–0.66 (0.39–0.65) | 1.45 (1.47) | 264 (260) | 1.13 (1.12) | 0.94–1.35 (0.93–1.33) | 3.23 (3.33) |
| < 15 | 41 (66) | 0.18 (0.27) | 0.12–0.27 (0.20–0.36) | 51 (75) | 0.22 (0.31) | 0.16–0.30 (0.24–0.40) | 1.22 (1.15) | 47 (72) | 0.20 (0.30) | 0.14–0.29 (0.23–0.39) | 1.11 (1.11) |
Note: eGFR in mL/min/1.73 m2 may be converted to mL/s/1.73 m2 by multiplying by 0.01667.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
Non-parenthesized values were obtained using the calculated eGFR for individuals indicating dialysis use; parenthesized values were obtained by classifying individuals indicating dialysis use into the < 15 category regardless of eGFR.
Mayo:MDRD
Cockcroft-Gault:MDRD
Table 5 displays prevalence estimates of microalbuminuria and macroalbuminuria as estimated by ACR using sex-specific and the non-sex-specific cut points. Sex-specific cut points gave a microalbuminuria prevalence estimate of 12.2%, compared with 8.4% using non-sex-specific cut points. Macroalbuminuria prevalence estimates did not differ substantially. These estimates are independent of GFR estimation.
Table 5.
Prevalence of Albuminuria
| ACR Cut Points |
|||||||
|---|---|---|---|---|---|---|---|
| Sex-Specific* |
Non-Sex-Specific† |
||||||
| Albuminuria Classification | n | Prevalence, %‡ | 95% CI | n | Prevalence, %‡ | 95% CI | Prevalence Ratio§ |
| Normal | 10,469 | 86.33 | 85.55–87.07 | 11,067 | 90.18 | 89.52–90.80 | 0.96 |
| Microalbuminuria | 1987 | 12.22 | 11.53–12.93 | 1403 | 8.38 | 7.78–9.03 | 1.46 |
| Macroalbuminuria | 322 | 1.46 | 1.22–1.75 | 308 | 1.43 | 1.19–1.72 | 1.02 |
Abbreviations: ACR, albumin-creatinine ratio; CI, confidence interval
Normal, men ACR (mg/g) < 17, women < 25; microalbuminuria, men 17–250, women 25–355; macroalbuminuria, men > 250, women > 355.
Normal, ACR (mg/g) < 30; microalbuminuria, 30–299; macroalbuminuria, ≥ 300.
Prevalence based on single measurement of ACR.
Sex-specific:non-sex specific
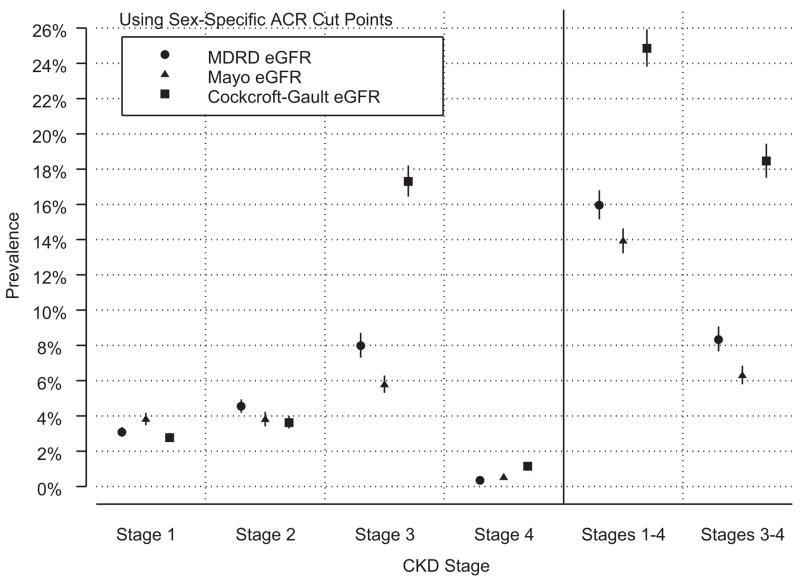
The KDOQI CKD classification guidelines use eGFR and albuminuria values to define CKD stages. Using sex-specific ACR cut points (Table 6), CKD stages 1–4 estimated prevalence is 15.97% by the MDRD Study equation, compared with 13.93% by the Mayo equation and 24.86% by the Cockcroft-Gault equation. In the 2006 estimated US population aged ≥ 20 years,21 the MDRD Study estimate is 34.7 million people compared with 30.3 million using the Mayo estimate and 54.0 million using the Cockcroft-Gault estimate. The estimated numbers for stages 3–4 are 18.1 million people using the MDRD Study equation, 13.7 million using the Mayo equation, and 40.1 million using the Cockcroft-Gault equation.
Table 6.
Prevalence of CKD Stages 1–4: Sex-Specific Albumin-Creatinine Ratio Cut Points: MDRD Study vs. Mayo vs. Cockcroft-Gault Estimating Equations
| Estimating Equation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDRD Study |
Mayo |
Cockcroft-Gault |
|||||||||
| CKD Stage |
Prevalence %* |
95% CI | Population n† |
Prevalence %* |
95% CI | Population n† |
Prevalence Ratio‡ |
Prevalence %* |
95% CI | Population n† |
Prevalence Ratio§ |
| 1 | 3.09 | 2.82–3.37 | 6,705,123 | 3.82 | 3.48–4.18 | 8,301,600 | 1.23 | 2.77 | 2.52–3.04 | 6,019,747 | 0.90 |
| 2 | 4.55 | 4.18–4.94 | 9,883,203 | 3.81 | 3.41–4.24 | 8,279,868 | 0.84 | 3.62 | 3.29–3.99 | 7,866,961 | 0.79 |
| 3 | 7.98 | 7.31–8.71 | 17,344,870 | 5.78 | 5.31–6.29 | 12,561,060 | 0.72 | 17.31 | 16.45–18.21 | 37,617,984 | 2.17 |
| 4 | 0.35 | 0.27–0.47 | 768,757 | 0.52 | 0.41–0.67 | 1,130,061 | 1.47 | 1.15 | 0.97–1.38 | 2,499,173 | 3.23 |
| 1–4 | 15.97 | 15.16–16.81 | 34,701,953 | 13.93 | 13.23–14.65 | 30,272,589 | 0.87 | 24.86 | 23.82–25.93 | 54,025,597 | 1.56 |
| 3–4 | 8.34 | 7.66–9.07 | 18,113,627 | 6.30 | 5.80–6.85 | 13,691,121 | 0.76 | 18.47 | 17.52–19.45 | 40,138,889 | 2.22 |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; MDRD, Modification of Diet in Renal Disease.
Prevalence estimates of stages 1 and 2 incorporate persistence estimates for microalbuminuria of 53.9% if estimated glomerular filtration rate ≥ 90 mL/min/1.73 m2 (≥ 1.50 mL/s/1.73 m2) and 72.7% if 60–89 mL/min/1.73 m2 (1.00–1.48 mL/s/1.73 m2).
Population n based on the estimated 2006 US population aged 20 years and older: 217,319,378.
Mayo:MDRD
Cockcroft-Gault:MDRD
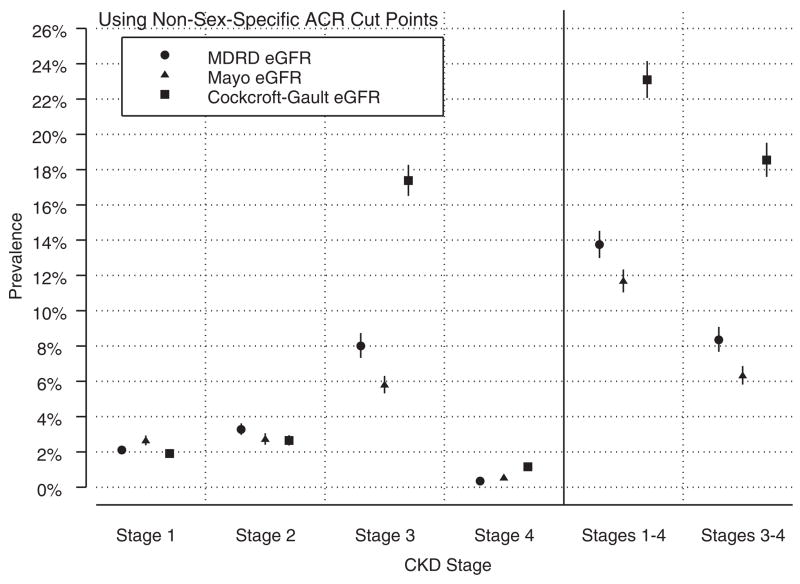
Using non-sex-specific ACR cut points to estimate CKD stages 1–4 prevalence (Table 7), values become 13.75% (29.9 million people) using the MDRD Study equation, 11.68% (25.8 million people) using the Mayo equation, and 23.10% (50.2 million people) using the Cockcroft-Gault equation. Stages 3–4 prevalence estimates did not change appreciably based on the ACR cut points used. Non-sex-specific ACR cut points reduce CKD stages 1–4 prevalence estimates by approximately 2 percentage points using each GFR estimating equation, a reduction of approximately 5 million people.
Table 7.
Prevalence of CKD Stages 1–4: Non-Sex-Specific Albumin-Creatinine Ratio Cut Points: MDRD Study vs. Mayo vs. Cockcroft-Gault Estimating Equations
| Estimating Equation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDRD Study |
Mayo |
Cockcroft-Gault |
|||||||||
| CKD Stage |
Prevalence %* |
95% CI | Population n† |
Prevalence %* |
95% CI | Population n† |
Prevalence Ratio‡ |
Prevalence %* |
95% CI | Population n† |
Prevalence Ratio§ |
| 1 | 2.12 | 1.93–2.32 | 4,607,171 | 2.64 | 2.38–2.93 | 5,737,232 | 1.25 | 1.91 | 1.70–2.13 | 4,150,800 | 0.90 |
| 2 | 3.28 | 2.97–3.62 | 7,128,076 | 2.72 | 2.41–3.06 | 5,991,087 | 0.83 | 2.65 | 2.37–2.95 | 5,758,964 | 0.81 |
| 3 | 8.00 | 7.33–8.74 | 17,385,550 | 5.80 | 5.32–6.31 | 12,604,524 | 0.73 | 17.39 | 16.52–18.28 | 37,791,840 | 2.17 |
| 4 | 0.35 | 0.27–0.47 | 760,618 | 0.53 | 0.41–0.68 | 1,151,793 | 1.51 | 1.16 | 0.97–1.38 | 2,520,905 | 3.33 |
| 1–4 | 13.75 | 12.99–14.54 | 29,881,414 | 11.68 | 11.04–12.34 | 25,832,903 | 0.85 | 23.10 | 22.07–24.16 | 50,200,776 | 1.67 |
| 3–4 | 8.36 | 7.68–9.09 | 18,167,900 | 6.32 | 5.82–6.87 | 13,734,585 | 0.76 | 18.55 | 17.60–19.53 | 40,312,745 | 2.22 |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; MDRD, Modification of Diet in Renal Disease.
Prevalence estimates of stage 1 and stage 2 incorporate persistence estimates for microalbuminuria of 50.9% if estimated glomerular filtration rate ≥ 90 mL/min/1.73 m2 (≥ 1.50 mL/s/1.73 m2) and 75.0% if 60–89 mL/min/1.73 m2 (1.00–1.48 mL/s/1.73 m2).
Population n based on the estimated 2006 US population aged 20 years and older: 217,319,378.
Mayo:MDRD
Cockcroft-Gault:MDRD
Regardless of ACR cut points, the 3 GFR estimating equations yield prevalence estimates within approximately one percentage point for stages 1, 2, and 4 (Figures 1 and 2), with their largest differences for stage 3. The Mayo equation estimates for stage 3 are 28% lower than the MDRD Study equation estimates, and the Cockcroft-Gault equation estimates are more than 2-fold higher than the MDRD Study equation estimates. These differences in the prevalence estimates for stage 3 CKD drive the large differences observed when considering stages 1–4 and stages 3–4 combined.
Figure 1.
Prevalence of chronic kidney disease (CKD) stages 1–4 using the Modification of Diet in Renal Disease (MDRD) Study, Mayo, and Cockcroft-Gault glomerular filtration rate (GFR) estimating equations and sex-specific albumin-creatinine ratio (ACR) cut points. Results were obtained from the primary model. Refer to Table 3 for modeling assumptions.
Figure 2.
Prevalence of chronic kidney disease (CKD) stages 1–4 using the Modification of Diet in Renal Disease (MDRD) Study, Mayo, and Cockcroft-Gault glomerular filtration rate (GFR) estimating equations and non-sex-specific albumin-creatinine ratio (ACR) cut points. Results were obtained from the primary model. Refer to Table 3 for modeling assumptions.
Including or excluding pregnant women, menstruating women, and individuals with unknown eGFR or ACR values affects prevalence estimates, but to a lesser extent than differences resulting from GFR estimating equations or ACR sex-specific cut points to define micro- and macroalbuminuria (data shown in figures S1 and S2).
Discussion
The results of this analysis demonstrate how varying assumptions can affect CKD prevalence estimates using continuous NHANES 1999–2004 data. At one end of the spectrum of assumptions, an estimated 25.8 million people aged > 20 years in the US have CKD stages 1–4. At the other end of the spectrum, an additional 28.2 million people, or 54.0 million total, have CKD.
The largest difference in estimated prevalence is due to differing GFR estimating equations. The MDRD Study equation is recommended in the KDOQI classification guidelines; it tends to underestimate GFR in the range above 60 mL/min/1.73 m2.13;22–24 The Mayo equation was developed in a population with known CKD and a population of healthy individuals (potential kidney donors).13 It appears to perform better in the healthy GFR range and is comparable to the MDRD Study equation in ranges consistent with CKD. In this analysis, the Mayo equation indeed yielded lower prevalence estimates for CKD stages 2 and 3, and an estimate only a slightly higher for stage 4. The Mayo equation yielded estimates 45% higher for GFR in the normal range (≥ 90 mL/min/1.73m2) than the MDRD Study equation. The higher prevalence of normal eGFR drives the slightly higher prevalence estimate of stage 1 CKD observed using the Mayo equation due to the albuminuria criterion used to define stage 1 CKD. The Cockcroft-Gault equation, adjusted for body surface area and corrected for the bias in the MDRD Study equation, yielded prevalence estimates for stages 3 and 4 CKD 2- and 3-fold higher than the estimates obtained using the MDRD Study equation.
Ibrahim and colleagues proposed an additional GFR estimating equation for use with Type 1 diabetic patients;25 however, it was not considered in the current analysis due to the small number of Type 1 diabetic patients in the NHANES population. Still other equations estimating creatinine clearance or GFR have been proposed by other investigators.26–33 We considered only 3 equations, and the equation used greatly influenced CKD prevalence estimates, particularly in the range of stage 3 CKD. In the absence of GFR measurement using a “gold-standard” technique such as iohexol clearance in a large random sample of the general population, further development and validation of GFR estimating equations can refine our estimates of CKD prevalence in the United States.
Bias due to serum creatinine standardization could have influenced our results. NHANES serum creatinine values are re-expressed for use with a standardized serum creatinine assay. While the Mayo equation was not developed on serum creatinine values standardized to IDMS-traceable reference standards, we used a previously published correction equation to transform the NHANES values back to the values that would have been obtained in the Mayo Clinic study. We also calculated prevalence estimates using the Mayo equation directly on the standardized NHANES serum creatinine values, and the prevalence of stages 1–4 CKD changed from 13.9% to 10.5 using sex-specific ACR cut points and from 11.7% to 8.2% using non-sex-specific ACR cut points, a decline of approximately 3 percentage points. These results highlight the importance of standardizing laboratory creatinine assays to National Institute of Standards and Technology Standard Reference Materials as recommended by the NKDEP.16;34
Using sex-specific cut points to determine albuminuria based on ACR also affects CKD prevalence estimates at stages 1 and 2. Sex-specific cut points give CKD stage 1 estimates approximately 50% higher than non-sex-specific cut points, and stage 2 estimates approximately 40% higher than non-sex-specific cut points, using each GFR estimating equation. KDOQI classification guidelines do not prescribe which cut points to use, but note, “creatinine excretion is higher in normal men compared with women; therefore, the values in the general population and cut-off values for abnormalities in urine albumin-to-creatinine ratio are lower for men than women” (p. S50).2 The cut points used have implications for population-based prevalence estimates.
Differences in inclusion criteria using NHANES data also affect prevalence estimates, although the effect is smaller than the effect of GFR estimating equation or ACR cut points. The most conservative estimates are obtained by excluding all participants with unknown eGFR or ACR from the calculations, and including pregnant or menstruating women in the ACR calculation. The highest prevalence estimates are obtained by including participants with unknown eGFR or ACR when possible and excluding pregnant or menstruating women from the ACR calculation. These modifications result in relatively minor differences in prevalence estimates, generally in the range of 10%. This 10%, however, translates to a population difference of approximately 2 to 3 million people.
The continuous NHANES 1999–2004 may be the best tool available to the research community for estimating national CKD prevalence, but it has limitations. It is a cross-sectional survey, and allows for GFR and ACR estimates at one point in time. The KDOQI CKD classification guidelines recommend consistent findings of CKD based on reduced GFR or signs of kidney damage over a period of 3 months. This analysis used estimates of albuminuria persistence based on previous analyses of the NHANES III data.11 These estimates may not be generalizable to the continuous NHANES dataset as NHANES III took place between 1988 and 1994 and the continuous NHANES from 1999 to 2004. Furthermore, the re-measurements used in a subset of the NHANES III population were taken within 2 weeks of the original estimates, not the 3-month period recommended by KDOQI. One could hypothesize that these persistence estimates are biased high, resulting in possible over-estimation of the population in stages 1 and 2. As a sensitivity analysis, we assumed the persistence estimates were 10% higher than measures obtained 3 months from the original measurement would be, and re-estimated stages 1–4 CKD prevalence using our primary model and sex-specific ACR cut points. This modification resulted in prevalence estimates of 15.3% (95% CI: 14.5%–16.1%) using the MDRD Study equation, 13.2% (95% CI: 12.6%–13.9%) using the Mayo equation, and 24.3% (95% CI: 23.3%–25.3%) using the Cockcroft-Gault equation. Comparing these with the original estimates of 15.97% using the MDRD Study equation, 13.93% using the Mayo equation, and 24.86% using the Cockcroft-Gault equation, the differences are relatively minor. Estimates of albuminuria persistence have no effect on the prevalence estimates of stage 3 and 4 CKD. Serum creatinine was also measured only once, so prevalence estimates do not incorporate any estimate of reduced GFR persistence; however, this would affect only patients with eGFR near 90 mL/min/1.73 m2 in estimating CKD prevalence in stages 1–4.
The complex, multistage, probability sampling design used in NHANES is meant to ensure generalizability to the US civilian, non-institutionalized population. The weights used to analyze the NHANES data also are designed to account for survey nonresponse. While the prevalence estimates obtained in this analysis are generalizable to the US civilian, non-institutionalized population, this analysis does not take into account the numerous cases of CKD that undoubtedly exist in the elderly population residing in long-term care facilities.
At a time when increasing attention is given to the public health burden of CKD in the US, estimating the prevalence of the disease in the population is especially important. This analysis is meant to highlight how varying methods of estimating the prevalence can have large effects on the resulting prevalence estimates. Qualitative assessments of how prevalence estimates are affected by various changes to the methodology are displayed in Table 8. The prevalence estimates published by Coresh and colleagues1 may be conservative, given the results of our analysis. Future refinements to GFR estimating equations may improve our ability to estimate the true prevalence of CKD in the US. Regardless of methodological nuances, CKD prevalence in the US is high. A conservative estimate may be 25.8 million people, with plausible estimates of more than 50 million people. Public health initiatives to identify individuals with signs of CKD, along with the availability of various treatments that have been shown to improve morbidity and mortality in this population, could have a large public health impact.
Table 8.
Qualitative Assessment of The Effect of Varying Prevalence Modeling Strategies.
| Factor | Qualitative Impact on Prevalence Estimates |
|---|---|
| GFR estimating equation: MDRD Study, Mayo, or Cockcroft-Gault | Large |
| Use of sex-specific or non-sex-specific ACR cut points to define albuminuria | Large, affecting only stages 1 and 2 |
| Including pregnant and menstruating women in the albuminuria calculation | Trivial |
| Excluding pregnant women from the GFR estimation | Trivial |
| Excluding or including persons with missing ACR or eGFR. | Small |
Abbreviations: ACR, albumin-creatinine ratio; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease
Supplementary Material
Figure S1: Prevalence of CKD stages 1–4 using the MDRD Study, Mayo, and Cockcroft-Gault GFR estimating equations, sex-specific ACR cut points, and 6 different modeling assumptions. Refer to Table 3 for modeling assumptions.
Figure S2: Prevalence of CKD stages 1–4 using the MDRD Study, Mayo, and Cockcroft-Gault GFR estimating equations, non-sex-specific ACR cut points, and 6 different modeling assumptions. Refer to Table 3 for modeling assumptions.
Note: The supplementary material accompanying this article (doi: _____) is available at www.ajkd.org.
Acknowledgments
The authors wish to thank Aaron R. Folsom, MD, MPH, David R. Jacobs, PhD, Richard H. Grimm, MD, PhD, David T. Gilbertson, PhD, and Eric D. Weinhandl, MS, for their guidance, and United States Renal Data System colleagues Beth Forrest, BBA, and Shane Nygaard, BA, for manuscript preparation, and Nan Booth, MSW, MPH, for manuscript editing. Support: This study was performed as a deliverable under Contract No. HHSN267200715002C (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland).
Footnotes
Financial Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39:S1–246. [PubMed] [Google Scholar]
- 3.Levey AS, Greene T, Kusek JW, Beck GL. A Simplified Equation to Predict Glomerular Filtration Rate from Serum Creatinine. J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 4.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 5.Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK) Am J Kidney Dis. 2003;42:617–622. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association: Standards of Medical Care in Diabetes. Diabetes Care. 2006;29 (Suppl 1):S4–S42. [PubMed] [Google Scholar]
- 7.Warram JH, Gearin G, Laffel L, et al. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs DR, Jr, Murtaugh MA, Steffes M, et al. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155:1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 9.Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 1998;32:992–999. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- 10.Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 11.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 13.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. [Accessed June 3, 2008];National Health and Nutrition Examination Survey: NHANES 1999–2000 Data Files. http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm.
- 16.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the laboratory working group of the national kidney disease education program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 17.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics and Centers for Disease Control and Prevention. [Accessed June 3, 2008];Analytic and reporting guidelines: the third National Health and Nutrition Examination Survey, NHANES III (1988–94) http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/analytical_guidelines.htm.
- 21.U.S. Census Bureau: Population Division. [Accessed June 3, 2008];Annual Estimates of the Population by Five-Year Age Groups and Sex for the United States: April 1, 2000 to July 1, 2006 (NC-EST2006-01) http://www.census.gov/popest/states/NST-ann-est.html.
- 22.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Poggio ED, Wang X, Greene T, et al. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 24.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim H, Mondress M, Tello A, et al. An alternative formula to the Cockcroft-Gault and the modification of diet in renal diseases formulas in predicting GFR in individuals with type 1 diabetes. J Am Soc Nephrol. 2005;16:1051–1060. doi: 10.1681/ASN.2004080692. [DOI] [PubMed] [Google Scholar]
- 26.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 27.Jelliffe RW. Letter: Creatinine clearance: bedside estimate. Ann Intern Med. 1973;79:604–605. doi: 10.7326/0003-4819-79-4-604. [DOI] [PubMed] [Google Scholar]
- 28.Mawer GE, Lucas SB, Knowles BR, et al. Computer-assisted prescribing of kanamycin for patients with renal insufficiency. Lancet. 1972;1:12–15. doi: 10.1016/s0140-6736(72)90005-0. [DOI] [PubMed] [Google Scholar]
- 29.Hull JH, Hak LJ, Koch GG, et al. Influence of range of renal function and liver disease on predictability of creatinine clearance. Clin Pharmacol Ther. 1981;29:516–521. doi: 10.1038/clpt.1981.72. [DOI] [PubMed] [Google Scholar]
- 30.Jelliffe RW. Estimation of creatinine clearance when urine cannot be collected. Lancet. 1971;1:975–976. doi: 10.1016/s0140-6736(71)91484-x. [DOI] [PubMed] [Google Scholar]
- 31.Gates GF. Creatinine clearance estimation from serum creatinine values: an analysis of three mathematical models of glomerular function. Am J Kidney Dis. 1985;5:199–205. doi: 10.1016/s0272-6386(85)80051-2. [DOI] [PubMed] [Google Scholar]
- 32.Bjornsson TD, Cocchetto DM, McGowan FX, et al. Nomogram for estimating creatinine clearance. Clin Pharmacokinet. 1983;8:365–369. doi: 10.2165/00003088-198308040-00007. [DOI] [PubMed] [Google Scholar]
- 33.Bjork J, Back SE, Sterner G, et al. Prediction of relative glomerular filtration rate in adults: new improved equations based on Swedish Caucasians and standardized plasma-creatinine assays. Scand J Clin Lab Invest. 2007;67:678–695. doi: 10.1080/00365510701326891. [DOI] [PubMed] [Google Scholar]
- 34.National Institute of Standards and Technology. [Accessed June 3, 2008];Technology Services Standard Reference Materials. http://ts.nist.gov/measurementservices/referencematerials/index.cfm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Prevalence of CKD stages 1–4 using the MDRD Study, Mayo, and Cockcroft-Gault GFR estimating equations, sex-specific ACR cut points, and 6 different modeling assumptions. Refer to Table 3 for modeling assumptions.
Figure S2: Prevalence of CKD stages 1–4 using the MDRD Study, Mayo, and Cockcroft-Gault GFR estimating equations, non-sex-specific ACR cut points, and 6 different modeling assumptions. Refer to Table 3 for modeling assumptions.
Note: The supplementary material accompanying this article (doi: _____) is available at www.ajkd.org.