Abstract
The metabolic syndrome (MetS) confers an increased risk for diabetes and cardiovascular disease. Although high-sensitive C-reactive protein (hsCRP) concentrations are higher and adiponectin concentrations lower in MetS, there is no reliable biochemical measure that can capture its various features. We evaluated whether hsCRP, adiponectin, or the ratio of adiponectin or its oligomers, especially the high-molecular-weight (HMW) oligomer, to hsCRP predict MetS in 123 subjects with MetS compared with that in 91 healthy control subjects. MetS subjects had significantly higher hsCRP levels and lower total adiponectin and oligomer levels relative to control subjects (P < .0001). The HMW/total adiponectin and adiponectin/CRP ratios were significantly lower in MetS subjects than control subjects (P < .005). The odds ratio (OR) of MetS using the 75th percentile cutoff for CRP was 3.8 (95% confidence interval [CI], 2.1–6.8) and equivalent to low total adiponectin (OR, 2.5; 95% CI, 1.3–4.5), its oligomers, or the adiponectin/hsCRP ratio (OR, 2.6; 95% CI, 1.5, 4.8). Thus, measurements of CRP, adiponectin, or its oligomers provide robust biomarkers for predicting MetS.
Keywords: C-reactive protein, Adiponectin, Biomarker, Metabolic syndrome
The metabolic syndrome (MetS) is a cluster of cardiovascular risk factors, which includes abdominal obesity, hypertension, hyperglycemia, dyslipidemia, and insulin resistance.1 In the United States, 1 in 4 people has MetS, and it is associated with an increased propensity for diabetes and cardiovascular disease.
Inflammation is pivotal to atherosclerosis. Recently, several lines of evidence implicate inflammation in the development of insulin resistance and MetS.2 Furthermore, abundant evidence has emerged demonstrating that high concentrations of high-sensitive C-reactive protein (hsCRP) are associated with MetS and may predict diabetes and cardiovascular events, independent of traditional risk factors. It has also been suggested that CRP may be included in the criteria for MetS.3 Concentrations of CRP predict increased cardiovascular events in MetS and diabetes.4–7 In addition, CRP concentrations correlate strongly with adiposity and insulin resistance.8–10
The adipocyte-derived hormone adiponectin is an important link among adiposity, type 2 diabetes, and cardiovascular disease.11 Circulating adiponectin concentrations are reduced in humans with obesity, type 2 diabetes, and coronary artery disease.12–14 Adiponectin-deficient mice exhibit diet-induced insulin resistance.15,16 In humans, low plasma adiponectin concentrations independently predict the development of type 2 diabetes and myocardial infarction.17,18 Hypoadiponectinemia is also associated with MetS.19 Furthermore, adiponectin concentrations have been found in a number of studies to be inversely associated with systemic inflammation, as evidenced by increased concentrations of hsCRP.20
In the circulation, adiponectin is found in 3 major forms: as trimers (low molecular weight [LMW]), as hexamers (medium molecular weight [MMW]), and as larger multimers of 12 to 18 subunits (high molecular weight [HMW]).21 HMW adiponectin, but not the MMW form, lowers blood glucose concentrations in adiponectin-deficient mice.11,21 Moreover, thiazolidinedione- and gastric bypass surgery–mediated improvements in insulin sensitivity are more closely associated with changes in HMW adiponectin than with total adiponectin concentrations.22,23 HMW adiponectin concentrations are also closely related to improvements in high-density lipoprotein cholesterol (HDL-C) following weight loss.23,24
Developing a robust biomarker that can predict MetS instead of examining individual features will be important from a population standpoint in screening, monitoring the natural history of the disease, and measuring the response to therapeutic interventions. However, the ability of HMW adiponectin to detect the presence of MetS or to predict individual MetS components has not been specifically assessed. Thus, while hsCRP concentrations are higher in MetS and adiponectin concentrations are lower in MetS and both seem to be highly relevant to the 2 major sequelae of MetS, diabetes and cardiovascular disease, existing studies have not examined the predictive power of these markers alone and/or in combination for MetS. In the present study, therefore, we tested whether CRP, adiponectin, or both HMW and LMW adiponectin/CRP ratios are valid and robust markers that predict MetS.
Materials and Methods
Subjects
All subjects were recruited following informed consent at the University of California Davis Medical Center, Sacramento. MetS was assessed in 214 adult subjects by modified criteria of the National Cholesterol Education Program Adult Treatment Panel III.25
Subjects had to have 3 or more of the following 5 features to be classified as having MetS: (1) Central obesity was measured by waist circumference, being greater than 40 inches in men and greater than 35 inches in women. (2) Elevated fasting triglyceride levels were categorized as greater than or equal to 150 mg/dL (1.7 mmol/L). (3) A low HDL-C level was defined as less than 40 mg/dL (1.0 mmol/L) in men and less than 50 mg/dL (1.3 mmol/L) in women. (4) High blood pressure was defined as a systolic blood pressure greater than 130 mm Hg and a diastolic blood pressure greater than 85 mm Hg. (5) An elevated fasting glucose level was defined as a concentration greater than or equal to 100 mg/dL (5.6 mmol/L). Exclusion criteria were as follows: smoking; use of lipid-lowering drugs or drugs known to effect lipid metabolism; diabetes and/or hypertension treated with drug therapy; aspirin therapy; anti-inflammatory drugs; liver, renal or uncompensated metabolic or hormonal disorders; infection; cancer; recent major surgery or illness; an hsCRP level of more than 10 mg/L; and/or an increased WBC count.
Anthropometric Measurements
Weight, waist circumference, height, and systolic and diastolic blood pressure were measured according to standard procedures by trained clinical research personnel. The body mass index (BMI) was calculated as weight (in kg) divided by height (in m2).
Biochemical Measurements
The lipid profile and glucose levels were measured in the clinical pathology laboratory using standard laboratory methods. Insulin was measured by radioimmunoassay (Linco, St Louis, MO; coefficient of variation [CV], <9%). HOMA-IR (homeostasis model assessment for insulin resistance), a marker of insulin sensitivity, was assessed as described previously.26 The hsCRP levels were measured using an automated immunoassay (Beckman Coulter LX PRO, Beckman Coulter, Fullerton, CA), which has an interassay and intra-assay CV of less than 7%.27 Adiponectin multimers were measured in duplicate by enzyme-linked immunosorbent assay (ALPCO Diagnostics, Salem, NH), a method recently validated against Western blot analysis.28 In addition to measuring total adiponectin, the enzyme-linked immunosorbent assay uses a protease treatment to digest the LMW and MMW adiponectin, enabling HMW adiponectin to be measured. MMW and LMW adiponectin are calculated by subtraction. In our laboratory, the interassay CVs for total and HMW adiponectin were 9.8% and 12.9%, respectively. The intra-assay CVs for each were less than 6.9% and less than 10%, respectively.
Statistical Procedures
Statistical procedures were performed by a general clinical research center biostatistician using SAS software, version 9.1 (SAS Institute, Cary, NC). All continuous variables were first assessed for normality using the Kolmogorov-Smirnov test. Comparisons between groups for continuous variables were performed using a t test or its nonparametric equivalent (Mann-Whitney or Wilcoxon rank sum test), as appropriate. Categorical variables were compared between groups by using the Fisher exact test. Relationships of total adiponectin, HMW, MMW plus LMW adiponectin, SA (HMW adiponectin/total adiponectin ratio), hsCRP, and the number of MetS components were assessed by 1-way analysis of variance (ANOVA) or the nonparametric Kruskal-Wallis test. Correlations between continuous variables were assessed by using the nonparametric Spearman rank. Linear regression was used to plot the relationships of CRP, total adiponectin, and HMW or MMW plus LMW adiponectin. In all cases, the level of significance was set at a P value of less than .05.
Multiple logistic regression was used to study the predictive value of total adiponectin, HMW, MMW plus LMW adiponectin, SA, and hsCRP. Receiver operating characteristic (ROC) curves were generated to evaluate the ability of the biochemical markers to discriminate subjects with and without MetS. The areas under the ROC curves were compared by using a nonparametric approach. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated from the estimates provided in the models.
Results
We studied 123 subjects with MetS and 91 healthy control subjects Table 1. As expected, the MetS group had significantly higher weight, BMI, waist circumference, glucose levels, blood pressure readings, and triglyceride levels and lower concentrations of HDL-C than the non-MetS group. In a subset of 172 subjects, fasting plasma insulin concentrations were also determined so that HOMA-IR, a measure of insulin resistance, could be calculated. As expected, plasma insulin concentrations and HOMA-IR were significantly higher in the MetS group than in the non-MetS group (P < .0001 for both).
Table 1.
Descriptive Statistics for Study Groups*
| Variable | Metabolic Syndrome (n = 123) | Control Subjects (n = 91) | P |
|---|---|---|---|
| Sex (M/F) | 47/76 | 36/55 | .888† |
| Age (y) | 51 ± 12 | 52 ± 12 | .779 |
| Waist circumference (cm) | 110 ± 16 | 87 ± 10 | <.0001‡ |
| Weight (kg) | 106 ± 25 | 87 ± 20 | <.0001 |
| BMI (kg/m2) | 36 ± 6 | 30 ± 6 | <.0001‡ |
| Systolic BP (mm Hg) | 131 ± 12 | 118 ± 10 | <.0001‡ |
| Diastolic BP (mm Hg) | 84 ± 8 | 77 ± 9 | <.0001‡ |
| Glucose level (mg/dL) | 105 ± 38 | 89 ± 12 | .0136‡ |
| Insulin level (μU/mL) | 27.1 ± 3.1 | 17.9 ± 1.2 | <.0001§ |
| HOMA-IR | 7.69 ± 1.20 | 4.09 ± 0.31 | <.0001§ |
| Total cholesterol level (mg/dL) | 228 ± 47 | 228 ± 42 | .99 |
| HDL cholesterol level (mg/dL) | 39 ± 9 | 51 ± 13 | <.0001‡ |
| LDL cholesterol level (mg/dL) | 147 ± 37 | 151 ± 37 | .50 |
| Triglyceride level (mg/dL) | 194 ± 12 | 122 ± 7 | <.0001‡ |
BMI, body mass index; BP, blood pressure; HOMA-IR, homeostasis model assessment for insulin resistance.
Data are given as mean ± SD. Laboratory values are given in conventional units; conversions to Système International units as follows: cholesterol, HDL, and LDL (mmol/L), multiply by 0.0259; glucose (mmol/L), multiply by 0.0555; insulin (pmol/L), multiply by 6.945; triglycerides (mmol/L), multiply by 0.0113.
Fisher exact test.
Wilcoxon rank sum test.
t test.
The hsCRP concentrations were significantly higher in MetS subjects than in non-MetS subjects (45% increase; P < .001). The levels of total adiponectin and HMW and other forms of adiponectin (MMW + LMW, SA) were significantly lower in MetS subjects than in control subjects (P < .0001). There was a significant inverse correlation between hsCRP and adiponectin concentrations and its oligomers (adiponectin and hsCRP, Spearman r = −0.34; P < .0001; HMW adiponectin and hsCRP, r = −0.30; P < .0001; non-HMW adiponectin and hsCRP, r = −0.35; P < .0001; and SA and hsCRP, r = −0.20; P = .0027). Because the hsCRP concentrations were higher and adiponectin concentrations lower in MetS, we examined the CRP/adiponectin ratio and the ratio of different adiponectin oligomers to CRP. There was a significant decrease in the adiponectin/CRP ratio in MetS subjects compared with that in control subjects (78% decrease; P < .0001). Similarly, the associations of the HMW adiponectin/CRP ratio and LMW plus MMW adiponectin/CRP ratio were also significantly decreased in MetS subjects Table 2.
Table 2.
Biomarkers of Inflammation*
| Variable | Metabolic Syndrome (n = 123) | Control Subjects (n = 91) | P† |
|---|---|---|---|
| hsCRP (mg/L) | 4.7 (2.9, 6.3) | 1.7 (0.6, 4.2) | <.0001 |
| Total adiponectin (μg/mL) | 5.1 (3.6, 6.7) | 7.0 (4.7, 10.4) | <.0001 |
| HMW adiponectin (μg/mL) | 2.0 (2.2, 3.5) | 3.4 (2, 5.7) | <.0001 |
| MMW + LMW adiponectin (μg/mL) | 2.9 (2.1, 3.5) | 3.5 (2.8, 4.4) | <.0001 |
| SA | 0.42 ± 0.14 | 0.49 ± 0.13 | .0003 |
| Adiponectin/hsCRP ratio | 1.2 (0.6, 2.2) | 4.6 (1.2, 15) | <.0001 |
| HMW adiponectin/hsCRP ratio | 0.5 (0.22, 1.1) | 2.3 (0.5, 7.2) | <.0001 |
| MMW + LMW adiponectin/hsCRP ratio | 0.96 ± 0.12 | 3.89 ± 0.54 | <.0001 |
HMW, high-molecular-weight; hsCRP, high-sensitive C-reactive protein; LMW, low-molecular-weight; MMW, medium-molecular-weight; SA, HMW adiponectin/total adiponectin ratio.
Data are given as mean ± SD or median (25th, 75th percentile).
Wilcoxon rank sum test.
Correlation of hsCRP and Adiponectin With Different Features of MetS
When subjects were grouped according to the number of MetS components (0–5), concentrations of total adiponectin, HMW, MMW plus LMW, SA, and ratios significantly and progressively decreased and hsCRP showed a progressive and highly significant increase with an increasing number of features of MetS Table 3.
Table 3.
Stratification of Subjects With or Without Metabolic Syndrome by Number of Metabolic Syndrome Components*
| No. of Components |
|||||||
|---|---|---|---|---|---|---|---|
| Variable | 0 (n = 33) | 1 (n = 35) | 2 (n = 23) | 3 (n = 66) | 4 (n = 35) | 5 (n = 22) | P† ANOVA |
| hsCRP (mg/L) | 1.9 ± 0.3 | 3.2 ± 0.5 | 4.7 ± 0.5 | 4.8 ± 0.3 | 5.2 ± 0.8 | 5.1 ± 0.8 | <.0001 |
| Total adiponectin (μg/mL) | 10.03 ± 0.83 | 7.02 ± 0.45 | 5.61 ± 0.54 | 5.65 ± 0.33 | 4.91 ± 0.35 | 3.01 ±0.29 | <.0001 |
| HMW adiponectin (μg/mL) | 5.88 ± 0.64 | 3.57 ± 0.33 | 2.57 ± 0.36 | 2.70 ± 0.25 | 2.18 ± 0.25 | 1.06 ± 0.16 | <.0001 |
| MMW + LMW adiponectin (μg/mL) | 4.15 ± 0.23 | 3.46 ± 0.16 | 3.04 ± 0.20 | 2.95 ± 0.13 | 2.73 ± 0.18 | 1.96 ± 0.19 | <.0001 |
| SA | 0.54 ± 0.02 | 0.47 ± 0.02 | 0.41 ± 0.02 | 0.43 ± 0.02 | 0.42 ± 0.03 | 0.35 ± 0.03 | .0001 |
| Adiponectin/hsCRP ratio | 15.5 ± 3.1 | 8.1 ± 1.8 | 2.8 ± 0.6 | 1.9 ± 0.3 | 1.6 ± 0.2 | 0.7 ± 0.1 | <.0001 |
| HMW adiponectin/hsCRP ratio | 8.68 ± 1.81 | 4.18 ± 1.04 | 1.23 ± 0.29 | 0.95 ± 0.15 | 0.63 ± 0.09 | 0.23 ± 0.04 | <.0001 |
| MMW + LMW adiponectin/hsCRP ratio | 6.82 ± 1.40 | 3.93 ± 0.83 | 1.58 ± 0.31 | 1.02 ± 0.16 | 0.89 ± 0.16 | 0.43 ± 0.09 | <.0001 |
ANOVA, analysis of variance; HMW, high-molecular-weight; hsCRP, high-sensitive C-reactive protein; LMW, low-molecular-weight; MMW, medium-molecular-weight; SA, HMW adiponectin/total adiponectin ratio.
Data are given as mean ± SEM.
Kruskal-Wallis test.
ORs for MetS Using the 75th Percentile Cutoff for CRP and the 25th Percentile for Adiponectin
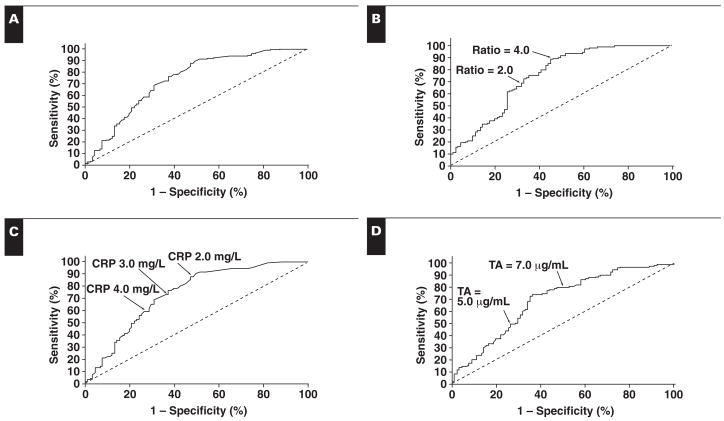
By using the 75th percentile cutoff for CRP and the 25th percentile cutoff for adiponectin, ORs were computed for having MetS Table 4. The OR of having MetS by using the 75th percentile cutoff for CRP was 3.8 and was equivalent to low adiponectin concentrations (OR, 2.5), the total adiponectin/CRP ratio (OR, 2.6), HMW adiponectin/CRP (OR, 3.0), and the LMW plus MMW adiponectin/CRP ratio (OR, 3.0). The area under the curve for CRP (0.74) was equivalent to that of the adiponectin/CRP ratio (0.75), the adiponectin oligomers/CRP ratio, and adiponectin alone (0.69) Figure 1. The sensitivity at an hsCRP level of more than 3 mg/L was 73% (95% CI, 64%–80%), and the specificity was 64% (95% CI, 53%–74%). The sensitivity of the adiponectin level at less than 6 mg/L was 68% (95% CI, 59%–76%), and the specificity was 66% (95% CI, 55%–76%). The sensitivity of an adiponectin/CRP ratio of less than 2 was 69% (95% CI, 60%–77%), and the specificity was 68% (95% CI, 58%–78%).
Table 4.
Odds Ratio for Metabolic Syndrome*
| Odds Ratio | 95% Confidence Interval | Comparison of ROC AUC P | |
|---|---|---|---|
| CRP (high) | 3.8 | 2.1–6.8 | Reference |
| Adiponectin (low) | 2.5 | 1.3–4.5 | .20 |
| Total adiponectin/CRP | 2.6 | 1.5–4.8 | .08 |
| HMW adiponectin/CRP | 3.0 | 1.7–5.4 | .33 |
| Non-HMW adiponectin/CRP | 3.0 | 1.6–5.4 | .22 |
AUC, area under the curve; HMW, high-molecular-weight; CRP, C-reactive protein; ROC, receiver operating characteristic.
75th percentile cutoff (25th for adiponectin).
Figure 1.
Receiver operating characteristic (ROC) curves for high-sensitive C-reactive protein (hsCRP) vs ratio (A; ROC area under the curve [AUC] = 0.50), ratio alone (B; ROC AUC = 0.752; 95% confidence interval [CI], 0.684–0.821), hsCRP alone (C; ROC AUC = 0.738; 95% CI, 0.668–0.808), and adiponectin alone (D; ROC AUC = 0.692; 95% CI, 0.619–0.765) for metabolic syndrome. TA, total adiponectin.
Discussion
MetS seems to be a proinflammatory state characterized by increased concentrations of CRP. Several earlier studies have shown that high CRP concentrations predict the development of diabetes. CRP has been shown to impair insulin signaling.29 Although increased CRP concentrations correlate most strongly with adiposity and insulin resistance, CRP also correlates significantly with the other features of MetS.8–10 Furthermore, CRP concentrations predict increased cardiovascular events in MetS and in diabetes.4–7 The level of adiponectin, the adipocyte-derived hormone, has been shown to be lower in MetS. Indeed, adiponectin concentrations correlate negatively with insulin resistance and adiposity. Recent lines of evidence indicate that oligomers of adiponectin, especially the HMW form, may be more important for the prediction of MetS or insulin resistance than measurements of total adiponectin.30 Prediction of MetS, defined by the presence of a cluster of metabolic abnormalities, including impaired glucose metabolism, increased central adiposity, dyslipidemia, and hypertension, seems to be important because of its association with the subsequent development of type 2 diabetes and cardiovascular disease. Developing a robust laboratory-based biomarker that will capture the various features of MetS instead of examining its individual components would be ideal from a population standpoint for screening and for testing novel therapeutic strategies.
Because CRP concentrations are higher and adiponectin concentrations are lower in MetS, we hypothesized that the ratio would emerge as a robust biomarker of MetS. Also, there seems to be cross-talk between CRP and adiponectin, with a recent article showing that CRP inhibits adiponectin expression and secretion from adipocytes in vitro.31 In the present cross-sectional study of 214 subjects with and without MetS, we found that hsCRP concentrations were significantly and progressively higher and concentrations of adiponectin and its oligomers and the ratio of hsCRP to adiponectin and its oligomers were significantly and progressively lower as the number of features of MetS present increased. Furthermore, measurement of the hsCRP level alone was equivalent to measuring the level of adiponectin or its oligomers and the ratio of hsCRP to adiponectin or its oligomers.
As previously demonstrated in several large trials,4,5,8,9 concentrations of hsCRP, an acute phase marker of inflammation, were higher in subjects with MetS. In particular, hsCRP concentrations exhibited a positive, significant, independent relationship with an increased number of features of MetS. In previous studies, strong associations between CRP and measures of body fat (BMI and waist circumference) and measures of insulin resistance (insulin sensitivity index, fasting insulin, and proinsulin) have been reported.4,5,8,10
MetS is also characterized by low adiponectin concentrations.19,32,33 Adiponectin concentrations in plasma are often found to be positively correlated with HDL-C and insulin sensitivity, whereas inverse correlations with adiposity (particularly central adiposity), triglyceride levels, and hsCRP concentrations have also been observed.34–36 However, attention has been focused on the different multimeric forms of adiponectin11,23 because these different molecular species are capable of activating different signaling pathways in muscle, liver, and endothelium.32,33,37 The HMW form is of particular interest for MetS because administration of the HMW form, but not the MMW form, reduces the blood glucose level in insulin-resistant, adiponectin-deficient mice.11 Because insulin resistance and/or low-grade inflammation are likely to be important underlying defects in MetS, we hypothesized that HMW adiponectin may be more indicative of MetS than measures of total or non-HMW (MMW + LMW) adiponectin. Furthermore, because we found that low adiponectin or HMW adiponectin and high hsCRP concentrations were independently associated with features of MetS, we hypothesized that the ratio of hsCRP to adiponectin or its oligomers may provide better predictive value for MetS.
Indeed, several lines of evidence point to the role of HMW adiponectin as a better predictor of insulin sensitivity. Swarbrick et al23 demonstrated that HMW adiponectin changes before total adiponectin in response to weight loss, and increases of HMW adiponectin are more closely related to the improvement of HOMA-IR and fasting insulin than changes of the LMW and MMW oligomers. Pajvani et al22 reported that changes in HMW adiponectin predict thiazolidinedione-mediated improvements in insulin sensitivity. Basu et al30 recently reported that HMW adiponectin and the HMW adiponectin/total adiponectin ratio are lower in people with diabetes than in people who do not have diabetes.
Furthermore, Fisher et al32 demonstrated the importance of the SA index as a better determinant of glucose intolerance than measurements of total adiponectin, suggesting that HMW adiponectin is the active form of the protein. On the other hand, Polak et al33 recently reported the absence of any correlation between changes in adiponectin complexes and indices of insulin sensitivity in diet-induced weight loss in 20 overweight or obese women who underwent 12 weeks of a low caloric diet. Abbasi et al38,39 reported parallel improvements in insulin resistance in thiazolidinedione-treated subjects, along with changes in the levels of total adiponectin and several specific adiponectin complexes; however, neither circulating adiponectin concentrations nor multimeric complexes changed in association with enhanced insulin sensitivity after moderate weight loss in 12 insulin-resistant, obese people.39 However, most of the studies detailed herein were performed in subjects with diabetes and also had sample sizes too small for examining the predictive power of adiponectin multimers for MetS.
Our findings contrast with those of Hara et al,40 who found that SA was more predictive than total adiponectin for the presence of MetS. The authors did not evaluate the absolute amount of HMW adiponectin as a predictor of MetS and did not examine the potential relationship of adiponectin oligomers to individual MetS components. Even though the assay used was identical,28 in the present study, we found SA to be less indicative of MetS than any of the other adiponectin measurements.
There are several possible reasons for the discrepancies observed. First, the patients studied by Hara et al40 had diabetes or were undergoing coronary angiography and so were likely to be in worse health than the subjects in the present study and receiving concomitant medications that could have affected the outcome of the study. In our study, we had a large sample and did not include patients with diabetes or people taking antidiabetic medications. Second, the subjects in the 2 studies are not directly comparable because the subjects studied by Hara et al40 had a lower average BMI (22–24 kg/m2 vs 30–36 kg/m2 in the present study) and were older. Also, the present study goes further in assessing not only adiponectin oligomers but also the ratio of hsCRP to adiponectin or its oligomers for predicting MetS.
In this study, although adiponectin concentrations, adiponectin oligomers, and ratios of hsCRP to adiponectin or its oligomers were significantly and progressively decreased with an increase in the number of features of MetS present, we had hypothesized that the ratio of hsCRP to adiponectin or its oligomers would be superior to either alone in predicting MetS; however, the OR and ROC curve analyses indicated that measurement of hsCRP is equivalent to measurement of adiponectin or its oligomers and the ratios of hsCRP to adiponectin or its oligomers in predicting MetS. Although a CRP cutoff of 3 mg/L provides low specificity (64%), the sensitivity is greater (73%) and seems to be slightly better than that of adiponectin and its oligomers. Furthermore, laboratory measurements that define MetS, such as triglyceride levels, have huge biologic variability (>25%), and HDL assays are far from optimum. Although adiponectin measurements need to be standardized, especially with respect to its oligomers, before they can be used in clinical practice, the measurement of hsCRP alone, which is automated and available on most clinical chemistry platforms, is standardized with a CV of less than 10%. Furthermore, measurement of the hsCRP concentration provides an objective and adjunctive biochemical marker for MetS that predicts cardiovascular events in this population that can be used to screen large populations for natural history of the disease and to assess response to therapeutic interventions targeting MetS, such as therapeutic lifestyle changes and pharmacotherapies.
hsCRP seems to be a robust biomarker for MetS. Ongoing studies with adiponectin oligomers, resistin, visfatin, and retinol binding protein-4 will hopefully help define a multimarker panel with greater sensitivity and specificity to identify MetS.
Acknowledgments
Supported by grants NIH K24 AT00596 and RO1 NIH HL 74360 from the National Institutes of Health, Bethesda, MD (Dr Jialal); grants NIH HL 075675, NIH 5R21AT002599, NIH AT 002993, and NIH AT 003545 from the National Institutes of Health (Dr Havel); and grants to Dr Jialal and Havel from the American Diabetes Association.
References
- 1.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Ann Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 2.Devaraj S, Rosenson RS, Jialal I. Metabolic syndrome: an appraisal of the pro-inflammatory and procoagulant status. Endocrinol Metab Clin North Am. 2004;33:431–453. doi: 10.1016/j.ecl.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 5.Rutter MK, Meigs JB, Sullivan LM, et al. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110:380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar D, Fisher MR, O’Connor CM, et al. Investigators in the Weekly Intervention with Zithromax for Atherosclerosis and Its Related Disorder Study. Metabolic syndrome, C-reactive protein, and prognosis in patients with established coronary artery disease. Am Heart J. 2006;152:298–304. doi: 10.1016/j.ahj.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Linnemann B, Voigt W, Nobel W, et al. C-reactive protein is a strong independent predictor of death in type 2 diabetes: association with multiple facets of the metabolic syndrome. Exp Clin Endocrinol Diabetes. 2006;114:127–134. doi: 10.1055/s-2006-924012. [DOI] [PubMed] [Google Scholar]
- 8.Festa A, D’Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 9.Hak AE, Stehouwer CD, Bots ML, et al. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler Thromb Vasc Biol. 1999;19:1986–1991. doi: 10.1161/01.atv.19.8.1986. [DOI] [PubMed] [Google Scholar]
- 10.Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 11.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(suppl 1):S143–S151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 12.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 13.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 14.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 15.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 16.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 17.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 18.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 19.Wannamethee SG, Tchernova J, Whincup P, et al. Associations of adiponectin with metabolic and vascular risk parameters in the British Regional Heart Study reveal stronger links to insulin resistance–related than to coronary heart disease risk–related parameters. Int J Obes (Lond) 2007;31:1089–1098. doi: 10.1038/sj.ijo.0803544. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 21.Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 22.Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 23.Swarbrick MM, Austrheim-Smith IT, Stanhope KL, et al. Circulating concentrations of high-molecular-weight adiponectin are increased following Rouxen-Y gastric bypass surgery. Diabetologia. 2006;49:2552–2558. doi: 10.1007/s00125-006-0452-8. [DOI] [PubMed] [Google Scholar]
- 24.Bobbert T, Rochlitz H, Wegewitz U, et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 25.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Devaraj S, O’Keefe G, Jialal I. Defining the proinflammatory phenotype using high sensitive C-reactive protein levels as the biomarker. J Clin Endocrinol Metab. 2005;90:4549–4554. doi: 10.1210/jc.2005-0069. [DOI] [PubMed] [Google Scholar]
- 27.Jialal I, Stein D, Balis D, et al. Effect of hydroxymethyl glutaryl coenzyme A reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–1935. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 28.Ebinuma H, Miyazaki O, Yago H, et al. A novel ELISA system for selective measurement of human adiponectin multimers by using proteases. Clin Chim Acta. 2006;372:47–53. doi: 10.1016/j.cca.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 29.D’Alessandris C, Lauro R, Presta I, et al. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser(307) and Ser(612) in L6 myocytes, thereby impairing the insulin signaling pathway that promotes glucose transport. Diabetologia. 2007;50:840–849. doi: 10.1007/s00125-006-0522-y. [DOI] [PubMed] [Google Scholar]
- 30.Basu R, Pajvani UB, Rizza RA, et al. Selective downregulation of the high molecular weight form (HMW) of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from non-diabetic subjects. Diabetes. 2007;56:2174–2177. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 31.Yuan G, Chen X, Ma Q, et al. C-reactive protein inhibits adiponectin gene expression and secretion in 3T3-L1 adipocytes. J Endocrinol. 2007;194:275–281. doi: 10.1677/JOE-07-0133. [DOI] [PubMed] [Google Scholar]
- 32.Fisher FF, Trujillo ME, Hanif W, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 33.Polak J, Kovacova Z, Jacek M, et al. An increase in plasma adiponectin multimeric complexes follows hypocaloric diet–induced weight loss in obese and overweight pre-menopausal women. Clin Sci (Lond) 2007;112:557–565. doi: 10.1042/CS20060296. [DOI] [PubMed] [Google Scholar]
- 34.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 35.Lara-Castro C, Luo N, Wallace P, et al. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 36.Matsubara M, Namioka K, Katayose S. Decreased plasma adiponectin concentrations in women with low-grade C-reactive protein elevation. Eur J Endocrinol. 2003;148:657–662. doi: 10.1530/eje.0.1480657. [DOI] [PubMed] [Google Scholar]
- 37.Tsao TS, Tomas E, Murrey HE, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity: different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 38.Abbasi F, Chang SA, Chu JW, et al. Improvements in insulin resistance with weight loss, in contrast to rosiglitazone, are not associated with changes in plasma adiponectin or adiponectin multimeric complexes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R139–R144. doi: 10.1152/ajpregu.00287.2005. [DOI] [PubMed] [Google Scholar]
- 39.Abbasi F, Lamendola C, McLaughlin T, et al. Plasma adiponectin concentrations do not increase in association with moderate weight loss in insulin-resistant, obese women. Metabolism. 2004;53:280–283. doi: 10.1016/j.metabol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Hara K, Horikoshi M, Yamauchi T, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]