Abstract
Accumulation of molecular damage and increased molecular heterogeneity are hallmarks of cellular aging. Mild stress-induced hormesis can be an effective way for reducing the accumulation of molecular damage, and thus slowing down aging from within. We have shown that repeated mild heat stress (RMHS) has anti-aging effects on growth and various other cellular and biochemical characteristics of normal human skin fibroblasts and keratinocytes undergoing aging in vitro. RMHS given to human cells increased the basal levels of various chaperones, reduced the accumulation of damaged proteins, stimulated proteasomal activities, increased the cellular resistance to other stresses, enhanced the levels of various antioxidant enzymes, enhanced the activity and amounts of sodium-potassium pump, and increased the phosphorylation-mediated activities of various stress kinases. We have now observed novel hormetic effects of mild heat stress on improving the wound healing capacity of skin fibroblasts and on enhancing the angiogenic ability of endothelial cells. We have also tested potential hormetins, such as curcumin and rosmarinic acid in bringing about their beneficial effects in human cells by inducing stress response pathways involving heat shock proteins and hemeoxygenase HO-1. These data further support the view that mild stress-induced hormesis can be applied for the modulation, intervention and prevention of aging and age-related impairments.
Keywords: anti-aging, hormesis, hormetin, proteasome, stress, blood vessels
INTRODUCTION
Low levels of stress from physical, chemical and biological stressors often, but not always, result in the functional improvement of cells, tissues, organs and organisms—a phenomenon termed physiological hormesis (Calabrese et al., 2007; Mattson, 2008a). The main hormetic agents identified so far are irradiation, heat, hypergravity, heavy metals, antibiotics, ethanol, pro-oxidants, exercise and food restriction (Calabrese and Baldwin, 2001; Le Bourg and Rattan, 2008). Based on the results obtained from a large number of studies showing the beneficial physiological and anti-aging effects of mild stress-induced hormesis, application of hormesis is suggested as a promising strategy to slow down aging and to prevent or delay the onset of age-related diseases (Rattan, 2005a; 2005b; 2008b; Le Bourg and Rattan, 2008). In this article, we present a brief review of our previous work and include some novel results from our ongoing studies on elucidating hormetic effects and the mode of action of thermal stress and of other inducers of stress, such as curcumin and other polyphenols, in different types of cultured human cells.
MILD HEAT STRESS HORMESIS IN HUMAN CELLS
Using a regimen of repeated mild heat shock (RMHS) at 41°C, for 1 hr twice a week, given to cultured normal human skin fibroblasts, keratinocytes and telomerase-immortalised bone marrow mesenchymal stem cells, we have reported a variety of hormetic effects. The choice of the above RMHS regimen was based on several pilot experiments performed for selecting conditions where 30% of the maximal HS response was elicited without affecting cell growth and survival (Rattan, 1998; Kraft et al., 2006). This does not imply that these are the ideal hormetic conditions for these cells. Other combinations of dose and duration may well have similar or even better effects, but that issue remains to be investigated. A summary of the results obtained and published previously in a series of papers since 1998 is as follows:
(1) Anti-aging effects in fibroblasts and keratinocytes
Various anti-aging effects of RMHS were observed in human skin fibroblasts and keratinocytes, as determined by the established criteria of cellular aging in vitro (Rattan, 2008a). These effects are:
a reduction in age-related alteration in cell morphology from a thin, long and spindle shape to irregularly enlarged and flattened shape (Rattan, 1998; Nielsen et al., 2006);
a 10–15% increase in cellular replicative lifespan, as measured by cumulative population doublings (Nielsen et al., 2006);
a 20–80% reduction in the accumulation of oxidatively damaged proteins, depending on the type of damage (Verbeke et al., 2000; 2001a; Verbeke et al., 2002); and
a 50–140% increase in intracellular antioxidative abilities including a significant increase in resistance to high levels of ethanol, hydrogen peroxide and UV-A irradiation (Fonager et al., 2002).
The main mechanisms involved in bringing about the above anti-aging effects of RMHS included an increase in and maintenance of higher levels of various heat shock proteins (HSP; (Fonager et al., 2002), and a stimulation of proteasomal activities. Whereas the increased levels of HSP may be protective against protein denaturing, an increase in the proteasomal activities of RMHS-treated cells is responsible for the removal of damaged proteins (Beedholm et al., 2004; Rattan, 2005a). We had also reported that the anti-aging hormetic effects of RMHS were accompanied by the activation of stress kinases and an increase in the content and activity of Na,K-ATPase sodium pump (Nielsen et al., 2006; Rattan and Ali, 2007). We are now extending these studies to find out the global changes in transcriptome (gene expression at the level of mRNA) and proteome (gene expression at the level of proteins), including post-translational modifications, to elucidate further the hormetic anti-aging effects and mechanisms of action of RMHS in human cells. The long term aim of these studies is to find effective hormetic treatments for delaying the onset and/or the prevention of age-related diseases related to replicative senescence, such as thinning of the skin, neurodegenerative diseases, immune deficiency, and muscle loss leading to sarcopenia.
(2) Enhanced differentiation in keratinocytes and bone marrow stem cells
RMHS enhanced the ability of serially passaged keratinocytes to enter into differentiation in the presence of calcium, as measured by the levels of differentiation markers involucrin, p38 and Hsp27 (Rattan and Ali, 2007; Berge et al., 2007; Berge et al., 2008). Another cell type in which we have tested whether differentiation can be improved hormetically by RMHS is the telomerase-immortalized bone marrow mesenchymal stem cell-line, hTERT-MSC (Simonsen et al., 2002). We have reported that single or multiple exposures to mild HS significantly enhanced the vitamin-D-induced differentiation of hTERT-MSC into osteoblasts, as measured by determining the levels of osteoblastic markers alkaline phosphatase and mineralised matrix (Nørgaard et al., 2006). Although the mechanistic aspects of the hormetic effects of HS on the differentiation of human cells are yet to be elucidated, such studies pave the way for developing novel means for the maintenance and improvement of differentiation abilities of various cell types and thus prevent age-related alterations leading to impairments such as thinning and excessive wrinkling of the skin, and a loss of bone mass leading to osteoporosis.
(3) Improved wound healing in vitro
Recently, we have observed a novel effect of mild HS-induced hormesis, in terms of enhanced wound healing in vitro, using the method for the wound healing assay as described earlier (Rattan et al., 2007). Briefly, human skin fibroblasts ASF-2 (Nielsen et al., 2006; Kraft et al., 2006) were seeded at a density of about 100,000 cells per well in a 6-well plate (growth area per well 10 cm2), and were allowed to attach, to grow and to make a near-confluent layer in about 24 hr. Thereafter, a “wound” or a scratch was made in the centre of the confluent layer by using a p1000 plastic tip. Phase-contrast photos of live cells in a selected part of the wounded area were taken by using an inverted microscope equipped with a digital camera (Zeiss-Axiovert25). Cells were then incubated at normal culturing conditions of 37°C, 5% CO2 and 95% humidity. After 24 hr, photos were again taken from exactly the same area as before and a composite image composed of at least 6 overlapping pictures was generated, which represented the wound area (about 3 mm2). The lines drawn on the pictures marked the areas which were not occupied by migrating fibroblasts, and these demarcations were used to quantify the extent of wound healing, by using an image analysis program “ImageJ” (downloaded from: http://rsb.info.nih.gov/ij/) (Rattan et al., 2007). The results of various combinations and the timing of HS and culture conditions are described below.
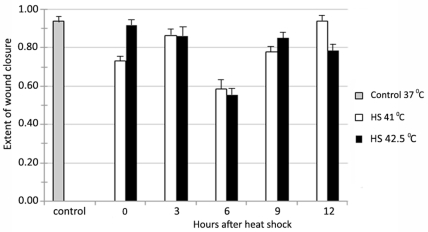
Exposure of human fibroblasts to a 1 hr mild or severe HS at 41°C and 42.5°C, respectively, followed by wounding at various times after HS (0, 3, 6, 9, and 12 hr) did not show any beneficial hormetic effects in terms of enhanced wound healing (Fig. 1). Rather, there was about 40% reduced wound healing in the following 24 hr if the wound was made up to 6 hr after HS, after which the extent of healing reached similar to the controls without HS (Fig. 1). The reason for this apparently opposite effect than that expected may be the fact that HS is known to induce preferentially the synthesis of new HSP, which is accompanied by the inhibition of most other metabolic processes in the cell for several hours (Verbeke et al., 2001b; Bakkenist and Kastan, 2004; Park et al., 2005). That is why the act of wounding, while the response of cells to the first stress of HS is not completely over, is a kind of additional stress that overshadows any beneficial effects that may be accrued from the synthesis of HSP during that period. Therefore, we undertook further studies by giving HS to one set of cells and using their medium after various times after HS to test for the effects of HS-induced HSP on wound healing.
FIGURE 1.
Effect of either 1 hr mild HS (41°C) or severe HS (42.5°C) before making the wound at different times after HS in the same set of cell cultures of mid-passage (less than 50% lifespan completed) human skin fibroblasts ASF-2.
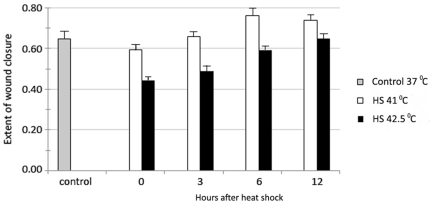
Fig. 2 shows that when the complete culture medium (containing 10% serum) was collected after various times from human fibroblasts exposed to 1 hr mild HS (41°C) or severe HS (42.5°C), and was given to a separate set of freshly wounded cells, there was some (17%) increase in the extent of wound healing in those cells receiving HS-conditioned medium (HSCM) collected after 6 hr. Furthermore, these stimulatory effects of HS on wound healing were best observed for mild HS as compared with the severe HS, thus meeting the non-linear dose response criterion for being hormetic.
FIGURE 2.
Effect of complete culture medium containing 10% serum collected at different times from cells exposed to either 1 hr mild HS (41°C) or severe HS (42.5°C) on the extent of wound healing in a separate set of cell cultures of mid-passage (less than 50% lifespan completed) human skin fibroblasts ASF-2.
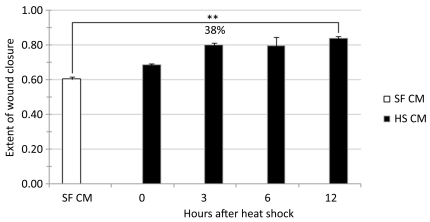
Since the presence of fetal calf serum in the culture medium can mask the effects of stress-induced secretory proteins (see for example, (Li et al., 2007), we undertook further studies by collecting HSCM in serum-free (SF) conditions. Fig. 3 shows that whereas SF conditioned medium (SFCM) without HS supported wound healing to about 60% of the levels reached by controls with serum, SF-HSCM for 6 and 12 hrs supported 38% additional wound healing in the following 24 hr period.
FIGURE 3.
Effect of serum free culture medium (SFCM) collected at different times from unstressed cells, and from cells exposed to 1 hr mild HS at 41°C (HSCM) on the extent of wound healing in a separate set of cell cultures of mid-passage (less than 50% lifespan completed) human skin fibroblasts ASF-2. Student’s t-test was applied for determining the statistical significance (p < 0.005)
Other observations that we have made with respect to the effects of HS conditioned medium on enhanced wound healing are summarized as follows (complete data not given):
Enhanced wound healing was not due to an induction of cell proliferation in HSCM-treated cells, as determined by bromo-deoxyuridine (BrdU) labelling and autoradiography method.
There was 68% increase in mobility and migration of HSCM-treated cells from the edge of the wound towards the wounded area, as determined by the colloidal gold particle assay (Albrecht-Buehler, 1977).
Increased migration of cells during wound healing was accompanied by about 54% elongation of cells in HSCM-treated cells, which was almost 5 times higher than elongation in SFCM-treated cells, measured by using the image analysis program.
HSCM alleviated almost completely (more than 95%) the negative effects of 0.25 mM and 0.5 mM glyoxal on wound healing reported previously (Rattan et al., 2007).
HSCM could also stimulate wound healing in late passage senescent cells, but it was about 50% less than that in early passage or middle-aged cells. However, it must be pointed out that late passage senescent cells had their wound healing capacity already reduced significantly due to aging, but could still get some benefits from mild HS-induced hormesis.
The above studies indicate that mild HS induces the synthesis of one or more gene-products which are secreted by the cells in the culture medium. Furthermore, these molecules can stimulate wound healing either as direct stimulants or as inhibitors of the negative modulators of wound healing, such as the plasminogen activator inhibitor PAI-1 (Kortlever and Bernards, 2006). However, the full range of secreted proteins, including HSP (Li et al., 2007), which may be responsible for enhanced wound healing and other biological effects are yet to be identified.
(4) Enhanced angiogenesis by mild HS
Angiogenesis is a physiological process involving the growth of new blood vessels from pre-existing vessels, by the active participation of vascular and microvascular endothelial cells. Since there is an age-related decrease in angiogenesis (Ashcroft et al., 2002), we have initiated studies to determine the effects of mild and severe stress on angiogenesis in vitro, using normal human umbilical vein endothelial cells (HUVEC) undergoing aging, and an immortal SV40-transformed human dermal microvascular cell line, HMEC-1.
A standardized tube-formation assay involving the growth of cells on commercially available basement membrane extract (Matrigel Matrix MM; BD Biosciences, USA) was employed to analyse the kinetics and extent of new blood vessel formation (Rattan et al., 2007). Pre-exposure of HMEC and HUVEC to 1 hr HS at 41°C or 42.5°C, followed by different periods of recovery at 37°C had hormetic effects with respect to angiogenesis. Interestingly, whereas the general extent and quality of the tubes formed by cells pre-exposed to 41°C was better than that in the controls, a pre-exposure at 42.5°C resulted in a relative worsening of tube structures (Rattan et al., 2007), indicating that only mild stress has hormetic effects. We now aim to elucidate whether the extent of hormetic effects of mild HS on angiogenesis are related to the levels of various HSP synthesised during this period, and what other pathways are involved in this. For example, there is some evidence that HSP90 stimulates tube formation by HUVEC via its role in enhancing the expression of nitric oxide synthase (NOS) gene and the production of nitric oxide (Sun and Liao, 2004).
HORMETINS AS MODULATORS OF AGING, DIFFERENTIATION AND WOUND HEALING
Various natural or synthetic compounds which can bring about biologically beneficial hormetic effects by activating one or more pathways of stress response, are termed as hormetins (Ali and Rattan, 2006; Rattan, 2008b). Accordingly, several dietary components, such as resveratrol, vitamins, and trace elements and minerals including iron, iodine, fluorine, selenium and copper and zinc are potential hormetins (Lamming et al., 2004; Putics et al., 2008; Hayes, 2007; Mattson, 2008b). Other potential hormetins are the so-called antioxidants such as alpha lipoic acid and coenzyme Q10 which, owing to their pro-oxidant activities in producing hydrogen peroxide, induce antioxidative defensive responses and ultimately may bring about beneficial effects (Linnane and Eastwood, 2006; Linnane et al., 2007). Another hormetin is a plant growth factor kinetin, which is known to have several anti-aging effects in human skin cells (Rattan and Clark, 1994), and promotes the differentiation of keratinocytes by inducing stress response pathways (Berge et al., 2006; 2008). Similarly, certain synthetic mimetics of superoxide dimutase/catalase, shown to have anti-aging effects, also appear to work as hormetins by inducing oxidative stress response (Melov et al., 2000; Melov et al., 2001; Liu et al., 2003). A DNA damage product, thymidine-dimer, is claimed to have protective effects in the skin by inducing DNA repair pathways (Goukassian and Gilchrest, 2004), and may be considered as a hormetin.
Similarly, some components of certain medicinal plants used frequently in the traditional Chinese medicine and in the Indian Ayurvedic system of medicine are claimed to have anti-aging effects, which appear to be achieved through hormetic pathways. For example, celasterols and paeoniflorin present in some medicinal herbs have cytoprotective effects and induce HSP in human cells (Westerheide et al., 2004; Yan et al., 2004). Leaf extracts of Ashwagandha (Withania somnifera) also induce HSP synthesis in mammalian cells (G. Kaur and A. Nagpal, personal communication), which may be one of the reasons for its observed beneficial medicinal effects (Winters, 2006).
Curcumin
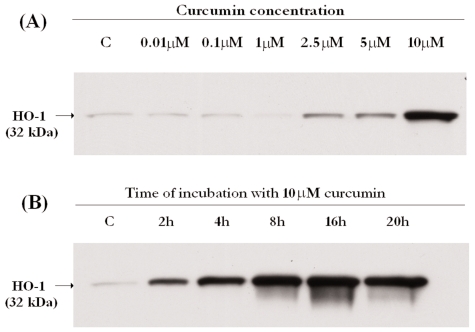
Another potential hormetin being tested for its biological effects is curcumin or diferuloylmethane. It is the active component in the commonly used food spice turmeric, and is derived from the roots of Curcuma longa. Curcumin is a co-inducer of HSP and has wide ranging biological effects depending on its dosage (Bala et al., 2006; Deorukhkar et al., 2007; Kunnumakkara et al., 2008). We have undertaken studies to check the hormetic effects of curcumin on human keratinocytes and fibroblasts. We have previously reported that at lower doses (0.3 and 1 μM) curcumin stimulates proteasome activity, enhances HSP induction after HS, and stimulates sodium pump activity (Ali and Rattan, 2006; Rattan and Ali, 2007). Since curcumin is not a direct inducer of HSP, we have tested whether it can induce any other stress response pathways in human cells. Fig. 4 shows the results of our recent observations that curcumin rapidly induces the synthesis of heme-oxygenease-1 (HO-1) in human skin fibroblasts in a dose response manner. HO-1 is a well known marker of intracellular stress (Schipper, 2000; Patriarca et al., 2007), and so the direct induction of HO-1 and co-induction of HSP by curcumin makes it a potential hormetin.
FIGURE 4.
Western blots showing the effect of curcumin on the induction of the stress response marker heme-oxygenase-1 (HO-1) in mid-passage (less than 50% lifespan completed) human skin fibroblasts ASF-2. The antibody used was a commercially purchased mouse monoclonal antibody anti-HO1 (osa110 from Nordic Biosite, Sweden), diluted 1:5000 in PBS containing 1% bovine serum albumin.
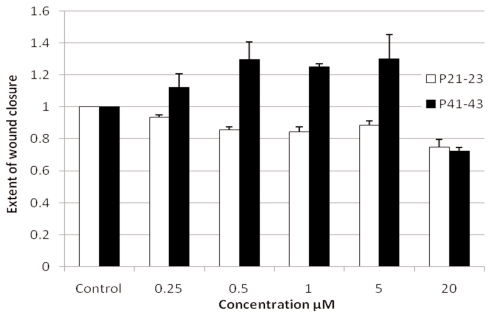
We are now testing the effects of curcumin on wound healing in vitro, using the wound healing assay as described above. Fig. 5 shows that cur-cumin has a hormetic dose response effect on the extent of wound healing, especially in late passage human fibroblasts ASF-2 (more than 95% lifespan completed). Whereas concentrations above 5 μM were inhibitory for cell migration and wound healing – a result similar to the one reported by other authors studying the growth inhibitory and anti-cancer effects of curcumin (Joe et al., 2004; Deorukhkar et al., 2007), our results show that at lower concentrations (0.25 to 5 μM) can promote wound healing in late passage cells (Fig. 5). In comparison, the middle aged cells (about 50% lifespan completed) showed either no effect or even inhibitory effects of curcumin on wound healing. The molecular explanation for this differentiated effect of curcumin between old and young cells requires further investigation. We are now extending these studies to find out long term effects of curcumin treatment on cellular aging, differentiation and angiogenesis.
FIGURE 5.
Effect of curcumin on the extent of wound healing in mid-passage (P21–23, about 50% lifespan completed) and late-passage (P41–43, more than 90% lifespan completed) human skin fibroblasts ASF-2.
Rosmarinic acid
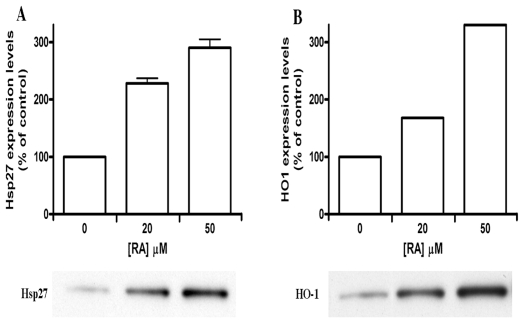
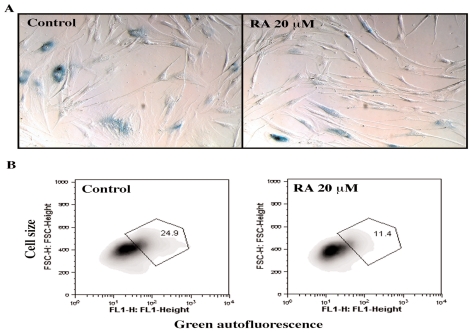
Another potential hormetin that we are testing is the rosmarinic acid (RA), which is a phenolic compound present in several medicinal plants such as those from the Labiatae family, for example Salvia officinalis (common sage) and Rosmarinus officinalis (Lima et al., 2007). Fig. 6 shows that human skin fibroblasts ASF-2 treated for 24 hr with 20 μM and 50 μM had 2 to 3 fold higher levels of stress proteins HSP27 and HO-1. We are now in the process of testing further the short term and the long terms effects of the hormetin RA on aging, wound healing, angiogenesis and differentiation of human cells. Our preliminary results show that human skin fibroblast cultures grown continuously in the presence of 20 μM RA for 50 days have a significant (33%) reduction in the number of cells showing senescence-specific β-galactosidase marker (Dimri et al., 1995) (Fig. 7A). Furthermore, there was 50% reduction in the proportion of enlarged cell population with high levels of green autofluorescence in RA-treated cultures, as measured by flow cytometery (Fig. 7B).
FIGURE 6.
Induction of stress proteins Hsp27 and HO-1 in mid-passage (less than 50% lifespan completed) human skin fibroblasts ASF-2 treated with 20 μM rosmarinic acid (RA) for 72 hr. The antibodies used in Western blots were commercially purchased mouse monoclonal antibody osa110 anti-HO1, and spa800 for Hsp27 (Nordic Biosite, Sweden), diluted 1:5000 in PBS containing 1% bovine serum albumin.
FIGURE 7.
Effect of 20 μM rosmarinic acid (RA) treatment for 50 days on senescence markers in late-passage human skin fibroblasts, ASF-2. (A) Light microscopic pictures of ASF2 cells stained for senescence-specific β-galactosidase (microscopic magnification 20x); (B) The proportion of ASF2 cells showing green autofluorescence in FL1 channel.
CONCLUSIONS
Using human fibroblasts, keratinocytes, endothelial cells and bone marrow mesenchymal stem cells we have provided evidence in support of the view that hormesis can be applied successfully to aging research and intervention. This includes the effects of mild HS on various parameters of cellular aging and other functional characteristics, such as differentiation, wound healing and angiogenesis. At the mechanistic level, the induction of HSP as mediators of hormetic effects is only a partial explanation and cannot account for the wide ranging and long lasting biological effects (Vermeulen and Loeschcke, 2007). The same may apply to other stresses, such as mechanical stress, irradiation, intermittent fasting, and hormetins. Therefore, it is important to determine how various components of the homeodynamic machinery respond and interact during stress-induced hormesis, and how relatively small individual hormetic effects lead to a significant biological amplification that results in an overall improvement of the living system (Rattan, 2006; 2008b; Rattan, 2008c). Such understanding will be essential for developing effective means of slowing down aging and preventing the onset of age-related diseases.
ACKNOWLEDGMENTS
Ricardo Fernandes was a SOCRATES/ERASMUS exchange student (February to August 2007), from the University of Porto, Portugal; Barbara Dymek was a SOCRATES/ERASMUS exchange student (February to June 2008), from the Jagiellonian University, Kraków, Poland; and Dr. Cristovao F. Lima was a visiting post-doctoral fellow (September 2007 to July 2008), from the University of Minho, Braga, Portugal, supported by the FCT grant SFRH/BPD/26316/2006. Laboratory of Cellular Ageing received research grants from the Danish Medical Research Council (FSS), and EU’s Biomed Health programme “Proteomage”.
REFERENCES
- Albrecht-Buehler G. Phagokinetic tracks of 3T3-cells. Cell. 1977;11:395–404. doi: 10.1016/0092-8674(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Ali RE, Rattan SIS. Curcumin’s biphasic hormetic response on proteasome activity and heat shock protein synthesis in human keratinocytes. Ann NY Acad Sci. 2006;1067:394–399. doi: 10.1196/annals.1354.056. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Mills SJ, Ashwoth JJ. Ageing and wound healing. Bigerontology. 2002;3:337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Bala K, Tripathy BC, Sharma D. Neuroprotective and anti-ageing effects of curcumin in aged rat brain regions. Biogerontology. 2006;7:81–89. doi: 10.1007/s10522-006-6495-x. [DOI] [PubMed] [Google Scholar]
- Beedholm R, Clark BFC, Rattan SIS. Mild heat stress stimulates proteasome and its 11S activator in human fibroblasts undergoing aging in vitro. Cell Stress & Chaperones. 2004;9:49–57. doi: 10.1379/475.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge U, Behrens J, Rattan SIS. Sugar-induced premature aging and altered differentiation in human epidermal keratinocytes. Ann NY Acad Sci. 2007;1100:524–529. doi: 10.1196/annals.1395.058. [DOI] [PubMed] [Google Scholar]
- Berge U, Kristensen P, Rattan SIS. Kinetin-induced differentiation of normal human keratinocytes undergoing aging in vitro. Ann NY Acad Sci. 2006;1067:332–336. doi: 10.1196/annals.1354.045. [DOI] [PubMed] [Google Scholar]
- Berge U, Kristensen P, Rattan SIS. Hormetic modulation of differentiation of normal human epidermal keratinocytes undergoing replicative senescence in vitro. Exp Gerontol. 2008;43:658–662. doi: 10.1016/j.exger.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: a generalizable and unifying hypothesis. Crit Rev Toxicol. 2001;31:353–424. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- Deorukhkar A, Krishnan S, Sethi G, Aggarwal BB. Back to basics: how natural products can provide the basis for new therapeutics. Expert Opin. Investg Drugs. 2007;16:1753–1773. doi: 10.1517/13543784.16.11.1753. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonager J, Beedholm R, Clark BFC, Rattan SIS. Mild stress-induced stimulation of heat shock protein synthesis and improved functional ability of human fibroblasts undergoing aging in vitro. Exp Gerontol. 2002;37:1223–1238. doi: 10.1016/s0531-5565(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Goukassian DA, Gilchrest BA. The interdependence of skin aging, skin cancer, and DNA repair capacity: a novel perspective with therapeutic implications. Rejuven Res. 2004;7:175–185. doi: 10.1089/rej.2004.7.175. [DOI] [PubMed] [Google Scholar]
- Hayes DP. Nutritional hormesis. Eur J Clin Nutr. 2007;61:147–159. doi: 10.1038/sj.ejcn.1602507. [DOI] [PubMed] [Google Scholar]
- Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin - cellular and molecular mechanism of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Bernards R. Senescence, wound healing and cancer: the PAI-1 connection. Cell Cycle. 2006;5:2697–2703. doi: 10.4161/cc.5.23.3510. [DOI] [PubMed] [Google Scholar]
- Kraft DC, Deocaris CC, Rattan SIS. Proteasomal oscillation during mild heat shock in aging human skin fibroblasts. Ann NY Acad Sci. 2006;1067:224–227. doi: 10.1196/annals.1354.028. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-KB-regulated gene products. Clin Canc Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Le Bourg E, Rattan SIS, editors. Mild stress and healthy aging: applying hormesis in aging research and interventions . Springer; Dordrecht, The Netherlands: 2008. [Google Scholar]
- Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, et al. Extracellular heat shock protein-90α: linking hypoxia to skin cell motility and wound healing. EMBO J. 2007;26:1221–1233. doi: 10.1038/sj.emboj.7601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima CF, Valentao PCR, Andrade PB, Seabra RM, Fernandes-Ferreira M, Pereira-Wilson C. Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage. Chemico-Biological Interactions. 2007;167:107–115. doi: 10.1016/j.cbi.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Linnane AW, Eastwood H. Cellular redox regulation and prooxidant signaling systems. A new persepctive on the free radical theory of aging. Ann N Y Acad Sci. 2006;1067:47–55. doi: 10.1196/annals.1354.008. [DOI] [PubMed] [Google Scholar]
- Linnane AW, Kios M, Vitetta L. Healthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: the essential roles of superoxide anion and hydrogen peroxide. Biogerontology. 2007;8:445–467. doi: 10.1007/s10522-007-9096-4. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl. Acad. Sci USA. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008a;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008b;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, et al. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Nielsen ER, Eskildsen-Helmond Y, Rattan SIS. MAP-kinases and heat shock-induced horme-sis in human fibroblasts during serial passaging in vitro. Ann NY Acad Sci. 2006;1067:343–348. doi: 10.1196/annals.1354.048. [DOI] [PubMed] [Google Scholar]
- Nørgaard R, Kassem M, Rattan SIS. Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortlized mesenchymal stem cells. Ann NY Acad Sci. 2006;1067:443–447. doi: 10.1196/annals.1354.063. [DOI] [PubMed] [Google Scholar]
- Park HG, Han SI, Oh SY, Kang HS. Cellular responses to mild heat stress. Cell Mol Life Sci. 2005;62:10–23. doi: 10.1007/s00018-004-4208-7. [DOI] [PubMed] [Google Scholar]
- Patriarca S, Furfaro AL, Cosso L, Pesce Maineri E, Balbis E, Domenicotti C, et al. Heme oxygenase 1 expression in rat liver during ageing and ethanol intoxication. Biogerontology. 2007;8:365–72. doi: 10.1007/s10522-006-9079-x. [DOI] [PubMed] [Google Scholar]
- Putics A, Végh EM, Csermely P, Soti C. Resveratrol induces the heat-shock response and protects human cells from severe heat stress. Antiox Red Sign. 2008;10:1–11. doi: 10.1089/ars.2007.1866. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Repeated mild heat shock delays ageing in cultured human skin fibroblasts. Biochem Mol Biol Int. 1998;45:753–759. doi: 10.1080/15216549800203162. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Hormetic modulation of aging and longevity by mild heat stress. Dose-response. 2005a;3:533–546. doi: 10.2203/dose-response.003.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SIS. Principles and practice of hormesis as an aging intervention. In: Rattan SIS, editor. Aging Interventions and Therapies. World Scientific Publishers; Singapore: 2005b. pp. 365–378. [Google Scholar]
- Rattan SIS. Theories of biological aging: genes, proteins and free radicals. Free Rad Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Cellular senescence in vitro. Encyclopedia of Life Sciences. 2008a doi: 10.1002/9780470015902.a0002567.pub2. [DOI] [Google Scholar]
- Rattan SIS. Hormesis in aging. Ageing Res Rev . 2008b;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Increased molecular damage and heterogeneity as the basis of aging. Biol Chem. 2008c;389:267–272. doi: 10.1515/BC.2008.030. [DOI] [PubMed] [Google Scholar]
- Rattan SIS, Ali RE. Hormetic prevention of molecular damage during cellular aging of human skin fibroblasts and keratinocytes. Ann NY Acad Sci. 2007;1100:424–430. doi: 10.1196/annals.1395.047. [DOI] [PubMed] [Google Scholar]
- Rattan SIS, Clark BFC. Kinetin delays the onset of ageing characteristics in human fibroblasts. Biochem Biophys Res Commun. 1994;201:665–672. doi: 10.1006/bbrc.1994.1752. [DOI] [PubMed] [Google Scholar]
- Rattan SIS, Sejersen H, Fernandes RA, Luo W. Stress-mediated hormetic modulation of aging, wound healing and angiogenesis in human cells. Ann N Y Acad Sci. 2007;1119:112–121. doi: 10.1196/annals.1404.005. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenease-1: role in brain aging and neurodegeneration. Exp Gerontol. 2000;35:821–830. doi: 10.1016/s0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- Simonsen JL, Rosada C, Serakinci N, Justesen J, Stendrup K, Rattan SIS, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotech. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- Sun J, Liao JK. Induction of angiogenesis by heat shock protein 90 mediated by protein kinase Akt and endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2004;24:2238–2244. doi: 10.1161/01.ATV.0000147894.22300.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke P, Clark BFC, Rattan SIS. Modulating cellular aging in vitro: hormetic effects of repeated mild heat stress on protein oxidation and glycation. Exp Gerontol. 2000;35:787–794. doi: 10.1016/s0531-5565(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Clark BFC, Rattan SIS. Reduced levels of oxidized and glycoxidized proteins in human fibroblasts exposed to repeated mild heat shock during serial passaging in vitro. Free Rad Biol Med. 2001a;31:1593–1602. doi: 10.1016/s0891-5849(01)00752-3. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Deries M, Clark BFC, Rattan SIS. Hormetic action of mild heat stress decreases the inducibility of protein oxidation and glycoxidation in human fibroblasts. Biogerontology. 2002;3:105–108. doi: 10.1023/a:1015284119308. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Fonager J, Clark BFC, Rattan SIS. Heat shock response and ageing: mechanisms and applications. Cell Biol Int. 2001b;25:845–857. doi: 10.1006/cbir.2001.0789. [DOI] [PubMed] [Google Scholar]
- Vermeulen CJ, Loeschcke V. Longevity and the stress response in Drosophila. Exp Gerontol. 2007;42:153–159. doi: 10.1016/j.exger.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Bosman JD, Mbadugha BNA, Kawahara TLA, Matsumoto G, Kim S, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- Winters M. Ancient medicine, modern use: Withania somnifera and its potential role. Altern Med Rev. 2006;11:269–277. [PubMed] [Google Scholar]
- Yan D, Saito K, Ohmi Y, Fujie N, Ohtsuka K. Paeoniflorin, a novel heat shock protein-inducing compound. Cell Stress & Chaperones. 2004;9:378–389. doi: 10.1379/CSC-51R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]