Abstract
The hormesis concept has broad implications for biology and the biomedical sciences. This perspective on hormesis concentrates on toxicology and toxicological risk assessment and secondarily explores observations from other fields. It considers the varied manifestations of hormesis in the context of a broad family of biological stress responses. Evidence for hormesis is reviewed, and the hormesis model is contrasted with more widely accepted dose-response models in toxicology: a linear nonthreshold (LNT) model for mutagenesis and carcinogenesis, and a threshold model for most other toxicologic effects. Scientific, philosophical, and political objections to the hormesis concept are explored, and complications in the hormesis concept are analyzed. The review concludes with a perspective on the current state of hormesis and challenges that the hormesis model poses for risk assessment.
Keywords: hormesis, dose-response, toxicology risk assessment, threshold, high-risk groups, biphasic curve
DOSE-RESPONSE MODELS FOR EFFECTS AT LOW DOSES
Three models have dominated thinking about effects of exposures to low doses of toxicants and radiation: a threshold model, a linear model with no threshold (LNT), and a hormetic model. The threshold model is widely considered to be the standard in toxicology, except that LNT prevails in mutagenesis and carcinogenesis. Hormesis, which entails a biphasic dose-response, is a challenge to the threshold and LNT models (Calabrese and Baldwin 2001a, 2003a, 2003b; Calabrese and Blain 2005).
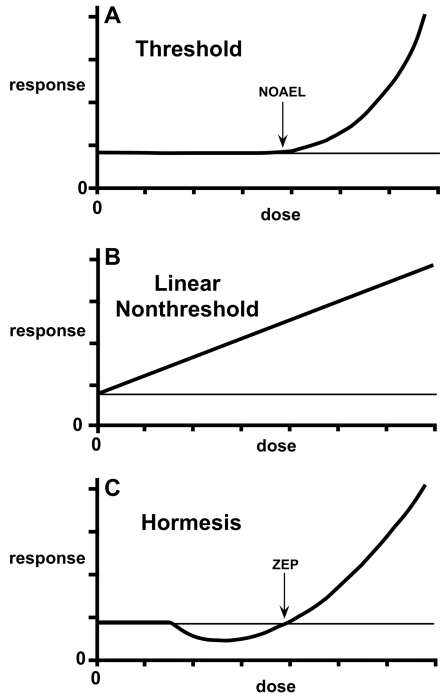
Figure 1 shows dose-response relationships representing the three models. The threshold model (Figure 1A) has a classical NOAEL (no observed adverse effect level) or a true threshold below which there is no effect. When expressed for a quantal characteristic in a finite population, the threshold model takes the form of the classic sigmoid curve shown in essentially all toxicology textbooks (Eaton and Gilbert 2008). The threshold model is considered a basic principle of toxicology, despite the fact that its predictions about low doses are based heavily on theory and assumptions. Mutagenesis and carcinogenesis differ from most of toxicology, in that the prevailing assumption has been low-dose linearity, reflected in the LNT model (Figure 1B). The LNT model has a linear decline in adverse effect with decreasing dose down to the spontaneous frequency, such that all doses are considered to have an effect. Hormesis is described by a dose-response curve with effects at low doses opposite to those at high doses (Figure 1C). Thus, the hormetic curve is nonmonotonic, such that the dependent variable—response—changes in more than one direction with a unidirectional change in the independent variable—dose (Davis and Svendsgaard 1994). In contrast, the threshold and linear models are monotonic, showing an increase or decrease in biological response over the entire range of doses that have an effect. The biphasic hormetic curve is often described as J-shaped or U-shaped (Davis and Svendsgaard 1994; Calabrese 2002; Conolly and Lutz 2004). It shows a zero equivalent point (ZEP), which is a variation on the concept of a NOAEL befitting the hormesis concept, in that it does not imply a threshold in the classical sense.
FIGURE 1.
Three dose-response models: (A) threshold model; (B) linear model; (C) hormesis model. The curves compare responses to the spontaneous frequency of the toxicologic effect being measured. A NOAEL (no observed adverse effect level) and ZEP (zero equivalent point) are shown for the threshold and hormesis models. The figure is adapted from Hoffmann and Stempsey 2008.
The dominance of the LNT model in mutagenesis and carcinogenesis stems from target theory, early observations at moderate to high doses that were consistent with linearity, and the historical roots of these fields (Auerbach 1976; National Research Council 1980, 2006; Brenner et al. 2003; Brenner and Sachs 2006; Friedl and Rühm 2006). The induction of mutations by ionizing radiation, discovered by H.J. Muller in 1927 (Muller 1927), remained a model for understanding mutagenesis even after the burgeoning of research in chemical mutagenesis after World War II. Chemical mutagenesis was in many ways the offspring of radiation biology, which shaped the way that geneticists viewed mutagenesis in general. If mutations resulted from a direct interaction between the mutagen and its target, DNA, following one-hit kinetics, one should expect linearity (Auerbach 1976). While there are exceptions, early studies of mutagenesis were largely compatible with this expectation (Friedl and Rühm 2006).
An interesting historical note is that Charlotte Auerbach, who, together with J.M. Robson, discovered chemical mutagenesis through work with mustard gas in Drosophila during World War II (Auerbach and Robson 1946, 1947), argued clearly in her 1976 book “Mutation Research: Problems, Results, and Perspectives” that mutagenesis is a complex cellular process, involving the processing of lesions by organisms, not a unitary interaction between mutagen and target (Auerbach 1976). Although the correctness of her argument was clear to most workers in chemical mutagenesis, it has taken time to shake the foundation of an expectation of linearity. Increasing awareness of the complexity of mutagenesis as a biological process and the diversity of its mechanisms has led to growing receptiveness to nonlinear dose-response relationships and thresholds, even for such mutagens as alkylating agents that are a model of direct mutagen-DNA interaction (Kodell 2001; Bolt et al. 2004; Fukushima et al. 2005; Jenkins et al. 2005). Factors in mutagenesis include the uptake and metabolism of mutagens, direct and indirect interactions between mutagen and DNA, recognition and processing of damage by enzymes of repair and recombination, influences of cellular proliferation and apoptosis, and conditions for mutant expression. These introduce many possibilities for deviations from linearity. Thus, mutagenesis should be considered an integral part of the spectrum of biological effects of toxicants and radiation, not an effect requiring fundamentally different expectations.
The somatic mutation theory of cancer became established early in the twentieth century, having been espoused by one of the founders of the Chromosome Theory of Inheritance, Theodor Boveri, between 1902 and 1914 (Boveri 1929; reviewed in Manchester 1995). Given the many linkages between mutagenesis and carcinogenesis, it was natural to think that what is true of one should be true of the other. When one concentrates on doses for which responses are readily measured—that is, higher doses—results in radiation carcinogenesis are largely compatible with the expectation for linearity (Brenner et al. 2003; Redpath et al. 2003a; Redpath 2006). Even at low doses, epidemiology may not detect U-shaped curves or other deviations from linearity because modest responses merge with random variation, and many studies are affected by mixed qualities of radiation and the pooling of different endpoints (Redpath et al. 2003a). Therefore, the LNT model has persisted, resting heavily on observations at higher doses combined with its theoretical foundation (Brenner and Sachs 2006).
LNT remains the dominant model in radiation risk assessment, as is reflected in the latest report of the U.S. National Academy of Sciences (NAS) on ionizing radiation (National Research Council 2006). However, the debate has intensified in light of evidence contrary to LNT, both from epidemiologic findings and from studies of carcinogenesis and longevity in laboratory animals (Duport 2003; Tubiana and Aurengo 2006; Tubiana et al. 2006). The recent Joint Report of the French Academies reached a decidedly different conclusion from the NAS, rejecting LNT as a realistic model for low doses of ionizing radiation (Aurengo et al. 2005; Tubiana and Aurengo 2006; Tubiana et al. 2006).
The French report, which shows receptiveness to the concept of radiation hormesis, holds that LNT is apt to strongly overestimate the carcinogenic effects of doses below ~100 mSv, and the overestimation is undoubtedly much greater at very low doses (<10 mSv) (Tubiana et al. 2006). Expecting linearity for carcinogenicity is questionable because of the diversity of mechanisms by which genotoxic and nongenotoxic carcinogens induce cancer. Epigenetic processes are important in carcinogenesis, and the outcome is modulated by such factors as cell proliferation, repair, apoptosis, intercellular communication, and cell signaling (Klaunig et al. 2000; Fukushima et al. 2005; Kinoshita et al. 2006). The process is far removed from the unitary interaction between agent and target envisioned in hit theory. Receptiveness to nonlinearity in carcinogenesis has therefore grown, especially for carcinogens with a nongenotoxic mode of action, but also for agents that act through genotoxic mechanisms (Kodell 2001).
DEFINING HORMESIS
Hormesis has been defined on different grounds, and this fact has undoubtedly contributed to confusion as to the nature of the concept. Most basically, hormesis is a dose response relationship in which effects at low doses are opposite to those at high doses. As a consequence, hormetic dose response curves are biphasic rather than being monotonic. The biphasic curve is so central that hormesis is often defined with respect to a dose-response curve that is essentially J-shaped or an inverted U (Davis and Svendsgaard 1990; Calabrese and Baldwin 2002a).
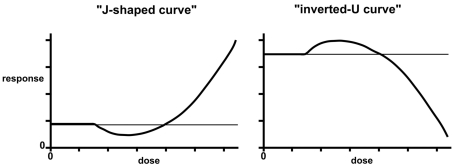
J-shaped and inverted-U biphasic curves
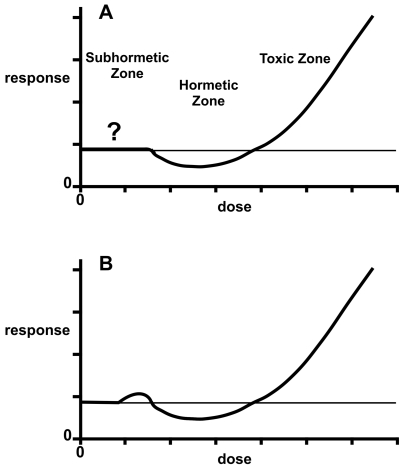
Figure 2 shows “J-shaped” and “inverted-U” curves typical of hormesis. The effect on the ordinate of the J-shaped curve is some dysfunction, such as carcinogenesis, with the spontaneous level of the event denoted by a horizontal line. The hormetic zone shows a frequency less than that occurring spontaneously with no exposure. If the endpoint were a normal biological function, such as growth, the hormetic curve would appear as an inverted U, in which the hormetic zone is represented by effects above the background level and the toxic zone by effects below it (Calabrese and Baldwin 2003c; Hoffmann and Stempsey 2008). Terms that have been used to describe U- or J- shaped curves include hormesis, β-curve, bell-shaped, biphasic, diphasic, bitonic, bidirectional, sinusoidal, subsidy gradient, functional antagonism, dual response, nonmonotonic, and stimulatory-inhibitory (Calabrese and Baldwin 2002a; Calabrese et al. 2007).
FIGURE 2.
Dose-response curve showing hormesis in the form of a J-shaped curve and an inverted-U curve. In the former the effect on the ordinate is a biological dysfunction, such as carcinogenicity or any other indicator of toxicity. In the latter, the response is a normal biological function such as growth.
Biological stress responses, adaptive response, and preconditioning
Besides its biphasic nature, hormesis may be defined on the basis of evolutionarily conserved biological responses to stress (Calabrese et al. 2007). Stress responses are processes by which a small exposure to a stressful stimulus (e.g., a low dose) increases the resistance of the cell or organism to a moderate or severe level of stress (Arumugam et al. 2006). The relationship of hormesis to stress responses was foreseen by T.D. Luckey who proposed in 1968 that “subharmful quantities of any stressing agent will be stimulatory to the organism” (Luckey 1968). Through the years, different terminology has come into use to describe different aspects of a family of related biological responses, including hormesis, preconditioning, and adaptive response (Calabrese et al. 2007; Calabrese 2008a). Among the agents that induce stress responses are toxicants, heat, hypoxia, caloric restriction, metabolic products, oxidants, and ionizing radiation (Wolff 1989; Miura 2004; Calabrese et al. 2007; Rattan 2008). Such responses are phylogenetically widespread, occurring in prokaryotes, fungi, plants, and animals (Miura 2004; Calabrese et al. 2007).
A feature that distinguishes adaptive responses and preconditioning from hormesis as originally defined is that the latter occurs at low doses without dependence on an earlier exposure (Calabrese et al. 2007). Adaptive responses and preconditioning have also been called xenohormesis, autoprotection, and heteroprotection, the latter being distinguished by cross-resistance to agents other than the inducer itself (Calabrese et al. 2007). Because of their historical origins, hormetic responses have also been referred to as the Arndt-Schulz Law, Hueppe’s Rule, and the Yerkes-Dodson Law (Calabrese et al. 2007). A group of over 50 scientists recently made recommendations to achieve greater conceptual coherence in dose-response terminology and better interdisciplinary communication (Calabrese et al. 2007). The proposed terminology uses prefixes to identify the inducing agent (chemical, radiation, or physiological stress) and to distinguish whether a prior exposure is involved. Examples include radiation hormesis, radiation conditioning hormesis, chemical hormesis, and physiological conditioning hormesis.
Stress responses are commonly called an adaptive response in genetics (Jeggo et al. 1977; Cairns 1980; Wolff 1998; Mitchel et al. 2004; Chen et al. 2006a) and preconditioning in other areas of biomedical sciences (Murry et al. 1986; Arumugam et al. 2006; Calabrese et al. 2007; Lin et al. 2008). An adaptive response refers to a phenomenon whereby a small dose of a toxicant or radiation renders cells or organisms less susceptible to effects of a subsequent larger dose. The first adaptive response was discovered in E. coli, where the methylating agent N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) was found to have a smaller mutagenic effect if the bacteria had previously been exposed to a low dose of the same agent (Samson and Cairns 1977; Jeggo et al. 1977). The rate of arg reversion in E. coli strain AB1157 was about 6000-fold higher in an ada5 mutant that lacks the adaptive response (Cairns 1980). The adaptive response also confers resistance to other alkylating agents that operate by a similar mechanism; it involves more rapid repair of the premutational lesion O6-methylguanine in the exposed cells (Cairns 1980; Volkert 1988; Shevell et al. 1990; Kleibl 2002). The finding in bacteria was extended to mammalian cells, where chronic treatment with MNNG rendered the cells less susceptible to the induction of sister chromatid exchange by higher doses of MNNG (Samson and Schwartz 1980). Adaptive responses have also been characterized for agents that cause oxidative damage in bacteria (Demple and Halbrook 1983; Winquist et al. 1984; Christman et al. 1985; vanBogelen et al. 1987), yeast (Davies et al. 1995), and mammalian cells (Wiese et al. 1995; Chen et al. 2006a).
Ionizing radiation induces an adaptive response in mammalian cells (Wolff et al. 1988; Wolff 1998). Exposure to low doses of low linear-energy-transfer (LET) radiation (0.5–2 cGy x rays) makes the cells less susceptible to the induction of chromosomal damage by larger doses of either low-LET (Shadley and Wolff 1987; Shadley et al. 1987) or high-LET (α particle) radiation (Wolff et al. 1993). Studies in which damage was induced by restriction enzymes showed that double-strand breaks in DNA trigger the adaptive response (Wolff 1998). Low doses of radiation can also alter susceptibility to genotoxic and carcinogenic effects of chemicals, depending on the nature of the lesions induced and their intracellular processing. For example, γ-radiation was observed to reduce the clastogenic effects of bleomycin and mitomycin C in human lymphocytes but to increase their susceptibility to methyl methanesulfonate (Wolff et al. 1988), and extended exposure to γ-radiation at a low dose-rate (1.2 mGy / h) suppressed the skin carcinogenicity of 3-methylcholanthrene in mice (Sakai et al. 2006).
Thus, diverse agents are involved in adaptive responses, both as inducers and as agents protected against (Wolff et al. 1988; Wolff 1989; Ishii and Watanabe 1996; Mitchel 2006; Calabrese et al. 2007). The different adaptive responses have distinctive attributes and specificities, yet they share some overlapping components and functions (vanBogelen et al. 1987; vanBogelen and Neidhardt 1990; Miura 2004; Seo et al. 2006). They encompass diverse biological endpoints (Mitchel 2006; Seo et al. 2006; Calabrese et al. 2007). Adaptive responses have been studied most extensively in cell cultures and microorganisms, but they have also been reported in mammals in vivo (Kang et al. 2002; Mitchel et al. 2003, 2004; Sakai et al. 2006; Mitchel 2006, 2007), including effects on germ-cell mutagenesis (Boreham et al. 2006; Mitchel 2006).
A stress response called preconditioning, conceptually similar to the adaptive response, was first described by Murry et al. in 1986. Through experiments in dogs, they found that brief exposures to hypoxia resulting from mild coronary occlusion reduced the severity of myocardial infarction caused by a subsequent longer episode of coronary occlusion (Murry et al. 1986). Similar observations have since been made for other species and organ systems (Calabrese et al. 2007; Lin et al. 2008).
Relationship to the concepts of NOEL, NOAEL, and ZEP
The relationship of hormesis to the concepts of a NOEL (no observed effect level), NOAEL (no observed adverse effect level), and zero equivalent point (ZEP) warrants consideration. A NOEL holds that there is a dose below which there is no effect, an assumption that fits a threshold model. A NOAEL can fit either a threshold model or a hormetic model depending on whether lower doses have no effect or effects opposite to those of high doses. The ZEP has been defined for hormetic curves as a point that is equivalent to the control (Calabrese 2005a). As such, it is essentially equivalent to the NOAEL, but it can be useful conceptually, in that NOAEL includes “adverse” and can be confused with NOEL, while the ZEP only states that the curve reaches the control level at this point. A biphasic curve arises from responses above and below the ZEP being on opposite sides of the control or background level. Thus, the designation of a ZEP is well suited to the hormesis concept. Unlike a NOEL, it does not imply a threshold model, and unlike a NOAEL, it excludes “adverse” and is therefore neutral to notions of benefit and harm.
Relationship to the prediction of low-dose effects
In suggesting a qualitative difference between responses at low doses and those at high doses, the hormesis model runs counter to the assumption that effects at low doses can be predicted from those at high doses in a reasonably straightforward way. This often-unchallenged assumption is a keystone in risk assessment because direct measurements may not resolve what actually happens at low doses. Biological effects at extremely low doses are not readily measured experimentally or epidemiologically (Redpath et al. 2003a; Redpath 2006), and low-dose data are often compatible with more than one of the three basic models. Monotonic curves, whether linear or nonlinear, have the appealing feature of getting around this problem by substituting prediction for measurability. Loss of the assumption that high-dose responses can predict those at low doses would make risk estimation more difficult. Biphasic curves do not necessarily exclude the possibility of predicting low-dose effects, in that one could, in principle, extrapolate on the basis of a hormetic curve if the features of hormesis were quantitatively consistent and known. Moreover, in pointing out qualitative differences between the different parts of a dose-response curve, biphasic curves can highlight important responses that may otherwise be overlooked.
DEFAULT ASSUMPTIONS, BENEFIT, AND HARM
Whether a threshold model, LNT, or a hormetic model can serve as a default model for effects at low doses is controversial. In its simplest form, a “default” model refers to the most reasonable expectation for responses at low doses when there is insufficient evidence to know what actually happens in the low-dose range. To be used as a surrogate for low-dose data in risk assessment, one needs strong evidence that the model is generalizable, reliable, and quantitatively predictive of the specific biological response of interest for which data are unavailable (Calabrese 2004; Mushak 2007). A default model is typically driven by theory that explains the totality of the curve, such as target theory in the case of LNT, and by experimental evidence albeit with limited power at low doses. The kind of response predicted by a model would need to be highly prevalent for the model to qualify as a default assumption (Crump 2001).
Unfortunately, discussions of default assumptions sometimes leave vague whether the model is intended to shape expectations for the scientific interpretation of low doses or to define risks and allowable exposures. Striving for conservatism is common in risk assessment, in accordance with the view that it is better to overestimate risk than to underestimate it (National Research Council 1980). While the correctness of the model is essential for scientific purposes, it may be less so for the purposes of policy. One could conceivably favor hormesis as a default assumption for scientific interpretation of low-dose effects but accept an LNT or a threshold model for risk assessment related to public policy. Such a dichotomy, although defensible, has pitfalls, including adverse effects that may stem from basing policy on something less than the best science available.
Discussions of hormesis are beset with difficulties owing to the failure to separate the phenomenon from whether its effects are beneficial or harmful. Because toxicologists and radiation biologists have historically emphasized adverse effects at high doses, there is a tendency to assume that hormesis means that low dosages are beneficial. Although sometimes true, this extension of the definition of hormesis is an oversimplification. If a low dose of an inhibitory drug stimulates a detrimental hyperplasia, the hormetic zone would be detrimental. The disruption of endocrine regulation has been identified as a case in which the high-dose and low-dose zones of a biphasic curve may both be harmful, and studies of bisphenol A, the monomer of polycarbonate plastic, have been cited in support of this interpretation (vom Saal and Sheehan 1998; vom Saal and Hughes 2005; Timms et al. 2005). If a high dose of a drug inhibits a parasite and a low dose stimulates it, the human benefit is associated with the toxic zone, and we had better be aware of hormesis to avoid the hazard of the hormetic zone. Focusing on the possibility of beneficial effects at low doses has led some to oppose the hormesis concept because of a concern that the recognition of hormesis may lead to a weakening of environmental standards or public health protection (Axelrod et al. 2004). Nevertheless, rigidly linking hormesis to benefit can undermine objective inquiry. Nothing in the hormesis concept requires that hormetic effects are beneficial. Hormesis is best defined on the basis of biological and quantitative attributes, not benefit or harm (Stebbing 2000; Calabrese and Baldwin 2002a; Calabrese 2008a).
EVIDENCE FOR HORMESIS
The history of hormesis extends into the nineteenth century and has been reviewed by Calabrese (Calabrese 2002, 2005b, 2008a). The modern era of hormesis may be traced to a paper by Southam and Ehrlich (1943), who coined the term “hormesis” to describe modest stimulatory effects of extracts from western red-cedar heartwood on cultured fungi that were strongly inhibited by high concentrations of the same extract. Despite its long history, hormesis was not a serious consideration in toxicology through much of the twentieth century for a combination of scientific and historical reasons (Calabrese 2002, 2005b, 2008a). This began to change in the 1980’s and 1990’s with recurring observations of hormetic effects, surveys of scientific literature, and commentaries by scientists convinced that these observations were important (Sagan 1989; Calabrese and Baldwin 2001a, 2001b). The evidence that hormesis is real is now strong.
Hormesis is common in surveys of scientific literature. Over 45 years ago, Townsend and Luckey (1960) examined the pharmacology literature for evidence of hormesis, defined as the “stimulatory action of a subinhibitory amount” of a toxicant. They identified hormesis, which they called hormoligosis, on the basis of a biphasic dose-response relationship, called a “β-pattern,” and they cited over 100 such responses caused by diverse agents. A few decades later, the studies of toxicological literature became more systematic in a survey by Davis & Svendsgaard (1994) and culminated in the massive efforts of Calabrese and Baldwin (2001a, 2001b, 2003a), who used carefully delineated criteria for choosing papers for evaluation and found biphasic dose-response relationships to be widespread. Roughly 3000 published examples of hormesis had been reported by 2001 (Calabrese 2002; Calabrese and Baldwin 2001a, 2001b), and by 2005 the number had swelled to over 5500 dose-response relationships (Calabrese and Blain 2005). A database of hormetic responses encompasses phylogenetically diverse organisms, including bacteria, fungi, plants, insects, fish, birds, and mammals, including humans (Calabrese and Blain 2005). Both sexes and organisms of varied ages are represented, and there are about 900 agents from diverse chemical classes (Calabrese and Blain 2005). Besides studies in intact organisms, hormetic effects are common in cultured mammalian cells, including many tumor cell lines (Calabrese 2005a).
The analysis of data from systematic chemical testing provides strong evidence of hormesis. The Developmental Therapeutics Program (DTP) of the U.S. National Cancer Institute (NCI) has a longstanding program of high-throughput screening of chemicals for toxicity in order to identify prospective cancer chemotherapy drugs (Holbeck 2004). Over 87,000 chemicals have been tested in yeast, of which 2189 compounds went from preliminary screening into multiple-dose screening in 13 genetically different strains (Holbeck 2004). The responses were measures of yeast growth in 12 h in 96-well plates, determined as optical densities, at each of 5 concentrations (1.2, 3.7, 10, 33, 100 μM). Each OD value was expressed as a ratio of the chemical well to the mean of 8 concurrent solvent-control wells on the same plate. The determination was repeated on another day, and the average of the two values was considered one replicate. The NCI database reported the average of two replicates and the difference between them.
The NCI yeast data, comprising 56,914 dose responses, were analyzed by Calabrese et al. (2006, 2008) to determine whether they are more compatible with hormesis or a threshold model. A Benchmark Dose (BMD), roughly equivalent to a NOAEL or ZEP, was calculated as an estimate of the minimum dose causing toxicity (Calabrese et al. 2006). If the threshold model were correct, one would expect responses for doses below those causing inhibition to be randomly distributed above and below the control level. In contrast, one would expect a predominance of responses greater than the control if hormesis were prevalent. Growth at doses below the BMD was significantly greater than the control in all yeast strains for both highly toxic compounds (low BMD) and relatively non-toxic compounds (high BMD). The data for average growth are summarized in Table 1. The fact that these responses are all over 100% of control growth can be ascribed to hormesis. The pattern of responses was consistent with the hormetic model but not with the threshold model. Hormetic response patterns occurred about four times more often than expected by chance (Calabrese et al. 2006). Several different methods of analysis were consistent with one another in showing that hormesis is common in the yeast database (Calabrese et al. 2006, 2008).
TABLE 1.
Mean percentage of control growth of yeast strains for chemical exposures below the BMD: Values over 100% suggest hormesisa
| Dosage Range of the BMDb |
||||
|---|---|---|---|---|
| Strain | 3.7–11 μM (high toxicity) | 11–33 μM | 33–100 μM | >100 μM (low toxicity) |
| Wild type | 102.6 | 107.2 | 105.8 | 105.1 |
| SPY50780 | 106.1 | 108.3 | 108.8 | 105.5 |
| CLN2oe | 101.7 | 103.7 | 104.6 | 104.8 |
| mgt1 | 102.7 | 106.6 | 106.5 | 105.0 |
| mec2 | 105.4 | 107.3 | 105.8 | 106.0 |
| mlh1 | 103.8 | 107.2 | 105.9 | 104.5 |
| rad14 | 103.9 | 107.4 | 106.5 | 106.4 |
| bub3 | 104.8 | 106.0 | 106.8 | 106.0 |
| rad50EPP+ | 102.2 | 105.3 | 106.3 | 107.7 |
| sgs1 | 103.3 | 106.7 | 106.8 | 104.8 |
| rad52 | 103.6 | 106.8 | 105.9 | 104.0 |
| rad18 | 103.9 | 106.2 | 106.5 | 106.6 |
| rad50 | 102.9 | 105.5 | 104.4 | 104.7 |
| ∑(mean ± SEM)c | 103.6 ± 0.18 | 106.6 ± 0.18 | 106.2 ± 0.20 | 105.5 ± 0.17 |
Table based on Calabrese et al., 2006.
All values are based on a BMD(10) as defined in Calabrese et al., 2006. Other BMD criteria (2.5, 5, 7.5, 12.5) support the same interpretations. The column for 3.7<BMD<11 is based on responses at 1.2 μM; 11<BMD<33 is based on 1.2 and 3.7 μM; 33<BMD<100 is based on 1.2, 3.7, and 11 μM; and the BMD>100 column is based on responses at 1.2, 3.7, 11, and 33 μM.
The average number of responses on which a single mean value is based is 402 (196–572 range). The overall means are based on 5326, 6266, 4544, and 4763 responses for the four BMD ranges shown left to right.
Hormetic responses show descriptive and quantitative similarities beyond the biphasic curve that suggest conceptual coherence. Analysis of a database of over 5500 examples of hormetic inverted-U dose responses (Calabrese and Blain 2005) showed that hormetic effects tend to be modest, commonly differing from the control by 30–60% (Calabrese 2002, 2005a; Calabrese and Blain 2005; Calabrese 2008a). Thus, if differences are twofold or more, one should suspect that something other than hormesis is involved (Calabrese 2002). The hormetic zone occurs at exposure levels immediately below the NOAEL, and its width is variable. It extends to doses several-fold below the NOAEL, often 10- to 20-fold, but only rarely approaches 100-fold (Calabrese 2002, 2005a; Calabrese and Blain 2005). These patterns observed in the scientific literature are also supported by an analysis of an NCI database on 136 human tumor cell lines derived from over 30 tissues treated with 120 agents, including antineoplastic drugs, other drugs, pollutants, endogenous agonists, and plant compounds (Calabrese 2005a).
PHILOSOPHICAL QUESTIONS ABOUT HORMESIS
One may distinguish several bases for argument about hormesis: philosophical questions related to the hormesis concept, scientific issues, and controversial political or ethical issues. Philosophical objections to hormesis center around the question of whether there is epistemological warrant for the hormesis concept. Hormesis has diverse manifestations, and these may obfuscate conceptual clarity.
Those skeptical of the existence of hormesis or of its implications have argued that hormesis has suffered from the lack a stable definition, thus creating confusion about what hormesis is (Elliott 2000, 2006; Mushak 2007). Elliott (2000) has argued that there are seven distinct concepts of hormesis in the literature (Elliott 2000). He describes three of these as operational, three mechanistic, and one adaptive. The operational concepts are defined with respect to biological endpoints; the mechanistic concepts focus on causal interactions among constituents of a system; and the adaptive concept is a generalized response to biological stress. He concludes that none of these is sufficient to provide epistemological warrant for hormesis (Elliott 2000). Such taxonomic splitting may not be necessary for exploring the scientific basis for hormesis, but it does suggest that the complexity of hormesis may hinder conceptual clarity. For policy purposes, Elliott’s operational definitions, which center on the predictiveness of outcomes for defined endpoints at low doses, may be most germane, whereas the mechanistic concepts may be most fruitful for exploring the basic nature of the phenomenon (Hoffmann and Stempsey 2008).
If one takes the multifaceted nature of a concept as a philosophical limitation, one might similarly raise doubts about other central biological concepts. Evolution is the central paradigm of modern biology. As stated famously by Theodosius Dobzhansky about 40 years ago, “Nothing in biology makes sense except in the light of evolution” (Dobzhansky 1964). One may define evolution in various ways and recognize it as a multifaceted phenomenon, yet this does not alter its essential truth as a phenomenon and its ability to explain diverse observations in the biological sciences. A look at two well-known textbooks shows evolution to be defined as “change in the genetic makeup of a population with time” (Keeton and Gould 1986) or as “all the changes that have transformed life on Earth from its earliest beginnings to the diversity that characterizes it today” (Campbell et al. 1999). Evolution is a multifaceted concept (Kardong 2008), and one might distinguish at least three levels of discussion about it: 1) the fact of evolution—evidence that organisms change through time; 2) phylogeny, the historical course of evolution; and 3) mechanisms of evolution—Darwinian natural selection and other elements, including mutation, chromosomal alterations, and random changes in the genetic structure of populations. There is no informed disagreement about the first, but debate continues among evolutionary biologists concerning the second and third.
The parallel between evolution and hormesis leads me to discount arguments that the multifaceted nature of hormesis undermines its epistemological justification (Elliott 2000). Some concession should be made, however, that clarity about the meaning of hormesis is essential. If we are thinking of hormesis in the narrow sense of a biphasic dose response, we should be explicit about this, just as we should be explicit when we are using hormesis in the broad sense of a family of stress responses that manifest themselves in diverse ways. For the purposes of policy related to toxicology, the operational definitions of hormesis—centered squarely on the biphasic dose response—have the most central importance.
SCIENTIFIC ISSUES GENERATING DEBATE ABOUT HORMESIS
The first question that one may ask about hormesis is whether it is real. An accumulation of evidence calls for an affirmative answer. Questions that continue to generate debate about hormesis center around impediments to detecting hormesis, artifacts that resemble hormesis, difficulties in evaluating the prevalence of hormesis from scientific literature, the paucity of experiments specifically designed to study hormesis, and insufficient understanding of mechanisms.
Impediments to detecting and measuring hormesis
Most scientific objections to hormesis stem from its being a low-dose phenomenon. Toxicology is centered on studying adverse effects, and this led to a focus on high doses where toxic responses are readily detected and measured. If preliminary experiments include low doses, these are commonly underrepresented in follow-up studies where adverse effects are of interest. Even if a study includes low doses, unequivocal evidence of hormesis may be elusive because of a lack of statistical power. Hormetic effects tend to be modest, and differences from background levels of the biological effect are small (Calabrese and Baldwin 2001b; Calabrese 2003). Without systematic analysis of large data sets or large experiments with multiple sub-NOAEL doses, hormetic effects are commonly ascribed to random variation. Data that appear to be hormetic do not necessarily exclude other models.
Even studies conducted on a large scale to increase the power to detect small differences can be consistent with several models. The large ED01 study, in which the National Center for Toxicological Research (NCTR) used over 24,000 mice to study the dose-dependence of the carcinogenicity of 2-acetylaminofluorene (AAF), gave responses that were described as nonthreshold for liver carcinogenesis and threshold for bladder (reviewed in Eaton and Gilbert 2008). The ED01 study was also controversial with respect to hormesis. A task force of the Society of Toxicology (SOT) pointed out that lifespan was longer and the incidence of bladder tumors lower in animals receiving low doses of AAF than in the controls (Society of Toxicology Task Force 1981; Bruce et al. 1983), a claim contested by the NCTR (Kodell et al. 1983). The SOT Task Force (Bruce et al. 1983) concluded credibly that even in such a large study “statistical uncertainty makes it impossible to establish the true shape of the dose response curve.” In a later debate about thresholds in ED01 (Waddell 2003), Melvin Andersen and colleagues noted that the existence of thresholds is unlikely to be fully resolved by empirical modeling of dose-response data or by experiments using more animals per group but, rather, will depend on mechanistic evidence for thresholds (Andersen et al. 2003). This prediction has bearing on low-dose responses more broadly, including an understanding of hormesis.
Apparent hormesis arising as an artifact
Among the difficulties that affect the observation of hormesis is the existence of artifacts that can falsely give the appearance of hormesis where it does not exist. If one pools endpoints into a composite (e.g., carcinogenesis, pooling tumors at several sites), one may generate hormetic curves in the absence of real hormesis; for example, if a modest decline in the first endpoint at low dose (e.g., tumors at site A) is offset by a larger increase in another endpoint at higher doses (e.g., tumors at site B), the composite (i.e., tumors in general) can take the form of a hormetic J-shaped curve (Thayer et al. 2005). In measuring a decline in biological function owing to toxicity, a control that is atypically low may cause measurements at low doses, even if slightly toxic, to seem hormetic (Thayer et al. 2005). Likewise, in measuring an adverse effect, a control with an atypically high spontaneous incidence can create the illusion of hormesis at low doses. The existence of such possibilities does not argue against the existence of hormesis in general, but it does emphasize the need for critical judgment and an awareness of potential artifacts in the assessment of hormesis.
The distinction between an essential substance and a xenobiotic toxicant is usually clear (Davis and Svendsgaard 1990), but there is some potential for confusion (Kefford et al. 2008). Adverse effects occur when physiologically essential chemicals are present at doses that are either too low or too high (Gaylor 1998). Essentiality can mimic hormesis when nutritional stimulation merges with toxicity (Kefford et al. 2008). If one measures growth or another indicator of biological function at different concentrations of an essential chemical, one sees no growth at dose zero, increases in growth with increasing concentration in a zone of deficiency, an optimum, and then a decline with the onset of toxicity at high dose. In natural environments, many substances are present at doses above zero, so the ordinate is effectively moved to the right, and this can create a curve resembling a hormetic inverted U. Confusion between essentiality and hormesis is unlikely if the optimum is broad, because the effects would appear as two curves in different dosage ranges. In ecological settings, however, essentiality may artifactually resemble hormesis. For example, if a toxicant or complex mixture contains a mineral nutrient that stimulates plant growth in the subtoxic zone, the dose-response curve may not differentiate this stimulatory response from hormesis.
Evaluating the prevalence of hormesis
Hormesis is commonly observed in toxicological literature, but debate continues about its generality. The existence of many biphasic curves does not demonstrate the prevalence or universality of hormesis. Difficulties in using published literature to evaluate the prevalence of hormesis are that many studies have too few doses in the low-dose range, and the choice of studies for evaluation may introduce bias. Disagreement persists on whether hormesis can be described as rare, occasional, common, highly prevalent, or even universal. The evidence of the last decade argues that rare or occasional are not apt descriptions, but the approach to universality is a high hurdle.
The accumulation of examples of hormesis has led proponents, especially Edward Calabrese, to argue with growing confidence that the fundamental nature of dose-response relationships in toxicology needs to be reevaluated (Calabrese and Baldwin 1999; Calabrese et al. 1999) and that hormesis should be the “default” assumption in toxicology (Calabrese and Baldwin 2003c) and toxicological risk assessment (Calabrese 2004, 2008a). Skeptics challenge the assertion that hormesis is highly prevalent or universal, do not agree that it is broadly generalizable, and reject the idea that it can serve as a default assumption (Kitchin and Drane 2005; Thayer et al. 2005; Mushak 2007). Crump (2001) noted the lack of a statistical test that specifically evaluates hormesis. While this assertion is essentially correct, it tends to minimize the value of statistical measures that have been used to analyze specific extensions of the hormesis hypothesis, such as whether low-dose responses differ significantly from the control in the direction consistent with hormesis. Such statistical measures, however, are not free of controversy; for example, there is disagreement about whether appropriate corrections are made for multiple comparisons (Kitchin and Drane 2005). Recent analyses of biphasic dose responses have increasingly taken advantage of statistical modeling techniques (Schöllnberger et al., 2001, 2006; Cedergreen et al., 2005; Cox, 2005; Calabrese et al., 2008; Leonard, 2008).
Crump (2001) has argued that one cannot judge the generality of hormesis on the basis of there being many examples in the scientific literature because of the lack of a reliable denominator that serves as a basis for quantifying the prevalence of hormetic curves. This criticism is justified when applied to the many papers that point out examples of hormesis but do not rigorously evaluate its prevalence relative to other models in a clearly defined number of studies. Such studies do not consider rates of false positive responses; and they often do not correct adequately for possible bias in the selection of studies that seemed hormetic.
Progress in addressing these criticisms was made by Calabrese and Baldwin (2001a) in a survey of over 20,000 articles in three toxicology journals. Rigorous a priori entry and evaluative criteria were used, and studies were chosen for inclusion on the basis of whether they permitted an evaluation of the low-dose zone, not on whether they seemed to show hormesis. Of roughly 20,000 articles, only 1% (195/ 20,285) had sufficient experimental designs, and they contained 668 dose-response relationships that could be evaluated. The majority of studies were excluded because they did not have appropriate controls, at least two doses below the NOAEL, or a toxic response at high dose (Calabrese and Baldwin 2001a,b). Most dose-responses that met the entry criteria were inconclusive, but about 37% (245/668) provided evidence of hormesis, as indicated either by statistical significance or by a 10% difference from the control in ≥3 sub-NOAEL doses (Calabrese and Baldwin 2001b). The issue of false positives was tackled by evaluating the relative number of positive and negative responses at sub-NOAEL doses, using the assumption that if random variation caused false positives, their number would be equal to responses deviating from the control in the opposite direction. The occurrence of false positives could not explain the excess of apparently hormetic responses. While 80% of responses below the NOAEL did not differ significantly from the control, 19.5% differed significantly from the control in the direction expected for hormesis, while 0.6% differed from the control in the opposite direction, a 32-fold difference in support of hormesis (Calabrese and Baldwin 2001a).
Depending on the stringency of the criterion used for declaring a response nonmonotonic, one might conclude that 19.5 to 37% of the responses in the large survey of Calabrese and Baldwin (2001a) gave evidence of hormesis. A smaller survey by Davis and Svendsgaard (1994) had earlier estimated 12 to 24%. In a subsequent analysis by Calabrese and Baldwin (2003a), predictions of a threshold model were compared with those of the hormesis model for 1800 published doses below the NOAEL in 664 dose-responses. The a priori criteria for inclusion were having a toxic dose, a NOAEL, two doses below the NOAEL, and a concurrent control. Whereas the threshold model would predict a ratio of approximately 1:1 for responses above and below the control in the sub-NOAEL zone, a ratio of 2.5:1 was observed (Calabrese and Baldwin 2003a).
Although it is now clear that hormesis is evident in literature studies that have a well-defined denominator, it should be kept in mind that other tabulations of hormetic responses still have an inherent limitation if the denominator is not defined (Crump 2001, Mushak 2007). Moreover, there is no agreement on the frequency at which hormesis must be demonstrable to make a persuasive case that it is a generalizable phenomenon. Thus, findings that 12–24% (Davis and Svendsgaard 1994) or 19.5–37% (Calabrese and Baldwin 2001a) of papers that qualify for evaluation are nonmonotonic have been used both as an argument for (Calabrese and Baldwin 2001a) and against (Mushak 2007) the prevalence of hormesis. Such differences of interpretation call for restraint in asserting the generalizability of hormesis, but they do not negate the fact that hormetic responses are widespread.
Hormesis is observed throughout the phylogenetic spectrum, including bacteria, fungi, algae, plants, insects, other invertebrates, fish, birds, and mammals (Calabrese and Baldwin 2001a, 2003a; Calabrese and Blain 2005). It encompasses diverse biological endpoints, including those that have been categorized as metabolic, growth, reproductive, molecular, behavioral, physiological, or survival (Calabrese and Baldwin 2001a, 2003a). The diversity includes radiation and varied organic and inorganic chemicals and mixtures (Calabrese and Baldwin 2001a, 2001b, 2003d, 2003e, 2003f; Calabrese and Blain 2005). No broad category of organism, endpoint, or agent consistently fails to give evidence of hormesis. Such diversity suggests that the phenomenon of hormesis is generalizable beyond the many specific examples (Calabrese 2008a).
Paucity of experiments designed to evaluate whether responses are hormetic
Despite the interest that hormesis has generated, there are relatively few experiments specifically designed to evaluate whether responses are hormetic. Therefore, the occurrence of hormesis is often identified in studies conducted for reasons other than measuring hormesis. As a consequence, there is a shortage of data on the reproducibility of hormetic responses (Kitchin and Drane 2005). More attention to low doses is a pressing need (Calabrese and Baldwin 2002a; Calabrese 2008a), both for evaluating the nature of hormesis and for evaluating the hypotheses of those who claim that adverse effects of low-doses may sometimes be greater than expected under linear or threshold models. The latter include the possibility that endocrine disruptors have adverse effects at doses that would otherwise be considered nontoxic (vom Saal and Sheehan 1998; Timms et al. 2005; Welshons et al. 2003; vom Saal and Hughes 2005). The ability to detect hormesis is favored by there being several closely-spaced doses immediately below the NOAEL (Calabrese 2008a), such as half-log or twofold increments, and enough statistical power to resolve small changes. Many toxicological studies do not fulfill these requirements, and even if they do, the observation of hormesis may depend on temporal components in the relationship of exposure to response that were not included in the study design, such as time required for induction of an adaptive response (Calabrese and Baldwin 2001b).
While it is true that there are relatively few studies specifically designed to measure hormesis, it is not true that experiments clearly showing hormesis are lacking. What is needed is an assay with appropriate background levels of response and the sensitivity to measure small changes. Unequivocal evidence of hormesis has been obtained by Redpath and colleagues using an in-vitro assay for neoplastic transformation in the HeLa x human skin fibroblast hybrid cell line CGL1. X rays of an energy widely used in diagnostic radiology (60 kVp) caused hormesis, indicated by transformation frequencies less than the spontaneous frequency at doses <10 cGy (Redpath et al. 2003a). The maximum hormetic effect was at 0.1 cGy (Redpath et al. 2003a; Redpath 2006). The observation of hormesis depended on a delayed plating scheme, whereby cells were seeded after 24h of post-irradiation holding (Redpath et al. 2003a). The time requirement for expression of the hormetic effect is parallel to the importance of time for the expression of adaptive responses in conditioning hormesis.
Hormesis was also observed after exposure of CGL1 cells to low doses (≤10 cGy) of 137Cs γ rays (Redpath et al. 2003a; Redpath 2006). While not exactly parallel, the two studies provide a preliminary comparison of the two treatments (Redpath et al. 2003a). The higher-LET 50 kVp x rays had a lower threshold for damage and caused greater damage at high doses than the more energetic γ rays. However, their relative biological effectiveness (RBE) appeared to be higher not only for inducing damage at high dose but for protecting against damage at low dose (Redpath et al. 2003a). Both the X- and γ-ray assays clearly show hormesis. While hormesis is usually observed at doses <5-10 cGy (Redpath et al. 2003a; Redpath and Elmore 2007), very low dose rates of low-LET radiation (~ 0.5 mGy/min) produced hormetic effects at higher doses, and no transformation was induced even at doses as high as 1 Gy (Elmore et al. 2006; Redpath and Elmore 2007). Mechanisms of radiation hormesis in the HeLa x fibroblast transformation assay apparently involve the induction of DNA repair and the selective death of a subpopulation of cells prone to spontaneous transformation at low doses (Redpath et al. 2003a, 2003b; Redpath 2006).
Another exquisitely sensitive assay has permitted the detection of hormetic effects for a genetic endpoint in mice in vivo. Sykes and colleagues have developed assays for the detection of chromosomal inversions that arise by somatic intrachromosomal recombination (SICR). The SICR assays are based on a genetic construct that permits the detection of inversions in various tissues of pKZ1 transgenic mice or in pKZ1 cell cultures in vitro (Sykes et al. 2006). The transgene has the E. coli lacZ gene in reverse orientation with respect to a chicken β-actin promoter. It is flanked by mouse immunoglobulin gene recombination signal sequences Jκ5 and Vκ21C. When an inversion occurs in the transgene, the lacZ gene is brought into correct association with the β-actin promoter, and expression of the lacZ gene can be detected by a blue staining reaction (Sykes et al. 2006). Inversions in the pKZ1 assay probably arise through the activity of proteins involved in repair of DNA double-strand breaks by nonhomologous end joining (Sykes et al. 2006). The pKZ1 assay is especially sensitive to low doses of DNA-damaging agents in lymphoid tissue (Sykes et al. 2006). Genetic effects are detected at doses over 1000-fold lower than those detected in other mouse assays (Hooker et al. 2002; Sykes and Day 2007). High spontaneous frequencies of inversions in the pKZ1 assay (~1.6 x 10−4 in spleen) make it practical to measure decreases in inversion frequency, which occur with hormesis, as well as the increases induced by genetic damage at high doses (Hooker et al. 2002; Sykes et al. 2006; Sykes and Day 2007).
Etoposide, a DNA topoisomerase II inhibitor that interferes with DNA and RNA synthesis and is genotoxic in various assays, is an effective inducer of SICR in the pKZ1 assay (Hooker et al. 2002). Doses of 0.05 to 50 mg/kg in a single intraperitoneal injection increased SICR inversions 1.4- to 3.1-fold. However, inversion frequencies after treatment with 0.0005 and 0.005 mg/kg were decreased significantly to 0.67 and 0.43 of the control levels (Hooker et al. 2002). Thus, there was a hormetic effect. Hormesis is also detected in pKZ1 cells in vitro. A pKZ1 mouse hybridoma cell line exposed to etoposide showed 2.3- and 4.6-fold increases in inversion frequencies at 100 and 1000 nM, respectively. However, there was hormesis at low doses, with inversion frequencies decreased to 0.31 and 0.5 of control levels at 1 and 10 nM, respectively (Hooker et al. 2002).
Understanding of mechanisms
A criticism that has been raised about the hormesis concept is that there is insufficient understanding of mechanisms to form a coherent view of hormesis within the broader context of biology (Stebbing 2003; Kitchin and Drane 2005; Thayer et al. 2005). It is true that establishing the mechanistic foundations of hormesis is critical. On the other hand, hormesis is well-documented at the phenomenological level, and it increasingly appears to be a manifestation of a broad family of stress responses (Calabrese et al. 2007), including adaptive responses and preconditioning. Some of these responses are reasonably understood at a mechanistic level. While hormesis is still primarily defined on the basis of dose-response relationships, there is a growing appreciation for mechanisms that can explain hormetic effects. The evidence suggests that there is no single mechanism of hormesis but, rather, many mechanisms that yield the common features of the hormetic dose response (Calabrese 2008a). Table 2 gives examples of mechanisms that can explain hormetic effects.
TABLE 2.
Mechanisms Contributing to Hormetic Responses at Low Doses
| Overcompensation to a disruption in homeostasis by overshooting homeostatic feedback controls. |
| Adaptive responses based on inducible repair processes. |
| Enhanced defenses against oxidative stress. |
| Activation of transcription factors and upregulation of genes for cytoprotective proteins, growth factors, cytokines, and enzymes involved in various signaling pathways. |
| Interaction of exogenous agents with stimulatory and inhibitory receptor subtypes of endogenous regulatory systems. |
| Interactions among cell proliferation, cell-cycle delay, apoptosis, and DNA damage. |
| Selective induction of apoptosis in transformed cells and death of cells predisposed to spontaneous transformation. |
| Preferential induction of adaptive responses in normal cells relative to cancer cells. |
| Enhancement of gap junction intercellular communication at low doses but inhibition at high doses. |
| Enhanced immune responses. |
One might expect the first interaction between a toxicant and its target to follow the law of mass action and exhibit a monotonic response. However, in a complex biological system various mechanisms can lead to nonlinearity. Deviations from linearity are favored by the occurrence of multiple concurrent and sequential events in toxicologic responses. In some instances, a biphasic response can be understood as the superimposition of monotonic responses of the system’s components on one another (Conolly and Lutz 2004). In other cases, biphasic curves are likely to result from complex interactions of an exogenous agent with a unitary biological system (Conolly and Lutz 2004; Fukushima et al. 2005). Mechanisms that can contribute to hormesis are found in literature on the hormesis concept itself (Calabrese and Baldwin 2001c; Calabrese 2002), quantitative aspects of dose-response curves (Conolly and Lutz 2004; Fukushima et al. 2005) and stress responses (Mattson et al. 2004; Miura 2004; Arumugam et al. 2006; Calabrese et al. 2007).
A combination of experimental evidence and credible speculation suggests that hormesis occurs when a system overcompensates for a disruption in homeostasis, effectively overshooting homeostatic feedback controls (Calabrese 2002; Conolly and Lutz 2004) and that the modest overcompensation reestablishes homeodynamic balance (Rattan 2008). Hormetic mechanisms for coping with oxidative stress are apt to involve enhanced antioxidant defenses, including the generation of endogenous scavengers, quenching agents, and enzymes of detoxication (Benzie 2000; Miura 2004; Arumugam et al. 2006; Chen et al. 2006b). Hormesis and adaptive responses can arise from the activation of transcription factors and upregulation of genes encoding cytoprotective proteins (e.g., heat-shock proteins), growth factors, cytokines, and enzymes involved in various signaling pathways (Stecca and Gerber 1998; Mattson et al. 2004; Miura 2004; Arumugam et al. 2006). Interactions of exogenous substances with stimulatory and inhibitory receptor subtypes of endogenous regulatory systems have been identified as mechanisms underlying some hormetic responses (Calabrese and Baldwin 2001c; Calabrese 2002, 2008a; Conolly and Lutz 2004; Joëls 2006), as have enhanced immune responses (Conolly and Lutz 2004). Differences in minimal doses required to trigger protective and detrimental effects involving interactions among cell proliferation, cell-cycle delay, apoptosis, and DNA damage (Rouse and Jackson 2002; Conolly and Lutz 2004; Fukushima et al. 2005; Kinoshita et al. 2006) could lead to hormesis in several ways. Selective induction of apoptosis by low levels of oxidative damage or radiation in transformed cells compared to normal cells (Heigold et al. 2002; Bauer 2007; Portess et al. 2007) and death of cells predisposed to spontaneous transformation (Redpath et al. 2003a; Redpath 2006) fit this general pattern. Preferential induction of adaptive responses in normal cells relative to cancer cells may similarly contribute to hormetic responses (Ishii and Watanabe 1996; Park et al. 1999).
Some adaptive responses, also called conditioning hormesis (Calabrese et al. 2007), have been ascribed to the induction of repair processes that remove damage caused by the inducer (Shevell et al. 1990; Wolff 1998; Kleibl 2002; Miura 2004). If the repair mechanism also removes damage caused by background levels of exposure or endogenous agents, it can yield a biphasic hormetic curve (Kodell 2001; Conolly and Lutz 2004). Alteration of cell cycle regulation after a conditioning dose is another mechanism favoring an adaptive response through more effective DNA repair (Miura 2004). Gap-junction intercellular communication (GJIC) is implicated in hormetic mechanisms, in that its disruption can block an adaptive response to ionizing radiation (Ishii and Watanabe 1996). Enhancement of GJIC at low doses, combined with its inhibition and loss of function at high doses, is a mechanism associated with hormesis for nongenotoxic carcinogens (Fukushima et al. 2005) and physiological preconditioning (Lin et al. 2008). A possible mechanistic basis for U-shaped curves with endocrine disruptors is that high doses may trigger a reduction in the number of hormone receptors in hormone-responsive cells, while lower doses lead to an increase in receptors (vom Saal and Sheehan 1998).
POLITICAL AND ETHICAL ISSUES CAUSING CONTROVERSY ABOUT HORMESIS
The intensity of the debate about hormesis is often centered on its societal implications rather than on the science itself. There is much argument about whether hormesis should enter into policy related to effects of low-dose exposures. Emotions run high owing to fear that the recognition of hormetic effects can undermine protections of human health and environmental quality. The hormesis concept suggests that low doses may be beneficial, but defining what is actually beneficial, considering both short-term and long-term consequences, is complex. It can be a serious error to conclude overall benefit on the basis of a single biological endpoint at a particular point in time. Thorough analysis is critical to avoid a premature claim of hormesis. Ignoring benefit, a hormetic model also suggests that low doses pose less risk than predicted by an LNT model. While this may be seen as good news, it can also be seen as an argument for weaker protective standards. Thus, the intensity of the debate is fueled by concerns about the ethical, social, and legal implications of hormesis. Disagreements about hormesis are often exacerbated by a tendency to conflate science and policy, blending what are fundamentally scientific questions with what are essentially social or political questions. Separating the two cleanly may be nearly impossible owing to the social factors and value judgments that enter into scientific practice. Nevertheless, failing to maintain an awareness of the boundaries can hinder both scientific understanding and the path to sound policies (Hoffmann and Stempsey 2008).
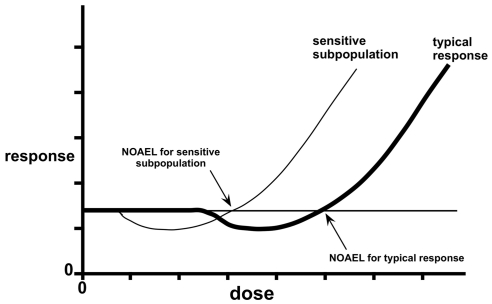
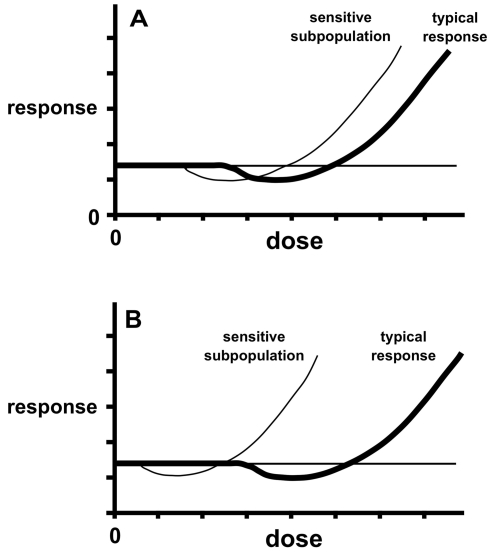
The unique attributes of biphasic responses are central to the controversy. A threshold model (Figure 1A) holds that there is a dose, approximated by a NOAEL, below which there is no net biological effect, either positive or negative. Under an LNT model (Figure 1B), all doses are considered to have an adverse effect. Thresholds and LNT are inherently simpler than hormesis for risk assessment because they lend themselves to the sole objective of avoiding harm. Under a threshold model, one seeks to be in the no-effect zone, and a safety factor may be imposed below the NOAEL to ensure the avoidance of harm. In the case of LNT, the objective is to be low enough on the dosage scale that the risk is negligible or acceptable. Hormesis raises the question of whether one should strive to be far enough below the zone of biological activity to ensure avoidance of harm or whether to permit or even encourage low-dose exposures that may be beneficial. The questions escalate in complexity when one contemplates the relationship of biphasic curves to heterogeneity among endpoints and agents, sensitive subgroups in populations, interactions among agents, and the political and ethical questions raised by these issues (Hoffmann and Stempsey 2008).
FACTORS SHAPING VIEWPOINTS ON HORMESIS AND RISK ASSESSMENT
It is not surprising that hormesis has been an unwelcome intruder in toxicological risk assessment, given that biphasic curves pose new challenges (Hoffmann and Stempsey 2008), and most views of dose-response relationships have been shaped by linear and threshold models. Factors that affect viewpoints on hormesis in relation to risk assessment include divergent perceptions of benefit and risk associated with a hormetic response, the practicality of assimilating hormesis into the estimation of hazards, beliefs about whether policy decisions should be based on the best available science, and ethical principles.
Even if there is agreement that a response is hormetic, that knowledge may trigger different perceptions of risk and benefit. Table 3 presents divergent views in order of decreasing optimism about benefit and increasing perception of hazard. All these views can be found among scientists receptive to the hormesis concept. The difficult challenges that one would encounter in practical applications undoubtedly shape the broad range of views found among those who are persuaded of the reality and common occurrence of hormesis.
TABLE 3.
Divergent Perceptions of Benefit and Risk Associated with a Hormetic Response
| In order of perceived risk: |
|
If we allow threshold and linear models to dominate our thinking about low doses, we are at risk of basing policies on incorrect models in the face of growing evidence for hormesis. At the same time, the policies themselves may be in the public interest, in that the errors tend to overestimate risk rather than underestimate it. There is a longstanding preference for excessive caution, rather than too little caution, in policy decisions, which is the foundation on which the assimilation of a precaution-ary principle into regulatory decisions is based. On the grounds of uncertainty, one might make a deliberate policy decision not to apply hormesis to risk assessment (Hoffmann and Stempsey 2008). If so, one should do so without conflating science and policy to the extent of arguing against the existence of hormesis. Risk assessment should be undertaken with cognizance of the best available scientific evidence. Denying the existence or common occurrence of hormesis in the face of growing evidence does not protect public health or the environment, but thoughtful policy decisions made with cognizance of hormesis can.
RELATIONSHIPS TO ETHICAL PRINCIPLES
Biomedical ethics has traditionally recognized four clusters of ethical principles: respect for autonomy, beneficence, nonmaleficence, and justice (Beauchamp and Childress 2001). While autonomy and justice have had growing recent emphasis, nonmaleficence and beneficence have historically formed the core of medical ethics. Nonmaleficence entails avoiding the causation of harm, while beneficence entails the conferral of benefit, typically in balance with risks and costs (Beauchamp and Childress 2001).
The bioethical principles of nonmaleficence and beneficence are germane to the prospect of assimilating hormesis into regulatory policies (Hoffmann and Stempsey 2008). Most policies related to toxicology are based on nonmaleficence—avoidance of harm. As an ethical principle, nonmaleficence has its roots in the medical maxim “above all, do no harm.” Although the first book of the Epidemics of Hippocrates actually advised that physicians both avoid harm and do good, ethical theory typically gives a higher priority to nonmaleficence (Ross 1988; Beauchamp and Childress 2001).
The biphasic curve of hormesis (Figure 1C), unlike threshold and linear responses, holds the prospect not only of avoiding harm in the toxic zone but of accruing benefit in the hormetic zone. If we assume that the hormetic zone for a substance can be precisely defined without risk of inadvertently entering the toxic zone, policies could, in principle, be formulated so as to allow optimum exposures rather than maximum permissible exposures. Attempting to harvest the benefit of the hormetic zone would entail a shift in ethical principle to beneficence, displacing the nonmaleficence that characterizes most current policies on toxicants (Hoffmann and Stempsey 2008). There is precedent for public-health policy based on beneficence, such as fluoridation of water supplies and mandated vaccination programs. Such measures often entail controversy, but the public largely accepts them if the evidence is clear. Cook and Calabrese (2006) have argued that hormesis opens the possibility of improving public health, rather than striving to maintain disease and disability near background levels, thus promoting public health rather than only protecting it. Such a change from nonmaleficence to beneficence would have to resolve whether it is possible to define benefit precisely to optimize health without incurring an unacceptable risk of harm. In considering hormesis in a policy setting, it is necessary to balance nonmaleficence and beneficence with a higher priority given to the former.
HORMESIS AND THE ESTIMATION OF HAZARDS
Regulating for hormesis would pose formidable information demands. One must know that the hormetic zone can be precisely targeted without risking harm. Accruing a hormetic benefit implies regulating to a level closer to the ZEP or NOAEL than is currently done. Asymmetry around the NOAEL is an inherent problem, in that hormetic effects tend to be modest, whereas toxic effects can be large. The requirement for greater precision in identifying points of transition in dose-response relationships is a formidable challenge because of the difficulty of measuring effects at low doses.
Precise knowledge of a chemical’s effect for a given endpoint or genotype does not provide a basis for extrapolation to other situations. For example, the same doses of cadmium chloride that appear to show hormetic effects for testicular tumors (Waalkes et al. 1988; Calabrese and Baldwin 1998; Calabrese and Baldwin 2003d) induce prostate tumors (Waalkes et al. 1988; Thayer et al. 2006). Testicular carcinogenesis is apparently impeded at low doses because it depends on testicular degeneration (Waalkes et al. 1988), and prostate carcinogenesis is impeded at higher doses where testicular toxicity interferes with the testosterone production on which it depends (Goyer et al. 2004). To avoid concluding safety or benefit on the basis of inappropriate or insensitive indicators, one must know that a presumptive benefit is not offset by adverse effects elsewhere (Davis and Svendsgaard 1990; Davis and Farland 1998; Mushak 2007), and it may not be obvious which endpoints are most sensitive for a given exposure. Those who think that hormesis is ready for use in risk assessment for chemicals, including chemical carcinogens, might consider accepting the challenge of Paul Mushak, a critic of hormesis who has argued that proponents “have not yet laid out a convincing methodologic schematic that actually walks the reader or risk assessor through a hormesis-based quantitative risk assessment” (Mushak 2007).
There is little disagreement that underestimation of hazards can lead to insufficient protection of public health and the environment. In contrast, overestimation of risk is often considered benign, as reflected in the phrase “making errors on the side of safety.” However, there is room for disagreement on this point, as an overestimation of risks can have such costs as greater regulatory burden than needed and hindrance of the development and use of improved products and technology.
LNT has received support in radiation risk assessment even after mechanisms that can cause departures from linearity were known because it would tend to overestimate risk rather than underestimate it. This was stated directly in the 1980 report of the NAS Committee on the Biological Effects of Ionizing Radiation (National Research Council 1980), which recommended the use of LNT for “reasons of prudent conservatism,” but acknowledged that the available data did not permit a rigorous evaluation of what actually happens at low doses. The latest NAS report continues its support of LNT (National Research Council 2006). Arguments for LNT can be advanced on its merits as a conservative approach and a simple tool in risk assessment or on its merits as a model that describes actual dose-response relationships (Breckow 2006; Friedl and Rühm 2006). While there is disagreement on both counts, a coherent argument can be made for the former more readily than the latter, and the boundary between scientific interpretation and policy judgment should not be blurred.
Many would agree that the avoidance of health risks should be weighed more heavily than economic interests. However, the view that overestimating risk may lead to expenditures for minimizing exposures but cannot threaten public health is questionable. The Joint Report of the French Academies (Aurengo et al. 2005; Tubiana and Aurengo 2006) warned that there can be health risks associated with an exaggeration of hazards of low doses of ionizing radiation if it leads to an avoidance of beneficial diagnostic procedures or therapy. More pointedly, Scott and Di Palma (2006) have argued that trust in LNT has led to fear of diagnostic procedures that use doses for which hormesis is likely (e.g., dental x rays, chest x rays, mammograms, thyroid scans). Although there is disagreement on the likelihood that fear of radiation exposure will lead to unwise avoidance of diagnostic or therapeutic procedures (Scott and Di Palma 2006; Tubiana and Aurengo 2006; Hall and Brenner 2008), a balanced perspective should recognize that there can be detrimental effects both of underestimating and of overestimating risks.
POSSIBLE CONSEQUENCES OF IGNORING OR REJECTING HORMESIS
Ignoring or rejecting hormesis can be detrimental to science and public health. A better understanding of hormetic effects can yield benefits in various areas of the biomedical and environmental sciences.
Effects on low-dose research
Recognition of hormesis calls for more research on effects of low doses and biological stress responses. There is less incentive for research on these subjects if one believes that everything of interest occurs in the high-dose range and that effects at low doses are predictable from those at high doses. A failure to recognize the unique biological responses that occur in the low-dose zone can stifle progress in diverse areas of public health, medicine, agriculture, and environmental sciences.
Biological stress responses and health optimization
Stress responses are important to muscular, skeletal, cardiovascular, and neurological health (Arumugam et al. 2006; Radak et al. 2008). It has been suggested on this basis that biological systems must routinely experience mild stress for optimization of health (Calabrese 2008a) and that hormetins—agents that induce hormesis—can contribute to healthy aging, which is favored by mild and periodic physical and mental challenges but disfavored by severe or chronic stress (Rattan 2008). Even though the effectiveness of stress responses tends to decline with age (Miura 2004; Rattan 2008), such factors as exercise, cognitive stimulation, and calorie restriction may improve health, extend lifespan, and lessen the risk of such age-related disorders as Alzheimer’s disease, Parkinson’s disease, and stroke through what have been called “hormesis-like mechanisms” (Mattson et al. 2004; Arumugam et al. 2006).
The beneficial effects of exercise can be considered as the hormetic zone of a biphasic curve, with adverse effects on one side caused by inactivity and on the other by excessive exercise and overtraining (Radak et al. 2008). The generation of reactive oxygen species apparently plays a central role in causing modest levels of oxidative damage to macromolecules, triggering stress responses that combat oxidative stress (Radak et al. 2008). Common features of neurodegenerative disorders that make them subject to beneficial effects of conditioning are increased oxidative stress, impaired energy metabolism, and disruption of cellular calcium homeostasis (Mattson et al. 2004). Biphasic dose responses are reported in diverse areas of neurosciences, including neuroprotection, neurite outgrowth, and responses to drugs for Alzheimer’s disease, Parkinson’s disease, anxiety, pain, seizures, and stroke (Calabrese 2008b, 2008c). Accumulating evidence in support of a “hormesis hypothesis of disease resistance and longevity” (Arumugam et al. 2006) gives impetus to achieving a fuller understanding of biological stress responses, which can lead to practical dietary, behavioral, and therapeutic methods (Mattson et al. 2004).
Agricultural and environmental aspects of hormesis
A failure to recognize and understand hormesis can have adverse effects not only in the biomedical sciences but also in agricultural and environmental sciences. For example, the hormetic stimulation of pests peripheral to those targeted with high doses of pesticides may be a factor in secondary pest outbreaks (Morse 1998). Hormetic effects in insects, often called hormoligosis rather than hormesis, have been observed after exposure to various pesticides (Luckey 1968; Morse and Zareh 1991; Fujiwara et al. 2002). Citrus thrips, which are a major pest of California citrus, exhibit a hormetic response when fed on lemon leaves containing low doses of organochlorine (dicofol), organophosphate (malathion), carbamate (formetanate) and pyrethroid (fluvalinate) insecticides (Morse and Zareh 1991). Doses expected to cause 1–25% mortality of adult females reduced fecundity, but doses predicted to cause 0.01 – 1% mortality led to an increase in fecundity (Morse and Zareh 1991). It is widely recognized that the formulation of pest-control strategies requires an understanding of toxic effects in pests, including insects, mites, and nematodes, as well as in host species and other nontarget organisms. Although less widely recognized, it is also important to understand hormetic effects in pests and in their natural predators and competitors (Morse 1998; Kefford et al. 2008).
Understanding the agricultural and ecological implications of hormesis requires consideration of hormetic processes in plants. Plants are well-represented in the hormesis literature (Calabrese and Baldwin 2001a, 2003a; Belz et al. 2005; Calabrese and Blain 2005; Duke et al 2006; Cedergreen et al. 2007; Velini et al. 2008). Endpoints for which hormesis has been observed include growth of stems, roots, and leaves; dry weight; height; protein content; and resistance to pathogenic fungi. Progress has been made in describing and quantifying plant hormetic responses by nonlinear mathematical modeling (Belz et al. 2005; Cedergreen et al. 2005). Many herbicides are among the agents shown to induce hormesis in plants (Duke et al. 2006). Allelopathic compounds, which are generally known as plant metabolites that are inhibitory to other plants, are also hormetic, in that stimulatory responses are evoked by low doses of such compounds as parthenin or benzoxazolin-2(3H)-one (Belz et al. 2005; Duke et al. 2006), and allelopathic plants at low densities can stimulate rather than inhibit neighboring plants (Belz et al. 2005). Cedergreen et al. (2007) analyzed 687 dose-response curves for effects of 9 herbicides, a fungicide, and binary mixtures of the compounds on an alga (Pseudokirchneriella subcapitata) and three vascular plants (Lemna minor, Tripleurospermum inodorum, and Stellaria media). Hormetic responses were recorded in all species. They were most evident with the herbicides glyphosate and metsulfuron-methyl but were also observed with the other compounds. Possible artifacts that could lead to erroneous claims of hormesis were considered, and hormesis was observed independently of altered interspecific competition (Cedergreen et al. 2007), which can be an important factor in natural populations (Duke et al. 2006; Cedergreen et al. 2007).
Effects of glyphosate have been studied in greenhouse experiments with maize, soybean, Eucalyptus grandis, Pinus caribea, and Commelia benghalensis, all of which showed hormesis at low doses and toxicity at high doses (Velini et al. 2008). Hormesis was measured as increases in dry weight of different plant organs, and it was also observable by growth differences in Eucalyptus. Glyphosate-resistant soybeans did not show the hormetic effect. Hormesis can have unforeseen consequences if it occurs by herbicide drift at sites removed from the target. It may also be exploited agriculturally, and this may already be occurring in the widespread use of low doses of glyphosate to enhance sucrose accumulation in sugarcane (Dusky et al. 1986; Su et al. 1992; Duke et al. 2006; Velini et al. 2008).
Hormetic effects may be beneficial to the exposed organism if they confer such attributes as improved pathogen resistance, but their net effect would be less clear if different low-dose effects offset one another. For example, increased numbers of offspring may be counterbalanced by lower offspring survival (Duke et al. 2006). Such trade-offs are suggested by the observation that diamondback moths treated with the pyrethroid insecticide fenvalerate at moderately toxic dosages (e.g., LD12.5 –LD25) laid more eggs than untreated moths, but the eggs tended to be smaller and exhibited lower viability (Fujiwara et al. 2002). Thus, there may be a broader partitioning of resources among eggs rather than a net increase in resource allocation to reproduction. In studies of blowfly development, Nascarella et al. (2003) found that larvae exhibited hormesis when exposed to cadmium in their feed. Low doses led to enhanced pupation, whereas higher doses were toxic and reduced pupation. However, the hormetic effect at low doses was offset by stage specific toxicity later in development, manifesting itself as reduced emergence of adults from the pupae (Nascarella et al. 2003). Conflicting effects of toxicants on survival and reproduction and differences among life-history stages suggest that population growth rate may be a better measure of toxicant effects in ecological settings than are effects at the individual level (Forbes and Calow 1999). Such studies raise an evolutionary question going beyond short-term ecological or agricultural effects and requiring further analysis: How do hormetic responses for specific biological characteristics in a given species relate to general fitness (Forbes and Calow 1999; Forbes 2000; Nascarella et al. 2003; Duke et al. 2006; Kefford et al. 2008)?
Two faces of hormesis in public health and medicine
In public health and medicine, the risks of ignoring hormesis can fall on two sides—failure to recognize it when biological effects in the hormetic zone are harmful, and failure to derive hormetic benefit from low-level stressors. It may be easier to achieve agreement on the former. If patients suffer adverse effects when low drug doses cause hormesis in pathogens or parasites, few would argue against modifying therapeutic regimens so as to take corrective action. Such actions would be rooted in the principle of nonmaleficence. The likelihood of identifying such risks is greater if there is general awareness that hormesis is an expected consequence of low-dose exposures. Such cases are more likely to be overlooked if there is a lack of awareness of the nature of hormesis or resistance to it on the basis of tradition or fear of its implications.
Some current medical practices, rooted in nonmaleficence, may have beneficial effects through hormesis, although they are not generally recognized as such. Scott and Di Palma (2006) have described several means by which hormesis induced by low-dose ionizing radiation can have anti-cancer effects. The concept of medical radiation hormesis holds that diagnostic x rays and nuclear medicine prevent cancer not only through the benefits of diagnosis but biologically. In contrast, therapeutic radiation hormesis encompasses the curative capacity of low-dose fractionated exposures or low-dose, low-dose-rate therapy for pre-existing cancer, either alone or in combination with apoptosis-sensitizing chemicals. They also consider environmental radiation hormesis whereby exposure to background ionizing radiation may protect against cancer and other diseases (Scott and Di Palma 2006).
Encouraging exposures specifically to harvest the benefit of hormesis would be rooted in beneficence, and this poses a higher hurdle for acceptance. Even if one is convinced that hormesis should be expected at low doses and might serve as a default assumption for the scientific interpretation of effects at low doses (Calabrese et al. 2006), one need not agree that public policy should attempt to regulate to the hormetic zone. However, caution about changing exposure standards should not entail adherence to incorrect dose-response models as though they are true (Hoffmann and Stempsey 2008).
Excessive emphasis on benefit as a corollary of the hormesis concept may hinder the recognition of hazards when the hormetic zone works counter to human well-being. Bacterial infections in cystic fibrosis (CF) are a serious health problem that can be worsened by hormetic stimulation of bacteria (Linares et al. 2006). Pseudomonas aeruginosa is an opportunistic pathogen of people with CF and other hospitalized patients. It colonizes the bronchial tree of CF patients, and the recurrent infections cause lung deterioration and can lead to death. Different classes of antibiotics, represented by tobramycin, tetracycline, and ciprofloxacin, all stimulate bacteria at low doses. By means of a P. aeruginosa microarray, analyzing 555 genes relevant to colonization, infection, antibiotic resistance, transcriptional regulation, and stress response, it has been shown that subinhibitory concentrations of antibiotics can increase the expression of bacterial virulence determinants (Linares et al. 2006). The case of Pseudomonas and CF is but one instance of what is probably a common phenomenon of hormetic stimulation of pathogens by low drug doses. Davies et al. (2006) have asserted that “All antibiotics, regardless of their receptors and mode of action, exhibit the phenomenon of hormesis and provoke considerable transcription activation at low concentrations.”
The same reasoning suggests the possibility of hormetic stimulation of surviving tumor cells in some chemotherapy regimens (Calabrese 2005a). Clinical evidence that hormesis in hormone-related cancer therapy may sometimes cause stimulation of a tumor, such as “tamoxifen flare” in metastatic breast cancer, argues that an understanding of biphasic curves is relevant to achieving the desired inhibitory response (Brandes 2005). Failure to recognize hormesis can be an impediment to the development of medical remedies that exploit natural adaptive responses when they are beneficial or combat them when detrimental.
HETEROGENEITY IN SUSCEPTIBILITY TO TOXICANTS
Heterogeneity in susceptibility to toxicants exists at several levels, including differences among biological endpoints, tissues and organs, individuals, and species. A hormetic effect for one endpoint may be of dubious benefit if accompanied by detriment with respect to another endpoint. For example, a substantially increased risk of carcinogenesis would outweigh a modest benefit in another category of effect. It would be acceptable, however, if an important benefit is bought at the cost of mild detriment elsewhere, as in many drug side effects. One also needs to know how an exposure that is beneficial at one site affects other tissues or organs, such as the occurrence of carcinogenesis at one site and anticarcinogenesis at another (Haseman and Johnson 1996; Thayer et al. 2005).
It has been argued that the failure to consider heterogeneity among individuals in susceptibility to toxicants is a serious weakness of the hormesis model with respect to risk assessment (Axelrod et al. 2004). In fact, the problem of accommodating population heterogeneity is not unique to hormesis but applies to all models. It would nevertheless be valuable to know whether hormetic responses occur in sensitive subpopulations and whether they are comparable to those in the typical population. Comparisons of hormetic curves in published literature have led to the suggestion that individuals and species exhibiting greater sensitivity to toxicants usually exhibit hormetic responses, but they occur at lower doses (Calabrese and Baldwin 2002b; Calabrese 2008a). While difficult to evaluate rigorously, a shift of the dose-response curve to the left seems a reasonable expectation for sensitive groups.
Mitchel et al. (2003) have shown that a radiation-sensitive, cancer-prone strain of mice (B6.129S2-Trp53tm1Tyj/+) given a single low dose (10 mGy) of 60Co γ rays at a low dose rate (0.5 mGy/min) showed a longer latency period for the development of spontaneous lymphomas and spinal osteosarcomas. Preexposure to 10 mGy also made this strain less susceptible to adverse effects of a large acute dose (4 Gy) of γ rays (Mitchel et al. 2004). In an NCI yeast database, 11 strains with altered DNA repair or cell-cycle progression all displayed hormesis (Calabrese et al. 2006). Such findings support the view that stress responses and hormesis occur in sensitive genotypes, but they do not preclude the possibility that some genotypes will prove to be refractory.
There is clear evidence of genetic and physiological alterations that interfere with stress responses. For example, E. coli ada mutants that are deficient in a methyltransferase that repairs O6-methylguanine in DNA do not exhibit the adaptive response to methylating agents (Volkert 1988; Shevell et al. 1990; Kleibl 2002). Similarly, inhibition of poly(ADP-ribose)polymerase (PARP), an enzyme that is activated by DNA strand breaks and involved in their repair (Shall and de Murcia 2000), blocks the induction by ionizing radiation of an adaptive response that prevents radiation-induced clastogenic effects in mammalian cells (Shadley and Wolff 1987; Stecca and Gerber 1998; Miura 2004). Inhibition of PARP also eliminates the reduction in frequency of neoplastic transformation that occurs by radiation hormesis in mammalian cells (Pant et al. 2003).
Research in a simple whole-animal model, the nematode worm Caenorhabditis elegans, demonstrates the existence of mutants altered in hormetic responses. Exposure to low doses of stressors (e.g., heat, ionizing radiation, or hyperbaric oxygen) leads to increased longevity in wild-type worms (Cypser and Johnson 2003). Conserved genes of the insulin/insulin-like signaling pathway, including daf-16 and daf-18, are involved in lifespan determination and are required for hormetic life extension (Cypser and Johnson 2003; Cypser et al. 2006). The dauer stage in Caenorhabditis is resistant to unfavorable environmental conditions and occurs as an alternative to continued development to the adult stage. Mutations in daf-16, daf-18, or daf-12, which block the formation of dauers, also block the hormetic response to heat stress (Cypser and Johnson 2003).
BIPHASIC CURVES IN HETEROGENEOUS POPULATIONS
Sensitive subgroups in populations pose difficult challenges for risk assessment (Thayer et al. 2005, 2006). Differences in chemical responses owing to age, health status, and such genetic attributes as enzyme polymorphisms need to be considered under any dose-response model, but the biphasic curves of hormesis present unique challenges not encountered with monotonic responses. Figure 3 shows hormesis in a typical population and a sensitive subpopulation. The sensitive subgroup represents a small part of the population in which the adverse effect occurs at a lower dosage. Given the differences in sensitivity, most doses that are hormetic for the population as a whole would be toxic for the sensitive group. Such curves raise questions not seen with monotonic curves, in that part of the population would benefit in the hormetic zone, whereas another part would be harmed. If the dose-response were described by a threshold or LNT model, subpopulations can differ in the extent of harm, or even the presence or absence of harm, but they would not experience the opposite outcomes of harm versus benefit.
FIGURE 3.
Dose-response curves illustrating hormetic responses in a typical population and a small subpopulation with greater toxicant susceptibility. The response is an adverse effect occurring at a lower dosage in the sensitive subpopulation. The figure is adapted from Hoffmann and Stempsey 2008.
An optimal solution given these curves is elusive, even if one assumes that the NOAEL in both subpopulations can be measured with precision. In the spectrum of possible solutions, one pole is strict adherence to the aphorism “do no harm,” such that protection of the most sensitive element of the population is the highest priority. At the opposite pole is a course of utilitarian ethics, striving for the greatest good for the greatest number. To do so, one might contemplate using smaller safety factors below the NOAEL, and this may benefit the typical population (Gaylor 1998). The first pole sacrifices the hormetic benefit to the majority, whereas the second buys a lower incidence of adverse effect for the population as a whole by risking harm in the sensitive subpopulation. Intermediates between the poles can be envisioned, just as they can in other biomedical situations. In clinical trials, for example, a balance is sought between heeding warning signals and terminating a trial when there is evidence of adverse effects, but yet not so quickly that premature termination unnecessarily sacrifices the potential benefit of the study. The principle of nonmaleficence lends itself to a course closer to early termination in clinical trials and to protection of sensitive subgroups in heterogeneous populations.
Hormesis poses an especially difficult challenge in the case just considered (Figure 3) because the zone that is hormetic to both subpopulations is small, and its delineation may never be attainable. Figure 4 shows curves for populations differing in the extent of sensitivity of the sensitive subgroup. Figure 4A shows greater overlap between the hormetic zones of the two subpopulations. If one were to attempt to regulate to the hormetic zone, the challenge in this case would be to find a position sufficiently to the left to ensure that the exposure is in the hormetic zone for the sensitive subpopulation while remaining in the hormetic zone for the typical population. This is the scenario on which Cook and Calabrese (2006) suggested that exposures below the NOAEL for the sensitive population can be beneficial for the entire population. Figure 4B, showing a large difference between subgroups, raises interesting questions about how to respond when protecting the sensitive group becomes increasingly difficult, but it falls largely outside the scope of a perspective on hormesis because it poses comparable difficulty under any dose-response model.
FIGURE 4.
Dose response curves showing greater and smaller differences between a typical population and a small subpopulation with higher susceptibility to adverse effects.
The distribution of benefit and harm in different parts of a population would need to be considered if the hormesis model enters into risk management. Ethical considerations, especially the primacy of non-maleficence, place a high priority on protecting sensitive groups. If one looks beyond public health to environmental policy, a parallel problem exists, as heterogeneity in susceptibility among species is rarely known in a broad sense. Ecological effects of low doses and effects on sensitive species warrant continued consideration.
COMPLICATIONS OF HORMESIS
The diverse manifestations of hormesis introduce variation into how one encounters it, and the mechanistic complexity of the phenomenon can lead to complications of interpretation.
Some hormetic responses do not yield typical hormetic curves
In order to observe the J-shaped curve of hormesis, responses need to be measurable both above and below background levels (Calabrese and Baldwin 2001b). If an effect occurs at a low spontaneous frequency, such as some tumors, one may not be able to measure a decrease in its frequency. An agent that induces the effect at high dose would therefore not be seen to reduce its incidence at low doses, because the control incidence is zero or near to zero. In this instance, the measurable curve will appear to fit a threshold model.
Some adaptive responses may not reveal hormetic curves for mechanistic reasons. One of the mechanisms of hormesis is inducible repair of genetic damage. Besides removing damage induced by environmental agents, repair mechanisms remove damage caused by the inherent error rates of biological processes, endogenous stressors, and spontaneous DNA degradation (Lindahl 1993). Curves measuring damage can differ in form depending on whether spontaneous damage is a significant contributor to the total damage. If the signal is a premutational lesion that is infrequent or absent spontaneously but likely to cause mutation if unrepaired, the inducible response can repair the lesions and reduce the effect of a subsequent exposure, but it may not yield a J-shaped curve because there is little or no spontaneous damage to remove. In contrast, if the inducing agent induces a kind of damage that is common spontaneously and contributes to the background level of the effect, the adaptive response can reduce the damage below the spontaneous level, thus giving a nonmonotonic curve (Kodell 2001). It is therefore not surprising that adaptive responses (Demple and Halbrook 1983; Davies et al. 1995; Wiese et al. 1995; Chen et al. 2006a) and biphasic curves (Davies et al. 1995; Caporossi et al. 2003) have both been reported for hydrogen peroxide and other agents that induce oxidative DNA damage. These findings are consistent with other studies showing biphasic curves for the induction of lipid peroxidation by the superoxide radical (McCord 2002) and protection or exacerbation of cardiac ischemia-reperfusion injury by superoxide (McCord and Edeas 2005).
Hormetic curves may not be observed if background levels of exposure are already in the toxic zone. Although hormetic responses may occur in such cases at exposures smaller than control levels of exposure, they would be irrelevant under the prevailing conditions. Some examples cited as arguments against hormesis (Axelrod et al. 2004), such as human lead exposures, may fit this category, and they illustrate the need for refined exposure assessments (Davis and Farland 1998). Agents that mimic endogenous substances can pose a similar problem for certain endpoints if the endogenous substance is a risk factor, such as estrogens and breast cancer (vom Saal and Sheehan 1998).
Dose dependence of the induction of stress responses
The induction of a stress response may show evidence of hormesis independently of whether the response itself is demonstrably biphasic. Adaptive responses are absent without a conditioning exposure and are induced by low doses. There is evidence in some cases that the induction is biphasic (Feinendegen 2005). When an x-ray exposure makes human lymphocytes less susceptible to the induction of chromosomal damage by a second exposure, the protective effect is most prominent with small priming doses (~10 mGy), decreases with dose, and is no longer detected at doses over ~200 mGy (Shadley and Wolff 1987). Similarly, the induction of an adaptive response using growth as an endpoint in HE22 human embryonic cells showed a peak at 13 cGy (Ishii and Watanabe 1996). Despite the evidence that adapting exposures may occur within a window of dosage, thus being hormetic rather than monotonic, it would be premature to generalize that conditioning exposures uniformly fit this pattern. Doses spanning a broad range have been observed to be equally effective in inducing an adaptive response in other systems, including radiation-induced micronuclei in human fibroblasts (Broome et al. 2002) and the pKZ1 assay for chromosomal inversions in mice (Day et al. 2007). Systems that differ in cellular targets, endpoints, mechanisms, and temporal considerations apparently vary in the dose-dependence of induction of adaptive responses (Wolff 1998; Broome et al. 2002).
Interactions among agents
Toxicologists have long recognized that biological effects need not be additive, and interactions among agents can take diverse forms. One agent may potentiate another or, if both are themselves active, their combined effects can be synergistic (Hoffmann et al. 2007). Alternatively, the interactions can be antagonistic, such that one agent diminishes the effects of another. The latter includes antimutagenic and anticarcinogenic effects (Hartman and Shankel 1990; De Flora and Ferguson 2005). Such interactions are of interest for their potential application in the prevention of cancer or other disorders (De Flora and Ferguson 2005), but caution is required because the same agent that is protective under one set of conditions may be mutagenic, carcinogenic, or enhance the adverse activity of other agents under other conditions (Zeiger 2003; Hoffmann et al. 2007).
Interactions among agents are a mystery with respect to hormesis. If two agents are in the hormetic zone, do they combine to give a toxic effect? Alternatively, is there a hormetic effect equal to or greater than the sum of the separate hormetic effects? There is no basis for answering such questions at present, yet assumptions about the answer have figured into arguments against hormesis, based on speculation that multiple chemicals in the hormetic zone would be equivalent to a toxic exposure (Axelrod et al. 2004; Thayer et al. 2005; Shrader-Frechette 2008). Alternatively, one might speculate that hormetic interactions may be constrained by the plasticity of biological systems that gives hormesis similar quantitative features in diverse systems (Calabrese 2005a; Calabrese and Blain 2005; Calabrese 2008a). Assumptions and speculation are insufficient; potential interactions among agents with respect to hormesis need to be evaluated scientifically, and doing so will not be easy.
Nature of the subhormetic zone
Shapes of hormetic curves may be more varied than the typical U and J. At very low doses, the hormetic zone of a J or inverted-U curve must return to the background level, as the effect must be zero at the ordinate. A low, nonzero dose induces the hormetic response, and this raises the question as to what happens in the subhormetic zone. Figure 5A shows a typical J-shaped hormetic curve, pointing out the unknown nature of the subhormetic zone. If the doses that induce hormesis are so low that they cause no effect in themselves, one might expect such an idealized J or inverted-U curve. Alternatively, if the hormesis-inducing dose causes a low level of damage, one should expect to see curves more complex than the simple J or inverted U. An expanding triphasic wave, shown in Figure 5B, would make sense as a dose-response relationship for an agent that causes damage and an inducible repair response at low dose. One might think that such a hypothetical possibility could not be observed because it is hard enough to measure effects in the hormetic zone, let alone the subhormetic zone. However, there is evidence that exquisitely sensitive assays can offer insight into the nature of the subhormetic zone.
FIGURE 5.
Hormesis and the subhormetic zone: (A) Typical hormetic J-shaped curve indicating the unknown nature of the subhormetic zone. (B) Hypothetical triphasic curve that may occur if an agent causes damage and an inducible repair response at low dose.
The pKZ1 transgenic mouse assay was used to study the induction of inversions that arise by SICR at x ray doses from 1 μGy to 2 Gy (Hooker et al. 2004; Sykes et al. 2006). There was induction of inversions at ultra-low doses, a reduction below the spontaneous frequency at low doses, and a larger induction of inversions at higher doses. At the lowest effective doses, where few cells experience damage, effects may be mediated by bystander effects, triggered in nonirradiated cells by signaling from neighboring cells that experienced damage (Sykes et al. 2006). Hooker et al. (2004) offered a speculative but credible interpretation of the three phases of the curve. At ultra-low doses (~10 μGy), DNA damage leads to an increase in inversions. At low doses (1–10 mGy), there is hormesis. The induced DNA damage either directly or indirectly triggers a decrease in repair of double-strand breaks by nonhomologous end joining; the result may be an increase in apoptosis that could prevent carcinogenesis (Hooker et al. 2004). At doses >100 mGy, there is induction of SICR that causes inversions but repairs large amounts of induced DNA damage (Hooker et al. 2004). The results clearly do not fit LNT, and they resemble the hypothetical triphasic curve.
Temporal component of hormetic responses
A temporal component is often underestimated or overlooked in studies of hormesis, yet it can be critical if hormesis is the result of an initial disruption of homeostasis (Calabrese and Baldwin 2001b). If hormesis depends on the induction of an adaptive response, one might expect to see no immediate hormetic response at the time of exposure, an increase in the hormetic response after the required time for induction, a maximum expression of hormesis, and then a return to the base level of response (Calabrese and Baldwin 2002a; Calabrese 2005c, 2008a; Feinendegen 2005; Calabrese et al. 2007). Studies of the time course of hormetic effects are limited, yet experimental evidence supports the basic pattern illustrated in Figure 6, which provides a context for the discussion that follows.
FIGURE 6.
Time course of hormesis: The curves show no immediate hormetic effect, an increasing hormetic response after the requisite time for induction, and a maximum hormetic effect followed by a return to the ground state. The figure is adapted from Calabrese et al. 2007.
Time is typically required after a conditioning exposure for the full expression of an adaptive response that depends on protein synthesis (Stecca and Gerber 1998; Wolff 1998). For example, de Toledo and Azzam (2006) found that the protective effect conferred by a moderate dose of γ-rays at a low dose rate (0.5 Gy at 0.002 Gy/min) against the induction of micronuclei by a higher dose and dose rate (4 Gy at 1.8 Gy/min) in cultured human fibroblasts is greater if there is a delay between the conditioning and challenging doses. The adaptive response that makes dividing human lymphocytes that were exposed to low doses of x rays (~5–10 mGy) less susceptible to the induction of chromatid breakage by a subsequent high dose (Shadley and Wolff 1987; Shadley et al. 1987) requires about 4–6 h for induction to occur. The induced state disappears with time (Wolff 1998) after persisting through several cell divisions (Shadley and Wolff 1987; Shadley et al. 1987). The observation of hormesis in the cell transformation assay of Redpath and colleagues also depends on delayed plating to allow for postirradiation gene expression (Redpath et al. 2003a). The time-dependence of hormesis undoubtedly applies to chemicals as well as radiation. For example, a study of the cytotoxicity of chlorpromazine in mouse neuroblastoma cells showed toxicity at 30 μM and hormesis at 3 μM. The hormetic effect was not evident at 24 h, in that the response was 60% of the control, but it was 135% at 48h and declined to 120% at 72h (Andres et al. 1999). Other studies similarly show that the induction of adaptive responses is time-dependent and transient, and they suggest that the time course and absolute doses differ among cell types (Ishii and Watanabe 1996; Park et al. 1999).
Taken together, the evidence suggests that if one measures biological effects too early, the response may appear to fit a threshold or linear model rather than show hormesis because insufficient time has elapsed for the requisite gene expression for an adaptive response. If one makes measurements too late, the system may have already returned from the induced state to the ground state. Accounting for temporal factors is a complication that affects the ability to observe hormesis in laboratory studies. The time dimension is also relevant to nonlaboratory conditions and would have to be considered in any attempts to include hormesis in hazard assessment.
Genetic dissection of hormesis
There is evidence of hormesis for many biological endpoints, agents, and species but little evidence on whether there are specific effects, agents, or genotypes for which hormesis does not occur. Mutation has been a useful tool for dissecting biological phenomena, such as the use of repair-deficient mutants in the study of DNA repair. It is of great interest to identify hormesis-minus mutants or other mutants with altered hormetic responses in model organisms.
In the NCI drug-screening database, 11 of 13 yeast strains carry known alterations that affect DNA repair or cell-cycle progression, and all display hormesis (Calabrese et al. 2006, 2008). Finding hormesis-minus mutants is not easy because the phenotypic effect is quantitative, subtle, and typically small. Exploring gene expression associated with stress responses is apt to be more fruitful, and genetic functions required for adaptive responses (i.e., conditioning hormesis) have been identified in several biological systems (Shadley and Wolff 1987; Volkert 1988; Shevell et al. 1990; Stecca and Gerber 1998; Kleibl 2002; Cypser and Johnson 2003; Pant et al. 2003). The research in nematode worms discussed earlier (Cypser and Johnson 2003; Cypser et al. 2006), as well as that in other model organisms, offers great promise for elucidating the genetic underpinnings of hormesis in specific genetic systems. Nevertheless, gene functions central to the hormetic response can be elusive owing to the multiplicity of mechanisms that are likely to be involved. The genetic dissection of hormesis remains a difficult and important challenge for the future.
Nontargeted damage, bystander effects and genetic instability
Nontargeted damage, including bystander effects and genomic instability, raise interesting questions about risks of low-dose exposures. Strong evidence indicates that cells that are not themselves irradiated may experience cytotoxicity, apoptosis, chromosome aberrations, and other biological effects if they receive signals from other cells that have been exposed to ionizing radiation (Zhou et al. 2001; Morgan 2002, 2003a, 2003b, 2003c; Azzam and Little 2004; Mothersill and Seymour 2004a, 2004b, 2006a, 2006b; Belyakov et al. 2005; Morgan and Sowa 2005). These nontargeted effects, commonly called bystander effects, may be mediated by diffusible messengers from exposed cells or by direct cellular contact. At high-doses, bystander effects are apt to be eclipsed by direct effects of irradiation, but they can be important at low doses (Mothersill and Seymour 2006a). There is no reason to think that bystander effects are unique to radiation, yet such effects are technically difficult to detect and quantify for chemical exposures (Mothersill and Seymour 2004a, 2004b). Genomic instability is a special case of nontargeted damage in which biological effects continue to arise in the progeny of affected cells (Morgan 2003a, 2003b; Azzam and Little 2004; Mothersill and Seymour 2004a, 2004b, 2006a).
A concern about bystander effects and induced genetic instability is that they may cause previously unpredicted adverse effects at low doses. Because they imply a larger target, it has been suggested that adverse effects in nontargeted cells may manifest themselves as supralinear dose responses—deviations from LNT opposite to those expected for hormesis, thus implying greater risk (Brenner et al. 2001, 2003; Zhou et al. 2001; Morgan 2002). Adaptive responses and bystander effects have been described as conflicting phenomena because the former reduce risk at low doses, whereas the latter may increase it (Zhou et al. 2003). The complexity is increased by the fact that the two effects are apparently not independent of one another, in that low doses of x rays given before an exposure of mammalian cells to α particles decreased the bystander mutagenesis (Zhou et al. 2003).
Strong evidence indicates that low-dose irradiation of nontransformed cells enhances apoptosis in nearby transformed cells (Bauer 2007; Portess et al. 2007). Portess et al. (2007) used a coculture system to explore the mechanism underlying this bystander effect by which normal cells selectively eliminate transformed cells. The intercellular induction of apoptosis is modulated by reactive oxygen and nitrogen species, involves transforming growth factor type beta (TGF-β), and does not require cell-to-cell contact (Bauer 2007; Portess et al. 2007). Superoxide anion plays a central role in the signaling (Bauer 2007). Various normal cell types (e.g., fibroblasts, epithelial cells, endothelial cells, monocytes, B cells) can serve as effectors, and the occurrence of induced apoptosis is strictly dependent on the transformed state of the target cells (Bauer 2007). Irradiation of nontransformed cells with doses as low as 2 mGy γ rays or 0.29 mGy α-particles stimulated the induction of apoptosis in unir-radiated transformed cells, an effect that increased with dose to a plateau at ≥50 mGy γ or 25 mGy α (Portess et al. 2007).
To reach conclusions on risk, one needs a better sense of the dose-response relationships for the induction of bystander effects, how they are propagated, and most importantly, the balance between different elements of the bystander response. It is unclear how bystander effects affect carcinogenesis because bystander mutagenesis or transformation may increase risk while increased cell death may decrease risk (Friedl and Rühm 2006). Bystander effects include diverse endpoints, including mutations, chromosome aberrations, micronuclei, neoplastic transformation, reduced survival, and apoptosis. It is possible that increases in chromosome aberrations, mutagenesis, or transformation in the bystander population are overshadowed by increased cell death, repair capacity, or other protective factors. An evolutionary perspective makes protective effects seem likely but, like the proposition of heightened risk by bystander effects, this is speculative. There is much to learn about the implications of nontargeted damage for the interpretation of low-dose effects.
CONCLUSIONS
This perspective on hormesis supports the following conclusions:
Growing evidence supports the reality and common occurrence of hormesis.
The hormesis concept merits empirical evaluation and refinement independently of whether it is assimilated into policy on toxic substances.
It would be premature to regulate to the hormetic zone for chemical exposures now. How hormesis figures into policy needs to be revisited as risk assessment improves.
Using hormesis for regulatory purposes would require better understanding of the positions of the toxic and hormetic zones for diverse endpoints, tissues, individuals, and species.
Biphasic dose responses raise challenging ethical questions regarding sensitive subpopulations.
Ecological effects of low doses and differences among species with respect to hormesis warrant continued investigation.
Effective exploitation of hormesis in medicine and agriculture is likely to precede its use in toxicologic risk assessment.
Deeper understanding of stress responses can stimulate medical and technological advances.
The hormesis concept has important implications for antimicrobial and cancer therapy.
Better understanding of hormesis can foster effective public health and environmental policies.
ACKNOWLEDGMENTS
I thank Edward Calabrese for his invitation to present this perspective on hormesis at the annual meeting of the International Dose-Response Society. I thank Ed Calabrese, Barbara Callahan, Wayne Hoffmann-Ogier, Walter Kozumbo, Donald MacPhee, Marc Nascarella, Kenneth Prestwich, Michael Shelby, Ed Stanek, and Bill Stempsey for valuable and enjoyable discussions of hormesis and related topics. I also gratefully acknowledge Darlene Colonna for her excellent secretarial work and Carrie Peck for library assistance.
REFERENCES
- Andersen ME, Conolly RB, Gaylor DW. Letter to the Editor. Toxicol Sci. 2003;74:486–487. doi: 10.1093/toxsci/kfg134. [DOI] [PubMed] [Google Scholar]
- Andres MI, Repetto G, Sanz P, Repetto M. Biochemical effects of chlorpromazine on mouse neuroblastoma cells. Vet Human Toxicol. 1999;41:273–278. [PubMed] [Google Scholar]
- Arumugam TV, Gleichmann M, Tang S-C, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res Rev. 2006;5:165–178. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Auerbach C. Mutation Research: Problems, Results, and Perspectives . Chapman and Hall; London: 1976. [Google Scholar]
- Auerbach C, Robson JM. Chemical production of mutations. Nature. 1946;157:302. doi: 10.1038/157302a0. [DOI] [PubMed] [Google Scholar]
- Auerbach C, Robson JM. The production of mutations by chemical substances. Proc Roy Soc Edinburgh (B) 1947;62:271–283. [PubMed] [Google Scholar]
- Aurengo A, Averbeck D, Bonnin A, Le Guen B, Masse R, Monier R, Tubiana M, Valleron A-J, de Vathaire F. Dose-effect relationships and estimation of the carcinogenic effects of low doses of ionizing radiation. Joint Report of the Académie des Sciences (Paris) and of the Academie Nationale de Médecine. 2005 doi: 10.1016/j.ijrobp.2005.06.013. March 30, 2005. Available at http://www.radscihealth.org/rsh/Papers/FrenchAcadsFinal07_04_05.pdf. [DOI] [PubMed]
- Axelrod D, Burns K, Davis D, Von Larebeke N. “Hormesis” – an inappropriate extrapolation from the specific to the universal”. Int J Occup Environ Health. 2004;10:335–339. doi: 10.1179/oeh.2004.10.3.335. [DOI] [PubMed] [Google Scholar]
- Azzam EI, Little JB. The radiation-induced bystander effect: evidence and significance. Hum Exp Toxicol. 2004;23:61–65. doi: 10.1191/0960327104ht418oa. [DOI] [PubMed] [Google Scholar]
- Bauer G. Low dose radiation and intercellular induction of apoptosis: potential implications for the control of oncogenesis. Int J Radiat Biol. 2007;83:873–888. doi: 10.1080/09553000701727523. [DOI] [PubMed] [Google Scholar]
- Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 5th Edition. Oxford University Press; New York: 2001. pp. 114–116. [Google Scholar]
- Belyakov OV, Mitchell SA, Parikh D, Randers-Pehrson G, Marino SA, Amundson SA, Geard CR, Brenner DJ. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc Natl Acad Sci USA. 2005;102:14203–14208. doi: 10.1073/pnas.0505020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz RG, Hurle K, Duke SO. Dose-response—a challenge for allelopathy? Nonlinearity Biol Tox Med. 2005;3:173–211. doi: 10.2201/nonlin.003.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IFF. Evolution of antioxidant defence mechanisms. Eur J Nutr. 2000;39:53–61. doi: 10.1007/s003940070030. [DOI] [PubMed] [Google Scholar]
- Bolt HM, Foth H, Hengstler JG, Degen GH. Carcinogenicity categorization of chemicals—new aspects to be considered in a European perspective. Toxicol Lett. 2004;151:29–41. doi: 10.1016/j.toxlet.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Boreham DR, Dolling J-A, Somers C, Quinn J, Mitchel REJ. The adaptive response and protection against heritable mutations and fetal malformation. Dose-Response. 2006;4:317–326. doi: 10.2203/dose-response.06-104.Boreham. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveri T. The Origin of Malignant Tumors . Williams & Wilkins; Baltimore: 1929. (English translation by Marcella Boveri of T. Boveri’s. 1914. Zur Frage der Entstehung maligner Tumoren. Gustav Fischer, Jena) [Google Scholar]
- Brandes LJ. Hormetic effects of hormones, antihormones, and antidepressants on cancer cell growth in culture: in vivo correlates. Crit Rev Toxicol. 2005;35:587–592. doi: 10.1080/10408440500246801. [DOI] [PubMed] [Google Scholar]
- Breckow J. Linear-no-threshold is a radiation-protection standard rather than a mechanistic effect model. Radiat Environ Biophys. 2006;44:257–260. doi: 10.1007/s00411-006-0030-y. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Sachs RK. Estimating radiation-induced cancer risks at very low doses: rationale for using a linear no-threshold approach. Radiat Environ Biophys. 2006;44:253–256. doi: 10.1007/s00411-006-0029-4. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Little JB, Sachs RK. The bystander effect in radiation oncogenesis: II. a quantitative model. Radiat Res. 2001;155:402–408. doi: 10.1667/0033-7587(2001)155[0402:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc Natl Acad Sci USA. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome EL, Brown DL, Mitchel REJ. Dose responses for adaptation to low doses of 60Co γ rays and 3H β particles in normal human fibroblasts. Rad Res. 2002;158:181–186. doi: 10.1667/0033-7587(2002)158[0181:drfatl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Carlton WW, Clayson D, Ferber KH, Hughes DH, Quast JF, Salsburg DS, Smith JM, Brown WR, Cranmer MF, Sielken RL, Van Ryzin J, Barnard RC. The SOT Task Force’s response to the NCTR Letter. Fundam Appl Toxicol. 1983;3:9/a–12/a. [Google Scholar]
- Cairns J. Efficiency of the adaptive response of Escherichia coli to alkylating agents. Nature. 1980;286:176–178. doi: 10.1038/286176a0. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis: Changing view of the dose response; a personal account of the history and current status. Mutat Res. 2002;511:181–189. doi: 10.1016/s1383-5742(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Toxicological diversity: making room for the U-shaped dose-response. Hum Exper Toxicol. 2003;22:465–466. doi: 10.1191/0960327103ht386ed. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis: From marginalization to mainstream: A case for hormesis as the default dose-response model in risk assessment. Toxicol Appl Pharmacol. 2004;197:125–136. doi: 10.1016/j.taap.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Cancer biology and hormesis: Human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol. 2005a;35:463–582. doi: 10.1080/10408440591034502. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Historical blunders: How toxicology got the dose-response relationship half right. Cell Molec Biol. 2005b;51:643–654. [PubMed] [Google Scholar]
- Calabrese EJ. Paradigm lost, paradigm found: The re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Env Poll. 2005c;138:379–412. doi: 10.1016/j.envpol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008a;27:1451–1474. doi: 10.1897/07-541. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Neuroscience and hormesis: overview and general findings. Crit Rev Toxicol. 2008b;38:249–252. doi: 10.1080/10408440801981957. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Dose-response features of neuroprotective agents: an integrative summary. Crit Rev Toxicol. 2008c;38:253–348. doi: 10.1080/10408440801981965. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Can the concept of hormesis be generalized to carcinogenesis? Regulatory Toxicol Pharmacol. 1998;28:230–241. doi: 10.1006/rtph.1998.1267. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Reevaluation of the fundamental dose-response relationship. BioScience. 1999;49:725–732. [Google Scholar]
- Calabrese EJ, Baldwin LA. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001a;62:330–338. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: A generalizable and unifying hypothesis. Crit Rev Toxicol. 2001b;31(4 & 5):353–424. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Agonist concentration gradients as a generalizable regulatory implementation strategy. Critical Rev Toxicol. 2001c;31(4 & 5):471–473. doi: 10.1080/20014091111758. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exper Toxicol. 2002a;21:91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis and high-risk groups. Reg Toxicol Pharmacol. 2002b;35:414–428. doi: 10.1006/rtph.2001.1529. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol Sci. 2003a;71:246–250. doi: 10.1093/toxsci/71.2.246. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Toxicology rethinks its central belief. Nature. 2003b;421:691–692. doi: 10.1038/421691a. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: The Dose-Response Revolution. Annu Rev Pharmacol Toxicol. 2003c;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Inorganics and hormesis. Crit Rev Toxicol. 2003d;33:215–304. doi: 10.1080/713611040. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Chemotherapeutics and hormesis. Crit Rev Toxicol. 2003e;33:305–353. doi: 10.1080/713611041. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Peptides and hormesis. Crit Rev Toxicol. 2003f;33:355–405. doi: 10.1080/713611042. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol. 2005;202:289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA, Holland CD. Hormesis: A highly generalizable and reproducible phenomenon with important implications for risk assessment. Risk Analysis. 1999;19:261–281. doi: 10.1023/a:1006977728215. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Staudenmayer JW, Stanek EJ, Hoffmann GR. Hormesis outperforms threshold model in NCI anti-tumor drug screening database. Toxicol Sci. 2006;94:368–378. doi: 10.1093/toxsci/kfl098. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Stanek EJ, Nascarella MA, Hoffmann GR. Hormesis predicts low-dose responses better than threshold models. Internatl J Toxicol. 2008;27:369–378. doi: 10.1080/10915810802503735. [DOI] [PubMed] [Google Scholar]
- Campbell NA, Reece JB, Mitchell LG. Biology. 5th Edition. Addison Wesley Longman; 1999. [Google Scholar]
- Caporossi D, Ciafrè, Pittaluga M, Savini I, Farace MG. Cellular responses to H2O2 and bleomycin-induced oxidative stress in L6C5 rat myoblasts. Free Radic Biol Med. 2003;35:1355–1364. doi: 10.1016/j.freeradbiomed.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Cedergreen N, Ritz C, Streibig JC. Improved empirical models describing hormesis. Environ Toxicol Chem. 2005;24:3166–3172. doi: 10.1897/05-014r.1. [DOI] [PubMed] [Google Scholar]
- Cedergreen N, Streibig JC, Kudsk P, Mathiassen SK, Duke SO. The occurrence of hormesis in plants and algae. Dose-Response. 2007;5:150–162. doi: 10.2203/dose-response.06-008.Cedergreen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Yoshida Y, Saito Y, Noguchi N, Niki E. Adaptive response induced by lipid peroxidation products in cell cultures. FEBS Lett. 2006a;580:479–483. doi: 10.1016/j.febslet.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Yoshida Y, Saito Y, Sekine A, Noguchi N, Niki E. Induction of adaptive response and enhancement of PC12 cell tolerance by 7-hydroxycholesterol and 15-deoxy-delta(12,14)-prostaglandin J2 through up-regulation of cellular glutathione via different mechanisms. J Biol Chem. 2006b;281(20):14440–14445. doi: 10.1074/jbc.M600260200. [DOI] [PubMed] [Google Scholar]
- Christman MF, Morgan RW, Jacobson FS, Ames BN. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Conolly RB, Lutz WK. Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol Sci. 2004;77:151–157. doi: 10.1093/toxsci/kfh007. [DOI] [PubMed] [Google Scholar]
- Cook R, Calabrese EJ. The importance of hormesis to public health. Environ Health Perspect. 2006;114:1631–1635. doi: 10.1289/ehp.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LA. A model of cytotoxic dose-response nonlinearities arising from adaptive cell inventory management in tissues. Dose-Response. 2005;3:491–507. doi: 10.2203/dose-response.003.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump K. Evaluating the evidence for Hormesis: A statistical perspective. Crit Rev Toxicol. 2001;31:669–679. doi: 10.1080/20014091111947. (alternative citation: 2001. Hum Ecol Risk Assess 7:781–794) [DOI] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Hormesis in Caenorhabditis elegans dauer-defective mutants. Biogerontology. 2003;4:203–214. doi: 10.1023/a:1025138800672. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Spiegelman GB, Yim G. The world of subinhibitory antibiotic concentrations. Current Opin Microbiol. 2006;9:445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Davies JMS, Lowry CV, Davies KJA. Transient adaptation to oxidative stress in yeast. Arch Biochem Biophys. 1995;317:1–6. doi: 10.1006/abbi.1995.1128. [DOI] [PubMed] [Google Scholar]
- Davis JM, Farland WH. Biological effects of low-level exposures: a perspective from U.S. EPA scientists. Environ Health Perspect. 1998;106:379–381. doi: 10.1289/ehp.98106s1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Svendsgaard DJ. U-shaped dose-response curves: their occurrence and implications for risk assessment. J Toxicol Environ Health. 1990;30:71–83. doi: 10.1080/15287399009531412. [DOI] [PubMed] [Google Scholar]
- Davis JM, Svendsgaard DJ. Nonmonotonic dose-response relationships in toxicological studies. In: Calabrese EJ, editor. Biological Effects of Low Level Exposures: Dose-Response Relationships, Chap 5. Lewis Publishers/CRC Press; Boca Raton: 1994. pp. 67–85. [Google Scholar]
- Day TK, Zeng G, Hooker AM, Bhat M, Turner DR, Sykes PJ. Extremely low doses of x-radiation can induce adaptive responses in mouse prostate. Dose-Response. 2007;5:315–322. doi: 10.2203/dose-response.07-019.Day. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S, Ferguson LR. Overview of mechanisms of cancer chemopreventive agents. Mutat Res. 2005;591:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Demple B, Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983;304:466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- de Toledo SM, Azzam EI. Adaptive and bystander responses in human and rodent cell cultures exposed to low level ionizing radiation: the impact of linear energy transfer. Dose-Response. 2006;4:291–301. doi: 10.2203/dose-response.06-103.deToledo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky Th. Biology, molecular and organismic. Amer Zool. 1964;4:443–452. doi: 10.1093/icb/4.4.443. [DOI] [PubMed] [Google Scholar]
- Duke SO, Cedergreen N, Velini ED, Belz RG. Hormesis: is it an important factor in herbicide use and allelopathy? Outlooks Pest Management. 2006;17:29–33. [Google Scholar]
- Duport P. A database of cancer induction by low-dose radiation in mammals: overview and initial observations. Int J Low Radiat. 2003;1:120–131. [Google Scholar]
- Dusky JA, Kang MS, Glaz B, Miller JD. Response of eight sugarcane cultivars to glyphosine and glyphosate ripeners. J Plant Growth Regul. 1986;4:225–235. [Google Scholar]
- Eaton DL, Gilbert SG. Principles of toxicology. In: Klaassen CD, editor. Casarett & Doull’s Toxicology: The Basic Science of Poisons. 7th Edition. McGraw-Hill; New York: 2008. pp. 11–43. [Google Scholar]
- Elliott K. Conceptual clarification and policy-related science: The case of chemical hormesis. Perspect Sci. 2000;8:346–366. [Google Scholar]
- Elliott KC. A novel account of scientific anomaly: Help for the dispute over low-dose biochemical effects. Phil Sci . 2006:73790–802. [Google Scholar]
- Elmore E, Lao X-Y, Kapadia R, Redpath JL. The effect of dose rate on radiation-induced neoplastic transformation in vitro by low doses of low-LET radiation. Radiat Res. 2006;166:832–838. doi: 10.1667/RR0682.1. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;2005;78(925):3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- Forbes VE. Is hormesis an evolutionary expectation? Funct Ecol. 2000;14:12–24. [Google Scholar]
- Forbes VE, Calow P. Is the per capita rate of increase a good measure of population-level effects in ecotoxicology? Environ Toxicol Chem. 1999;18:1544–1556. [Google Scholar]
- Friedl AA, Rühm W. LNT: a never ending story. Radiat Environ Biophys. 2006;44:241–244. doi: 10.1007/s00411-006-0028-5. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Takahashi T, Yoshioka T, Nakasuji F. Changes in egg size of the diamondback moth Plutella xylostella (Lepidoptera: Yponomeutidae) treated with fenvalerate at sublethal doses and viability of the eggs. Appl Entomol Zool. 2002;37:103–109. [Google Scholar]
- Fukushima S, Kinoshita A, Puatanachokchai R, Kushida M, Wanibuchi H, Morimura K. Hormesis and dose-response-mediated mechanisms in carcinogenesis: evidence for a threshold in carcinogenicity of non-genotoxic carcinogens. Carcinogenesis. 2005;26:1835–1845. doi: 10.1093/carcin/bgi160. [DOI] [PubMed] [Google Scholar]
- Gaylor D. Safety assessment with hormetic effects. Hum Exp Toxicol. 1998;17:251–253. doi: 10.1177/096032719801700506. [DOI] [PubMed] [Google Scholar]
- Goyer RA, Liu J, Waalkes MP. Cadmium and cancer of prostate and testis. BioMetals. 2004;17:555–558. doi: 10.1023/b:biom.0000045738.59708.20. [DOI] [PubMed] [Google Scholar]
- Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- Hartman PE, Shankel DM. Antimutagens and anticarcinogens: a survey of putative interceptor molecules. Environ Mol Mutagen. 1990;15:145–182. doi: 10.1002/em.2850150305. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Johnson FM. Analysis of National Toxicology Program rodent bioassay data for anticarcinogenic effects. Mutat Res. 1996;350:131–141. doi: 10.1016/0027-5107(95)00098-4. [DOI] [PubMed] [Google Scholar]
- Heigold S, Sers C, Bechtel W, Ivanovas B, Schäfer R, Bauer G. Nitric oxide mediates apoptosis induction selectively in transformed fibroblasts compared to nontransformed fibroblasts. Carcinogenesis. 2002;23:929–941. doi: 10.1093/carcin/23.6.929. [DOI] [PubMed] [Google Scholar]
- Hoffmann GR, Stempsey WE. The hormesis concept and risk assessment: Are there unique ethical and policy considerations. Hum Exper Toxicol. 2008;27:613–620. doi: 10.1177/0960327108098487. (online in BELLE Newsletter 14(3):11–17, 2008— http://www.belleonline.com/newsletters/volume14/vol14-3.pdf) [DOI] [PubMed]
- Hoffmann GR, Gessner GS, Hughes JF, Ronan MV, Sylvia KE, Willett CJ. Modulation of the genotoxicity of bleomycin by amines through noncovalent DNA interactions and alteration of physiological conditions in yeast. Mutat Res. 2007;623:41–52. doi: 10.1016/j.mrfmmm.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Holbeck SL. Update on NCI in vitro drug screen utilities. Eur J Cancer. 2004;40:785–793. doi: 10.1016/j.ejca.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Hooker AM, Horne R, Morley AA, Sykes PJ. Dose-dependent increase or decrease of somatic intrachromosomal recombination produced by etoposide. Mutat Res. 2002;500:117–124. doi: 10.1016/s0027-5107(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Hooker AM, Bhat M, Day TK, Lane JM, Swinburne SJ, Morley AA, Sykes PJ. The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat Res. 2004;162(4):447–452. doi: 10.1667/rr3228. [DOI] [PubMed] [Google Scholar]
- Ishii K, Watanabe M. Participation of gap-junctional cell communication on the adaptive response in human cells induced by low dose of X-rays. Int J Radiat Biol. 1996;69:291–299. doi: 10.1080/095530096145841. [DOI] [PubMed] [Google Scholar]
- Jeggo P, Defais M, Samson L, Schendel P. An adaptive response of E. coli to low levels of alkylating agent: Comparison with previously characterized DNA repair pathways. Mol Gen Genet. 1977;157:1–9. doi: 10.1007/BF00268680. [DOI] [PubMed] [Google Scholar]
- Jenkins GJS, Doak SH, Johnson GE, Quick E, Waters EM, Parry JM. Do dose response thresholds exist for genotoxic alkylating agents? Mutagenesis. 2005;20:389–398. doi: 10.1093/mutage/gei054. [DOI] [PubMed] [Google Scholar]
- Joëls M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kang C-M, Park K-P, Cho C-K, Seo J-S, Park W-Y, Lee S-J, Lee Y-S. Hspa4 (HSP70) is involved in the radioadaptive response: results from mouse splenocytes. Radiat Res. 2002;157:650–655. doi: 10.1667/0033-7587(2002)157[0650:hhiiit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kardong KV. An Introduction to Biological Evolution. 2nd Edition. McGraw-Hill; 2008. [Google Scholar]
- Keeton JT, Gould JL. Biological Science. 4th Edition. Norton; 1986. [Google Scholar]
- Kefford BJ, Zalizniak L, Warne JS, Nugegoda D. Is the integration of hormesis and essentiality into ecotoxicology now opening Pandora’s Box? Environ Pollution. 2008;151:516–523. doi: 10.1016/j.envpol.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Wanibuchi H, Wei M, Fukushima S. Hormesis in carcinogenicity of non-genotoxic carcinogens. J Toxicol Pathol. 2006;19:111–122. [Google Scholar]
- Kitchin KT, Drane JW. A critique of the use of hormesis in risk assessment. Hum Exptl Toxicol. 2005;24:249–253. doi: 10.1191/0960327105ht520oa. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM, Xu Y. Epigenetic mechanisms of chemical carcinogenesis. Hum Exptl Toxicol. 2000;19:543–555. doi: 10.1191/096032700701546442. [DOI] [PubMed] [Google Scholar]
- Kleibl K. Molecular mechanisms of adaptive response to alkylating agents in Escherichia coli and some remarks on O6-methylguanine DNA-methyltransferase in other organisms. Mutat Res. 2002;512:67. doi: 10.1016/s1383-5742(02)00025-x. [DOI] [PubMed] [Google Scholar]
- Kodell RL. U-shaped dose-response relationships for mutation and cancer. Hum Ecol Risk Assessment. 2001;7:909–919. [Google Scholar]
- Kodell RL, Gaylor DW, Greenman DL, Littlefield NA, Farmer JH. Response to the Society of Toxicology task force re-examination of the ED01 study. Fundam Appl Toxicol. 1983;3:3/a–8/a. doi: 10.1016/s0272-0590(83)80150-x. [DOI] [PubMed] [Google Scholar]
- Leonard BE. A review: development of a microdose model for analysis of adaptive response and bystander dose response behavior. Dose-Response. 2008;6:113–183. doi: 10.2203/dose-response.07-027.Leonard. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH-C, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares JF, Gustafsson I, Baquero F, Martinez JL. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci USA. 2006;103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Insecticide hormoligosis. J Econ Entomol. 1968;61:7–12. doi: 10.1093/jee/61.1.7. [DOI] [PubMed] [Google Scholar]
- Manchester KL. Theodor Boveri and the origin of malignant tumours. Trends Cell Biol. 1995;5:384–387. doi: 10.1016/s0962-8924(00)89080-7. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx. 2004;1:111–116. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM. Superoxide dismutase in aging and disease: an overview. Methods Enzymol. 2002;349:331–341. doi: 10.1016/s0076-6879(02)49348-2. [DOI] [PubMed] [Google Scholar]
- McCord JM, Edeas MA. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomed Pharmacother. 2005;59:139–142. doi: 10.1016/j.biopha.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ. Low doses of radiation are protective in vitro and in vivo: evolutionary origins. Dose-Response. 2006;4:75–90. doi: 10.2203/dose-response.04-002.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel REJ. Low doses of radiation reduce risk in vivo. Dose-Response. 2007;5:1–10. doi: 10.2203/dose-response.06-109.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, Carlisle SM. Upper dose thresholds for radiation-induced adaptive response against cancer in high-dose-exposed, cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res. 2004;162:20–30. doi: 10.1667/rr3190. [DOI] [PubMed] [Google Scholar]
- Miura Y. Oxidative stress, radiation-adaptive responses, and aging. J Radiat Res. 2004;45:357–372. doi: 10.1269/jrr.45.357. [DOI] [PubMed] [Google Scholar]
- Morgan WF. Genomic instability and bystander effects: a paradigm shift in radiation biology? Mil Med. 2002;167(Suppl 1):44–45. [PubMed] [Google Scholar]
- Morgan WF. Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene. 2003a;22:7094–7099. doi: 10.1038/sj.onc.1206992. [DOI] [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003b;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. 2003c;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Morgan WF, Sowa MB. Effects of ionizing radiation in nonirradiated cells. Proc Natl Acad Sci USA. 2005;102:14127–14128. doi: 10.1073/pnas.0507119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse JG. Agricultural implications of pesticide-induced hormesis of insects and mites. Hum Exptl Toxicol. 1998;17:266–269. doi: 10.1177/096032719801700510. [DOI] [PubMed] [Google Scholar]
- Morse JG, Zareh N. Pesticide-induced hormoligosis of citrus thrips (Thysanoptera: Thripidae) fecundity. J Econ Entomol. 1991;84:1169–1174. [Google Scholar]
- Mothersill C, Seymour CB. Radiation-induced bystander effects and adaptive responses—the Yin and Yang of low dose radiobiology? Mutat Res. 2004a;568:121–128. doi: 10.1016/j.mrfmmm.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Radiation-induced bystander effects—implications for cancer. Nat Rev Cancer. 2004b;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Radiation-induced bystander effects and the DNA paradigm: An “out of field” perspective. Mutat Res. 2006a;597:5–10. doi: 10.1016/j.mrfmmm.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Radiation-induced bystander effects: evidence for an adaptive response to low dose exposures? Dose-Response. 2006b;4:283–290. doi: 10.2203/dose-response.06-111.Mothersill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ. Artificial transmutation of the gene. Science. 1927;66:84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Mushak P. Hormesis and its place in nonmonotonic dose-response relationships: some scientific reality checks. Environ Health Perspect. 2007;115:500–506. doi: 10.1289/ehp.9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascarella MA, Stoffolano JG, Stanek EJ, Kostecki PT, Calabrese EJ. Hormesis and stage specific toxicity induced by cadmium in an insect model, the queen blowfly, Phormia regina Meig. Environ Pollution. 2003;124:257–262. doi: 10.1016/s0269-7491(02)00479-7. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC Committee on the Biological Effects of Ionizing Radiations) The Effects on Populations of Exposure to Low Levels of Ionizing Radiation (BEIR III) National Academy Press; Washington, DC: 1980. [Google Scholar]
- National Research Council (NRC Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiations) Health Risk from Exposure to Low Levels of Ionizing Radiation (BEIR VII, Phase 2) National Academy Press; Washington, DC: 2006. [PubMed] [Google Scholar]
- Pant MC, Liao X-Y, Lu Q, Molloi S, Elmore E, Redpath JL. Mechanisms of suppression of neoplastic transformation in vitro by low doses of low LET radiation. Carcinogenesis. 2003;24:1961–1965. doi: 10.1093/carcin/bgg172. [DOI] [PubMed] [Google Scholar]
- Park SH, Lee Y, Jeong K, Yoo SY, Cho CK, Lee YS. Different induction of adaptive response to ionizing radiation in normal and neoplastic cells. Cell Biol Toxicol. 1999;15:111–119. doi: 10.1023/a:1007525531145. [DOI] [PubMed] [Google Scholar]
- Portess DI, Bauer G, Hill MA, O’Neill P. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67:1246–1253. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Hormesis in aging. Ageing Res Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Redpath JL. Suppression of neoplastic transformation in vitro by low doses of low LET radiation. Dose-Response. 2006;4:302–308. doi: 10.2203/dose-response.06-114.Redpath. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath JL, Elmore E. Radiation-induced neoplastic transformation in vitro, hormesis and risk assessment. Dose-Response. 2007;5:123–130. doi: 10.2203/dose-response.06-010.Redpath. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath JL, Lu Q, Lao X, Molloi S, Elmore E. Low doses of diagnostic energy X-rays protect against neoplastic transformation in vitro. Int J Radiat Biol. 2003a;79:235–240. doi: 10.1080/0955300031000096306. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Short SC, Woodcock M, Johnston PJ. Low-dose reduction in transformation frequency compared to unirradiated controls; the role of hyper-radiosensitivity to cell death. Radiat Res. 2003b;159:433–436. doi: 10.1667/0033-7587(2003)159[0433:ldritf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ross WD. The Right and the Good. Hackett Publishing Company; Indianapolis: 1988. pp. 21–22. [Google Scholar]
- Rouse J, Jackson SP. Interfaces between the detection, signaling, and repair of DNA damage. Science. 2002;297:547–551. doi: 10.1126/science.1074740. [DOI] [PubMed] [Google Scholar]
- Sagan LA. On radiation, paradigms, and hormesis. Science. 1989;245:574–621. doi: 10.1126/science.2669125. [DOI] [PubMed] [Google Scholar]
- Sakai K, Nomura T, Ina Y. Enhancement of bio-protective functions by low dose/dose-rate radiation. Dose-Response. 2006;4:327–332. doi: 10.2203/dose-response.06-115.Sakai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson L, Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977;267:281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Samson L, Schwartz JL. Evidence for an adaptive DNA repair pathway in CHO and human skin fibroblast cell lines. Nature. 1980;287:861–863. doi: 10.1038/287861a0. [DOI] [PubMed] [Google Scholar]
- Schöllnberger H, Ménache MG, Hanson TE. A biomathematical modeling approach to explain the phenomenon of radiation hormesis. Hum Ecol Risk Assess. 2001;7:867–890. [Google Scholar]
- Schöllnberger H, Stewart RD, Mitchel RE. Low-let-induced radioprotective mechanisms within a stochastic two-stage cancer model. Dose-Response. 2006;3:508–518. doi: 10.2203/dose-response.003.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Di Palma J. Sparsely ionizing diagnostic and natural background radiations are likely preventing cancer and other genomic-instability-associated diseases. Dose-Response. 2006;5:230–255. doi: 10.2203/dose-response.06-002.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H-R, Chung H-Y, Lee Y-J, Bae S, Lee S-J, Lee Y-S. p26/Cip/Kip is involved in Hsp25 or inducible Hsp70 mediated adaptive response by low dose radiation. J Radiat Res. 2006;47:83–90. doi: 10.1269/jrr.47.83. [DOI] [PubMed] [Google Scholar]
- Shadley JD, Wolff S. Very low doses of X-rays can cause human lymphocytes to become less susceptible to ionizing radiation. Mutagenesis. 1987;2:95–96. doi: 10.1093/mutage/2.2.95. [DOI] [PubMed] [Google Scholar]
- Shadley JD, Afzal V, Wolff S. Characterization of the adaptive response to ionizing radiation induced by low doses of x rays to human lymphocytes. Radiat Res. 1987;111:511–517. [PubMed] [Google Scholar]
- Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res. 2000;460:1–15. doi: 10.1016/s0921-8777(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Shevell DE, Friedman BM, Walker GC. Resistance to alkylation damage in Escherichia coli: Role of the Ada protein in induction of the adaptive response. Mutat Res. 1990;233:53–72. doi: 10.1016/0027-5107(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Shrader-Frechette K. Ideological toxicology: invalid logic, science, ethics about low-dose pollution. Hum Exper Toxicol. 2008;27:647–657. doi: 10.1177/0960327108098491. (online in BELLE Newsletter. 2008. 14(3):39–47 — http://www.belleonline.com/newsletters/volume14/vol14-3.pdf) [DOI] [PubMed]
- Society of Toxicology Task Force (no authors listed) Re-examination of the ED01 study—adjusting for time on study. Fundam Appl Toxicol. 1981;1:67–80. [PubMed] [Google Scholar]
- Southam CM, Ehrlich J. Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathol. 1943;33:517–524. [Google Scholar]
- Stebbing ARD. Hormesis: interpreting the β-curve using control theory. J Appl Toxicol. 2000;20:93–101. doi: 10.1002/(sici)1099-1263(200003/04)20:2<93::aid-jat640>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Stebbing ARD. A mechanism for hormesis – a problem in the wrong discipline. Crit Rev Toxicol. 2003;33:463–467. doi: 10.1080/713611038. [DOI] [PubMed] [Google Scholar]
- Stecca C, Gerber GB. Adaptive response to DNA-damaging agents. Biochem Pharmacol. 1998;55:941–951. doi: 10.1016/s0006-2952(97)00448-6. [DOI] [PubMed] [Google Scholar]
- Su LY, Dela Cruz A, Moore PH, Maretzki A. The relationship of glyphosate treatment to sugar metabolism in sugarcane: new physiological insights. J Plant Physiol. 1992;140:168–173. [Google Scholar]
- Sykes PJ, Day TK. Requirements for identification of low dose and non-linear mutagenic responses to ionising radiation. Dose-Response. 2007;5:308–314. doi: 10.2203/dose-response.07-018.Sykes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes PJ, Morley AA, Hooker AM. The pKZ1 recombination mutation assay: a sensitive assay for low dose studies. Dose-Response. 2006;4:91–105. doi: 10.2203/dose-response.05-035.Sykes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Melnick R, Burns K, Davis D, Huff J. Fundamental flaws of hormesis for public health decisions. Environ Health Perspect. 2005;113:1271–1276. doi: 10.1289/ehp.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Melnick R, Huff J, Burns K, Davis D. Hormesis: a new religion? Environ Health Perspect. 2006;114:A632–A633. doi: 10.1289/ehp.114-1665404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci USA. 2005;102:7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JF, Luckey TD. Hormoligosis in pharmacology. J Amer Med Assoc. 1960;173:44–48. doi: 10.1001/jama.1960.73020190007010. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A. Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionising radiation: the Joint Report of the Académie des Sciences (Paris) and of the Academie Nationale de Médecine. Int J Low Radiation. 2006;2:1–19. doi: 10.1016/j.ijrobp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A, Averbeck D, Masse R. Recent reports on the effects of low doses of ionizing radiation and its dose-effect relationship. Radiat Enviro Biophy. 2006;44:245–251. doi: 10.1007/s00411-006-0032-9. [DOI] [PubMed] [Google Scholar]
- vanBogelen RA, Neidhardt FC. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanBogelen RA, Kelley PM, Neidhardt FC. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987;169:26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velini ED, Alves E, Godoy MC, Meschede DK, Souza RT, Duke SO. Glyphosate applied at low doses can stimulate plant growth. Pest Management Science. 2008 doi: 10.1002/ps.1562. [DOI] [PubMed] [Google Scholar]
- Volkert MR. Adaptive response of Escherichia coli to alkylation damage. Environ Mol Mutagen. 1988;11:241–255. doi: 10.1002/em.2850110210. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Sheehan DM. Challenging risk assessment. Forum Appl Res Publ Policy. 1998;13(3):11–18. [Google Scholar]
- Waalkes MP, Rehm S, Riggs CW, Bare RM, Devor DE, Poirier LA, Wenk ML, Henneman JR, Balaschak MS. Cadmium carcinogenesis in male Wistar [Crl:(WI)BR] rats: Dose-response analysis of tumor induction in the prostate and testes and at the injection site. Cancer Res. 1988;48:4656–4663. [PubMed] [Google Scholar]
- Waddell WJ. Thresholds of carcinogenicity in the ED01 study. Toxicol Sci. 2003;72:158–163. doi: 10.1093/toxsci/kfg004. Letters to the Editor. 2003. Toxicol Sci 74:485–488; Letters to the Editor. 2003. Toxicol Sci 75:223–225. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese AG, Pacifici RE, Davies KJA. Transient adaptation of oxidative stress in mammalian cells. Arch Biochem Biophys. 1995;318(1):231–240. doi: 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- Winquist L, Rannug U, Rannug A, Ramel C. Protection from toxic and mutagenic effects of H2O2 by catalase induction in Salmonella typhimurium. Mutat Res. 1984;141:145–147. doi: 10.1016/0165-7992(84)90087-3. [DOI] [PubMed] [Google Scholar]
- Wolff S. Are radiation-induced effects hormetic? Science. 1989;245:575–621. doi: 10.1126/science.2762808. [DOI] [PubMed] [Google Scholar]
- Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect. 1998;106:277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A. Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double-strand breaks in DNA. Int J Radiat Biol. 1988;53:39–48. doi: 10.1080/09553008814550401. [DOI] [PubMed] [Google Scholar]
- Wolff S, Afzal V, Jostes RF, Wiencke JK. Indications of repair of radon-induced chromosome damage in human lymphocytes: an adaptive response induced by low doses of X-rays. Environ Health Perspect. 1993;101(Suppl 3):73–77. doi: 10.1289/ehp.93101s373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E. Illusions of safety: antimutagens can be mutagens, and anticarcinogens can be carcinogens. Mutat Res. 2003;543:191–194. doi: 10.1016/s1383-5742(02)00111-4. [DOI] [PubMed] [Google Scholar]
- Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Chen G, Trosko JE, Waldren CA, Hei TK. Radiation risk to low fluences of α particles may be greater than we thought. Proc Natl Acad Sci USA. 2001;98:14410–14415. doi: 10.1073/pnas.251524798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ, Hei TK. Interaction between radiation-induced adaptive response and bystander mutagenesis in mammalian cells. Radiat Res. 2003;160:512–516. doi: 10.1667/rr3083. [DOI] [PMC free article] [PubMed] [Google Scholar]