INTRODUCTION
Coronary artery disease (CAD) is the leading cause of death in women. Mammography is a common, inexpensive and widely accepted test that is recommended to all middle aged women to screen for breast cancer. Breast arterial calcification (BAC) detected in mammograms are frequently not included in the final reports because it is considered a benign finding that is not relevant to the diagnosis of breast cancer. The preponderance of studies in this topic, including large cohorts in Europe and the US, indicate that the detection and grading of BAC in mammograms may be a useful marker of atherosclerosis and therefore may have value for cardiovascular risk stratification (1–9). The only exception is a smaller, case-control study that reported no significant association between BAC and CAD detected on angiography (10).
BAC assessment could be performed during regular rounds of screening mammography with minimal added burden to radiologists. However, additional evidence from large-scale prospective studies is needed to guide recommendations for routine BAC reporting in clinical practice. Previous studies on breast arterial calcification have reported only presence or absence of calcification in a mammogram. The currently available full field digital mammography systems provide the capability to quantify BAC. We have recently developed a technique for quantification of BAC using standard full field digital mammography and performed an anthropomorphic breast phantom study that demonstrates the feasibility of quantifying vascular calcium mass using densitometry after taking into account the effects of scatter correction, breast thickness, breast density, anatomic background and magnification error on calcium mass (11).
The main objective of the current study was twofold: first, to determine the reproducibility and second, to assess the inter-reader agreement of our technique for quantification of breast arterial calcification, respectively. We have also introduced an automated technique for calcium calibration based on previous phantom measurements.
METHODS
Patient population
We recruited a convenient, consecutive sample of 42 women attending their regular mammographic examination at the KP San Francisco Medical Center Department of Radiology. To maximize the chances of measuring breast arterial calcium, we targeted women over age 49 years and, whenever a prior mammogram was available, women with apparent arterial calcifications in their prior mammogram. This study was conducted in a 2-week period starting May 7th, 2007. All the participants provided written consent form and the study protocol was approved by the Kaiser Foundation Research Institute Institutional Review Board. Of the 42 women who participated in the study, 3 women did not have any detectable BAC on either of the mammographic projections and were excluded from further analysis. Therefore calcium measurement was done in the remaining 39 women. The mean age of the 39 women was 65 years (range, 49–82 years). The study protocol was approved by the Kaiser Foundation Research Institute and all women provided informed written consent.
Image acquisition and processing
All images in this study were acquired using a full-field digital mammography system (Senographe 2000D, GE Medical Systems, Milwaukee, WI), which uses an aSi:H flat panel detector coupled with a CsI(Tl) converter layer with an anti-scatter grid. The anti-scatter grid consists of 5:1 grid-ratio and 31 lines/cm. Automatic exposure control (AEC) was used for image acquisition. The system can operate in one of three AEC or automated optimization of parameters (AOP) modes: AOP contrast (CNT), AOP standard (STD), or AOP dose (DOS) mode. The system makes a pre-exposure that is used to select the target material (molybdenum or rhodium), filtration material (molybdenum or rhodium), and peak kilovoltage (kVp) setting. All the images for this study were acquired using the AOP standard (STD) mode at the Department of Radiology, Kaiser Permanente San Francisco Medical Center and transferred to a workstation for image analysis at University of California, Irvine.
Calcium calibration
System calibration is necessary for quantification of calcium mass using densitometry. A calcium calibration phantom was constructed by mixing 1.50 grams of calcium hydroxyapatite (CaHA) (Ca5HO13P3, Sigma-Aldrich Inc, St. Louis, MO) powder with 4.50 grams of the plastic resin and 0.54 grams of curing agent. This mixture was used to make plastic rods of different diameters (0.6 –1.89 mm) with a known CaHA weight fraction. The dimension and calcium content of the rods were chosen to approximately simulate the observed breast arterial calcification in mammograms. The rods were then sealed into a plastic resin block of 3.00 mm thickness (11). Calcium calibrations were made on 2–9 cm uniform breast tissue equivalent material (BR12, GAMMEX RMI, Middleton, MI) using standard automatic brightness control. BR12 is a homogenous material that mimics the attenuation properties of an average density breast with 50% glandular/50% adipose breast tissue over the range of energies used in mammography (12). A previous study indicates that composition variations and differences of approximately 1 cm between calibration phantom and breast thickness introduce only minimal error in calcium measurement (11). A region of interest (ROI) was then drawn around the individual calcium phantom rods. The integrated signal intensity in the background subtracted images was then plotted with respect to the known CaHA mass in each rod.
Calcium calibrations were repeated to include a range of anticipated thicknesses and beam energies (kVp) for each x-ray tube anode. However, it is not feasible to sample every possible combination of breast thickness, kVp and x-ray tube anode. Since the calcium calibration slope is a function of beam energy and thickness, it is possible to fit the sampled calibrations slopes to a function which models this physical behavior. Using a Levenberg-Marquardt nonlinear least squares algorithm, the calibration data was fit to the following equation:
Where K and T are the kVp and thickness, respectively, S is the calcium calibration slope and a1, a2, b1, b2 are fitting parameters. The fit was performed for both Molybdenum and Rhodium x-ray tube anodes. The relative RMS error quantified by calculating the residuals was 0.84 % of the mean calibration slope for the Molybdenum anode and 1.05% of the mean calibration slope for the Rhodium anode. The fitting of the calibration data to this two dimensional surface permitted the calculation of calcium calibration slopes for points not explicitly sampled during the calibration process. The slope of the calibration curve was used to estimate CaHA mass from integrated signal intensity.
Calcium measurement
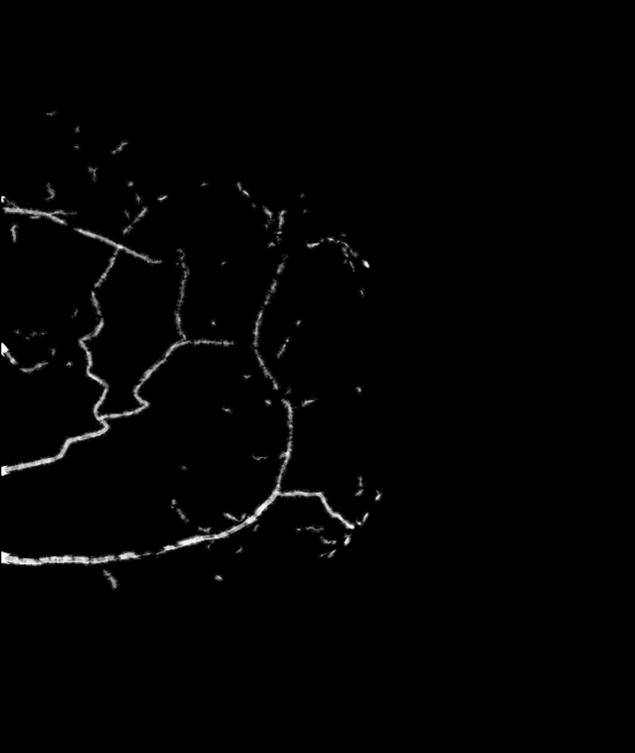
Full-field digital mammograms were acquired using standard mediolateral oblique (MLO) and craniocaudal (CC) views. The original raw images were initially logarithmically transformed (Figure 1A). An ROI was then drawn around each breast arterial calcification (Figure 1B). The surrounding local background was estimated and subtracted using the following linear interpolation algorithm (Figure 1B). Each pixel inside the ROI was assigned a shortest line that passed through the pixel and connected to pixels bordering the ROI. Bordering pixels that yielded the shortest line were found by searching eight radial directions surrounding the pixel in 45 degree increments. The gray value for pixels inside the ROI was linearly interpolated from the two closest pixel values found during the search. When more than one shortest path length existed, the average of the interpolated gray values from each path length was used (11, 13). This background estimate was subtracted from the original region of interest in order to produce an estimate of the breast arterial calcification alone (Figure 1D). The integrated signal intensity in the ROI was used for calcium mass measurement after converting it to CaHA mass using system calibration. Calcium calibration was determined based on compressed breast thickness, kVp, x-ray tube target and filtration from Dicom header for each digital mammogram. Previously measured calcium calibration slopes for different uniform phantom thicknesses, kVp, x-ray tube target and filtration was used for this purpose. A comprehensive calcium calibration of the system was done in advance of the mammograms. Therefore, the mammograms were acquired using the standard clinical protocol. The true depth of any calcium mass within the breast is unknown and therefore it must be assumed. In order to minimize the magnification error, the calcified vessel is assumed to be in the middle of the compressed breast (11).
Figure 1.
Original mammogram (A) is used to draw a region of interest around the calcified arteries (B). The background signal inside the region of interest is estimated using a linear interpolation technique based on the surrounding pixel values (C). This image is then subtracted from the original image to produce an image of the calcified arteries (D).
Ideally, in order to assess reproducibility of calcium measurement, it is necessary to acquire two sequential mammograms, but this was not possible due to the additional radiation dose to the patient. Instead, since standard mammograms routinely acquire two standard projections (MLO and CC), we were able to compare the measured calcium mass from the two projections. Linear regression analysis was used to estimate the degree of correlation between the standard projections, separately in right and left breasts.
In order to assess the inter-observer agreement of the technique, all the images were analyzed by two different observers. The calcium measurement technique is automated except drawing ROIs around breast arterial calcification, which was done by two different observers. Therefore, the calcium calibration process and the rest of the calcium measurement was the same. The measured calcium mass obtained by each observer in each projection for each breast was compared using intra-class correlation coefficients estimated as described by Shrout and Fleiss (14).
RESULTS
Calcium calibration
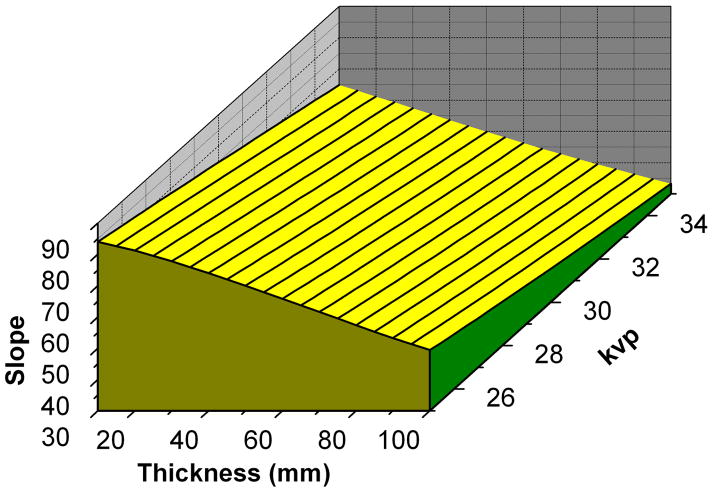
Figure 2 shows an image of the calcium calibration phantom and a typical calibration curve. The slope of the calibration curve is needed to convert the integrated signal intensity to CaHA mass. Figure 3 shows the slope of the calibration curve for different phantom thicknesses, beam energy and x-ray tube target material. The Mo/Mo anode is used for lower phantom thicknesses and beam energies (figure 3a), Mo/Rh anode is used for midrange phantom thicknesses and beam energies (figure 3b) while the Rh/Rh anode is used for the higher phantom thicknesses and beam energies (figure 3c). In order to assess the variability of the calcium calibration over time, a comparison was made of the measured calcium calibration slope during the patient study and the predicted slope from phantom studies made two months apart (figure 4). The comparison of the calibration slopes over time show that the calibration slope does not significantly change over the two month period.
Figure 2.
Image of the calcium calibration phantom (a) and an example of the calcium calibration curve for a 33 mm thickness phantom and a 28 Mo/Mo technique (b).
Figure 3.
3-D plot of calibration slope as functions of kVp and phantom thickness for Mo/Mo (a), Mo/Rh (b) and Rh/Rh (c) anode and filtration combinations.
Figure 4.
Measured calibration slope (Integrated signal intensity/mg CaHA mass) compared to predicted slope from phantom studies (N = 17). The predicted (Sp) and measured (Sm) calibration slopes were related by Sp = 1.114 Sm + 0.984 (r =0.998 and SEE = 0.613).
Calcium measurements
The comparison of calcium measurements from MLO and CC projections, during standard mammography in the 39 patients, is shown in figure 5. The measurements include results from both right and left breasts. The measured CaHA mass in CC (MCC) and MLO (MMLO) projections were related by MCC = 0.82 MMLO+ 0.27 mg (r =0.97 and SEE = 3.44 mg). The comparison of the calcium mass between left and right breasts is shown in figure 6. The average of the measured calcium mass from the two projections was used for this purpose. The measured CaHA mass in the left (ML) and right (MR) breasts were related by ML = 0.86 MR − 0.06 mg (r =0.95 and SEE = 4.30 mg). Thus, measured arterial calcium masses in the left and right breasts were also highly correlated.
Figure 5.
Comparison of the calcium mass measured (N = 78) in the CC perspective to the MLO projections. The measured CaHA mass in CC (MCC) and MLO (MMLO) projections were related by MCC = 0.82 MMLO+ 0.27 mg (r =0.97 and SEE = 3.44 mg).
Figure 6.
Correlation of calcium mass (N = 39) between right and left breasts. The measured CaHA mass in the left (ML) and right (MR) breasts were related by ML = 0.86 MR − 0.06 mg (r =0.95 and SEE = 4.30 mg).
The estimated intra-class correlation coefficients were: 0.94 for Left CC, 0.95 for Left MLO, 0.99 for Right CC and 0.98 for Right MLO. The ICC was 0.96 for the average calcium mass of the left breast, 0.99 for the average calcium mass of the right breast, and 0.97 for the total breast calcium mass (i.e., the sum of the average calcium mass of the left breast + the average calcium mass of the right breast). A plot of all the breast calcium mass measurements (MLO and CC projections for right and left breasts) performed by the two observers is shown in Figure 7.
Figure 7.
Comparison of the total calcium mass (N = 156) measured by two different observers. The estimated intra-class correlation coefficient is 0.97.
DISCUSSION
Breast arterial calcification in mammograms may be a useful marker of atherosclerosis and therefore may have value for cardiovascular risk stratification (1, 3–8). However, previous studies on breast arterial calcification have reported only presence or absence of calcification in a mammogram. The degree to which severity of BAC provides additional prognostic information above and beyond presence vs. absence of BAC is currently unknown. This study evaluated a densitometry technique for quantification of BAC by measuring calcium mass using standard full field digital mammograms. The technique may provide a more accurate metric for future studies relating severity of BAC and cardiovascular risk. In this technique, calcium mass measurements are based on calcium calibration on uniform breast tissue equivalent material. Calcium calibration was done for different phantom thicknesses, beam energies (kVp) and anode-filter combinations. The recorded breast thickness and beam parameters from the DICOM file was then used to determine the calcium calibration factor for each mammogram from previous measurements on uniform breast tissue equivalent phantoms. The reproducibility of the technique was evaluated by comparing calcium mass measurements from mammograms acquired in two different standard projections and the inter-observed agreement by comparing results derived by two different observers.
Interpretation of results
In an effort to simplify the clinical implementation of the technique, calcium calibration was done based on previous calibration measurements. Figure 3 shows the calcium calibration slope for different breast tissue equivalent thicknesses and beam energies. Figure 4 shows a comparison of the measured calcium calibration slope during the patient study and the predicted slope from phantom studies. The results indicate that the calibration slope does not change over time and it might be sufficient to only measure the calibration slope periodically.
Figure 5 shows the relation of the measured breast arterial calcification using mammograms from MLO and CC projections. The results show that calcium measurements made from two different projections are very highly correlated. The ideal measure of reproducibility would be comparing the same projection between repeated mammograms. However, the associated radiation dose to the patient precluded us from performing repeated mammograms. Figure 6 shows the correlation of calcium mass measured from right and left breasts. The high-level correlation of the calcium mass measured from the right and left breasts suggests a common systemic cause of breast arterial calcification.
The inter-observer agreement was excellent but not perfect (i.e., intraclass correlation coefficient=1). We believe that the main source of inter-observer variability is separating calcium structures from other soft tissue anatomical background. Separation of calcified structures from other soft tissue background can be done using dual energy mammography. This is particularly important in the case of breast arterial calcifications near the threshold of detectibility.
Limitations
Quantification of breast arterial calcification requires careful calcium calibration. Calibration was performed based on a previously measured calcium calibration at different breast phantom thicknesses and beam energies. This calibration method does not require any modification in the standard image acquisition for clinical mammography. Calibration can also be performed by including a calcium calibration phantom within each mammogram. This can be implemented by placing the phantom on a balloon filled with a mixture of water and oil. The balloon can be placed in the image field next to the breast. This calibration method does not require acquisition of an additional image for calibration. However, it slightly modifies the standard image acquisition for clinical mammography, which might hamper its clinical implementation.
The uncertainty in magnification can introduce error in quantification of breast arterial calcification. The magnification error increases as the breast thickness increases (11). Breast arterial calcification was assumed to be at the center layer of the compressed breast, which reduces the magnification error. The magnification error can potentially be further reduced by more accurate estimation of the location of calcification within the compressed breast. The location of a calcified artery within the breast can potentially be estimated using the MLO and CC views acquired during conventional mammography.
The calcium quantification technique was performed by drawing an ROI around the calcium signal in the MLO and CC projection mammograms. The images with the ROIs were reviewed by a radiologist to validate that non-vascular calcification was not included in the measurements. The surrounding local anatomical background was estimated and subtracted using a linear interpolation algorithm (11). Manual drawing of the region of interest around the visualized breast arterial calcification is time consuming. This is particularly challenging in the cases of mild breast arterial calcification where anatomical background structure noise limits its detection and in cases that calcification is present due to other pathophysiological conditions. It will be helpful to automate this process using computer aided diagnosis (CAD) and dual energy mammography.
In this study the reproducibility of quantification of breast arterial calcification was assessed by comparing measurements from MLO and CC projections. The measured calcium mass from the two projections should ideally be the same. However, there are a number of physical limitations that can change the measured calcium mass from the two projections. The main factors that can change the measured calcium mass from the two projections are differences in the extent of the breast arteries included from the two projections, differences in the overlying anatomical structures, differences in detection of arterial calcification and differences in scatter and beam hardening. Therefore, these factors are expected to attenuate the correlation of the mass measured from two different projections. The coverage of the breast from the two projections is slightly different because the truncation of the breast arterial calcification on the chest wall side is different for the two projections. Another factor is that the same breast arterial calcification might not be detectable from the two views. This is particularly the case for mild calcification where it might be near the threshold of detectability and it can only be detected from one view. Detectability and quantification of mild breast arterial calcification is limited by the background anatomical structure noise in standard digital mammography. Dual energy mammography can potentially improve the detectability and quantification of breast arterial calcification. Cone-beam CT is expected to further improve the detectability and quantification of breast arterial calcification. However, these techniques have not been implemented for routine clinical application.
Clinical implications
To date, two prospective cohort studies have evaluated clinical outcomes of women with BAC. Investigators from the Netherlands performed a prospective cohort study in 12,239 women aged between 50–68 years who participated in a breast cancer screening program between 1975 and 1977 (1). They found arterial calcification in 9% of all women. Data from 16–19 years of follow-up were analyzed to determine the relationship between BAC and cardiovascular disease (CVD) mortality. After adjustment for age, hypertension, obesity, smoking and parity, a 1.4 increased hazard of CVD mortality was found for all women with BAC. Notably, in diabetic women, the presence of BAC was associated with a 1.9 increased hazard of CVD death. In regard to overall mortality, they reported an increased hazard of 1.3 for all women with BAC and an increased hazard of 1.7 among diabetic women with BAC. These results indicate that BAC represents an independent risk marker for CVD mortality in women over 50 years of age, especially in those with diabetes. In another prospective study 12,761 women were followed for 25 years after the initial mammogram (3). After adjustment for age, race, educational level, cigarette smoking, alcohol use, body mass index, serum total cholesterol, hypertension, diabetes, family history of heart disease, parity, and hormone replacement therapy, BAC was associated with coronary heart disease (CHD) (hazard ratio [HR] = 1.32), ischemic stroke (HR = 1.41) and heart failure (HR = 1.52). Taken together, these two prospective studies highlight the potential of BAC for independent prediction of CVD morbidity and mortality. In the European cancer-Norfolk study among 1,590 women older than 55 years, BAC found on mammograms was present in 16% of women and was associated with prevalent CHD after adjustment for age, but with a sensitivity of 32% (specificity was higher, 85%) (4).
An association between BAC and coronary artery disease (CAD) has also been shown in studies which analyzed presence of BAC in women who underwent coronary angiography (5, 6). Significant CAD was defined in this study as a diameter narrowing of at least 50% in at least one coronary artery. Doerger et al., in a study of 1,803 women at the Mayo Clinic, found that BAC is a risk marker for coronary artery disease independent of age (5). Moshyedi et. al. found that positive predictive value of BAC for CAD in women aged less than 59 years was 0.88 and a negative predictive value was 0.65 (6). Another report based on 319 women who underwent coronary angiography and mammography within a two year period showed that the prevalence of BAC was non-significantly higher in the CAD group (p=0.13) (10). A report by Italian investigators demonstrates a strong correlation between the presence and severity of BAC and the coronary artery calcium score, suggesting that BAC may also be a surrogate marker of sub-clinical CVD (7). A prospective evaluation of 645 women undergoing consecutive routine screening mammography also demonstrates that the presence of breast vascular calcifications is significantly higher in women with peripheral vascular disease (8). In another recent study by Dale et al. among 819 women with no history of diabetes or coronary artery disease (CAD) and 395 women with a positive history of CAD, the odds of having coronary artery disease when BAC was present in screening mammography was 6.2 (95% CI, 4.3–8.2) (9). The authors concluded that mammography may be a useful screening tool for CAD and for identifying women who may benefit from early therapeutic intervention.
Previous studies on breast arterial calcification have reported only presence or absence of calcification in a mammogram. This study presents a technique for quantification of BAC using full field digital mammography. Approximately 30% of mammography systems in the USA are now based on digital technology and the fraction of digital mammography systems is rapidly increasing. Furthermore, approximately 62% of American women age 65 and older report having a mammogram in the past year (and this proportion is much higher in managed care), which indicates that quantification of BAC could be performed on the majority of women (15).
This technique for quantification of breast arterial calcification can be used to determine its correlation to coronary artery calcification (16, 17). It can also provide the capability to assess the progression or regression of BAC. The advantage of this technique for quantification of arterial calcification using standard digital mammography is that it does not require any extra x-ray exposure to the patient. In conjunction with a breast cancer screening program, the ability to provide additional information regarding cardiovascular disease risk can be of potentially great benefit to the public.
Acknowledgments
This research was supported in part by Grant No. R01 HL083295 awarded by the NHLBI, DHHS. The authors would like to thank Cathy Stern for image acquisition and Irina Tolstykh, M.S., for assistance with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sabee Molloi, Department of Radiological Sciences, University of California, Irvine, California 92697
Toufan Mehraien, Department of Radiological Sciences, University of California, Irvine, California 92697
Carlos Iribarren, Kaiser Permanente, Division of Research, Oakland, California 94697
Christopher Smith, Department of Radiological Sciences, University of California, Irvine, California 92697.
Justin L. Ducote, Department of Radiological Sciences, University of California, Irvine, California 92697
Stephen A. Feig, Department of Radiological Sciences, University of California, Irvine, California 92697
References
- 1.van Noord PA, Beijerinck D, Kemmeren JM, van der Graaf Y. Mammograms may convey more than breast cancer risk: breast arterial calcification and arterio-sclerotic related diseases in women of the DOM cohort. Eur J Cancer Prev. 1996;5:483–487. [PubMed] [Google Scholar]
- 2.Kemmeren JM, Beijerinck D, van Noord PA, et al. Breast arterial calcifications: association with diabetes mellitus and cardiovascular mortality. Work in progress Radiology. 1996;201:75–78. doi: 10.1148/radiology.201.1.8816524. [DOI] [PubMed] [Google Scholar]
- 3.Iribarren C, Go AS, Tolstykh I, Sidney S, Johnston SC, Spring DB. Breast vascular calcification and risk of coronary heart disease, stroke, and heart failure. J Womens Health (Larchmt) 2004;13:381–389. doi: 10.1089/154099904323087060. discussion 390–382. [DOI] [PubMed] [Google Scholar]
- 4.Kataoka M, Warren R, Luben R, et al. How predictive is breast arterial calcification of cardiovascular disease and risk factors when found at screening mammography? American Journal of Roentgenology. 2006;187:73–80. doi: 10.2214/AJR.05.0365. [DOI] [PubMed] [Google Scholar]
- 5.Doerger K, Whaley D, Berger P, Rowland C, Britain M. Breast arterial calcification detected on mammography is a risk factor for coronary artery disease. Radiology. 2002;225:553. [Google Scholar]
- 6.Moshyedi AC, Puthawala AH, Kurland RJ, O’Leary DH. Breast arterial calcification: association with coronary artery disease. Work in progress Radiology. 1995;194:181–183. doi: 10.1148/radiology.194.1.7997548. [DOI] [PubMed] [Google Scholar]
- 7.Pecchi A, Rossi R, Coppi F, Ligabue G, Modena MG, Romagnoli R. Association of breast arterial calcifications detected by mammography and coronary artery calcifications quantified by multislice CT in a population of post-menopausal women. Radiol Med (Torino) 2003;106:305–312. [PubMed] [Google Scholar]
- 8.Dale PS, Graham J, Nichols KW, Catchings T, Richards M. Mammography as a screening tool for peripheral vascular disease. Am J Surg. 2006;192:488–491. doi: 10.1016/j.amjsurg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Dale PS, Mascarhenas C, Richards M, Mackie G. Mammography as a screening tool for coronary artery disease. J Surg Res. 2008;148:1–6. doi: 10.1016/j.jss.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Henkin Y, Abu-Ful A, Shai I, Crystal P. Lack of association between breast artery calcification seen on mammography and coronary artery disease on angiography. J Med Screen. 2003;10:139–142. doi: 10.1177/096914130301000308. [DOI] [PubMed] [Google Scholar]
- 11.Molloi S, Xu T, Ducote JL. Quantification of breast arterial calcification using full field digital mammography. Medical Physics. 2008;35:1428–1439. doi: 10.1118/1.2868756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White DR. The formulation of tissue substitute materials using basic interaction data. Phys Med Biol. 1977;22:889–899. doi: 10.1088/0031-9155/22/5/008. [DOI] [PubMed] [Google Scholar]
- 13.Wong JT, Kamyar F, Molloi S. Quantitative coronary angiography using image recovery techniques for background estimation in unsubtracted images. Med Phys. 2007;34:4003–4015. doi: 10.1118/1.2779942. [DOI] [PubMed] [Google Scholar]
- 14.Shrout PE, Fleiss JL. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychological Bulletin. 1979;2:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society: Cancer Facts & Figures. 2007.
- 16.Molloi S, Detrano R, Ersahin A, Roeck W, Morcos C. Quantification of coronary arterial calcium by dual energy digital subtraction fluoroscopy. Med Phys. 1991;18:295–298. doi: 10.1118/1.596674. [DOI] [PubMed] [Google Scholar]
- 17.Xu T, Ducote JL, Wong JT, Molloi S. Feasibility of real time dual-energy imaging based on a flat panel detector for coronary artery calcium quantification. Med Phys. 2006;33:1612–1622. doi: 10.1118/1.2198942. [DOI] [PubMed] [Google Scholar]