Abstract
Somatic sensation relies on the transduction of physical stimuli into electrical signals by sensory neurons of the dorsal root ganglia. Little is known about how and when during development different types of sensory neurons acquire transduction competence. We directly investigated the emergence of electrical excitability and mechanosensitivity of embryonic and postnatal mouse sensory neurons. We show that sensory neurons acquire mechanotransduction competence coincident with peripheral target innervation. Mechanotransduction competence arises in different sensory lineages in waves, coordinated by distinct developmental mechanisms. Sensory neurons that are mechanoreceptors or proprioceptors acquire mature mechanotransduction indistinguishable from the adult already at E13. This process is independent of neurotrophin-3 and may be driven by a genetic program. In contrast, most nociceptive (pain sensing) sensory neurons acquire mechanosensitive competence as a result of exposure to target-derived nerve growth factor. The highly regulated process of mechanosensory acquisition unveiled here, reveals new strategies to identify molecules required for sensory neuron mechanotransduction.
Keywords: ion channels, mechanotransduction, nerve growth factor, nociception, touch
Introduction
Sensory neurons in the dorsal root ganglia (DRG) mediate somatic sensations such as touch, warmth, cold and pain and can be classified as mechanoreceptors, thermoreceptors and nociceptors (Lewin and Moshourab, 2004). The functional properties of sensory neurons are correlated with their anatomical and neurochemical features. For example, nociceptive neurons often contain neuropeptides and are defined by their expression of the nerve growth factor (NGF) receptor TrkA (Lewin and Barde, 1996; Luo et al, 2007; Marmigere and Ernfors, 2007). Mechanoreceptors, which also include proprioceptors that signal limb position and movement, have larger cell bodies, do not contain neuropeptides and express either the neurotrophin-3 (NT-3) receptor TrkC or brain-derived neurotrophic factor (BDNF) receptor TrkB (Marmigere and Ernfors, 2007). The achievement of neurochemical and anatomical diversity in the sensory lineage has been extensively investigated in recent years. Starting around embryonic day 9 (E9) in the mouse, there are two consecutive waves of neurogenesis that form the early DRG from migrating neural crest cells (NCCs). Putative mechanoreceptors and proprioceptors that are TrkC+ or TrkB+ are the first sensory neurons born, between E10 and E11, and arise from NCCs that express the transcription factors neurogenin 2 (ngn2) and runt-related transcription factor-3 (runx3). The second neurogenic wave (E11–E13) of NCCs express ngn1 and runx1 and gives rise to the entire range of DRG neurons the majority of which are TrkA+ nociceptive neurons (Ma et al, 1999; Chen et al, 2006; Kramer et al, 2006).
In mammals, adult sensory neurons can be reliably classified as nociceptive or mechanoreceptive by their characteristic action potential (AP) configuration (Koerber et al, 1988; Djouhri et al, 1998; Lawson, 2002). Low threshold mechanoreceptors with myelinated axons have very narrow APs (Koerber et al, 1988), whereas small- to medium-sized nociceptors in the adult are characterized by wider APs that exhibit an inflection or ‘hump' on the falling phase (Koerber et al, 1988; Lawson, 2002). At late embryonic stages, it has been shown that sensory neurons that are mechanoreceptors have narrow APs and nociceptors have humped APs similar to mature neurons (Mirnics and Koerber, 1997). However, the development of AP configuration and its relation to transduction properties and the molecular phenotype has not been investigated. Electrophysiological studies in the embryonic rat and chick indicate that mechanosensitivity, a property shared by most adult sensory neurons (Lewin and Moshourab, 2004), is present in most sensory neurons before birth or hatching (Scott, 1982; Fitzgerald, 1987b). The development of thermoreceptive function during embryonic development has also been studied recently albeit indirectly. Thus, the capsaicin and heat sensitive ion channel TRPV1 (Jordt et al, 2003) is present in early embryonic sensory neurons but the menthol and cold-gated channel TRPM8 appears much later (Bautista et al, 2007; Hjerling-Leffler et al, 2007). Mechanosensitive currents are present in sensory neurons and are thought to be necessary for these neurons to transduce mechanical stimuli in vivo (Hu and Lewin, 2006; Hu et al, 2006). Three types of mechanically activated currents, which can be distinguished on the basis of their inactivation kinetics, biophysical properties and pharmacological sensitivity, can be recorded from isolated sensory neurons (Hu and Lewin, 2006; Wetzel et al, 2007). The rapidly adapting (RA-type) mechanosensitive current is found in mechanoreceptors and nociceptors, whereas an intermediately adapting (IA-type) and slowly adapting (SA-type) current are found exclusively in nociceptors (Drew et al, 2002; Hu and Lewin, 2006).
Here, we directly investigated the emergence of mechanical and electrical excitability in embryonic mouse sensory neurons. Our data show that mechanosensitivity emerges in distinct waves during embryonic and postnatal development. Sensory neurons that are mechanoreceptors acquire mature mechanotransduction very early in development (E13) and this process is independent of NT-3. In contrast, most nociceptive sensory neurons acquire mechanosensitive competence as a result of exposure to target-derived NGF. The highly regulated process of mechanosensory acquisition unveiled here, reveals new strategies to identify molecules required for sensory neuron mechanotransduction.
Results
Maturation of receptor specific AP configuration
To study the diversification of electrical properties, we made current-clamp recordings from acutely dissociated DRG neurons taken from embryonic stage E11.5 and stages thereafter until birth. All recordings were made between 3 and 8 h after plating to ensure that the electrophysiological properties measured best reflect those found in vivo. At E11.5, only 34% of neurons (12/35 tested) fired normal APs in response to current injection (Figure 1A, blue trace). The remaining neurons (66%, 23/35 tested) typically exhibited an immature AP, which lacked a rapid upstroke and failed to repolarize normally (Figure 1A, grey trace). All cells with an AP recorded at E11.5 had narrow spikes typical of mechanoreceptors (Figure 1B and C). Immature neurons lacked a sufficient density of voltage-gated sodium channels as under voltage-clamp conditions inward current amplitudes measured at holding potentials of −10 mV were significantly smaller in neurons with immature APs (561±63 pA, Supplementary Figure S1B, grey example trace) compared with those in neurons with normal APs (1796±91 pA; P<0.001, t-test, n=11–21; Supplementary Figure S1B); the mean cell diameter of the two cell populations were not significantly different (13.5±0.45 μm versus 13.4±0.22 μm, P>0.5, t-test, n=11–21, see Supplementary Figure S1A, E11.5).
Figure 1.
Emergence and maturation of APs. (A) Example traces of APs. The first derivative of humped spikes exhibit two relative minima (dV/dt, black traces). (B) Summarizes changes in the mean half-peak AP duration, note significant changes in APs with humps (*P<0.05; **P<0.01; ***P<0.001, Mann–Whitney test; Bars represent mean±s.e.m., n⩾43). (C) The proportions of putative nociceptors (red squares), mechanoreceptors (blue triangles) and immature cells (grey circles) is plotted as a function of development. (D) The proportion of nociceptors with repetitive firing in response to a 240 pA/100 ms current injection (see inset) plotted as a function of development.
Between E11.5 and E14.5, the proportion of neurons with immature APs decreased from 66 to 5% of the total (3/55 tested) (Figure 1C). Starting at E12.5, a new population of neurons with APs with a hump on the falling phase of the spike appeared and their proportion steadily increased with each day until E15.5 and remained constant until birth at approximately 80% (Figure 1C). In contrast, cells with narrow APs, lacking a hump were found at the same or reduced frequency from E11.5 until birth (Figure 1C). The mean half-peak duration of the AP in cells lacking a hump decreased significantly from 1.94±0.13 ms at E11.5 to 1.47±0.07 ms at E12.5 (P<0.01, Mann–Whitney, n=11–24) (Figure 1B).
Thus, the first neurons to appear at E11.5 are likely mechanoreceptors with APs lacking a hump. In contrast, nociceptors, characterized by humped APs, appear from E12.5 onwards and gradually increase in number until E14.5. The half-peak duration of APs with and without a hump was not significantly different at stages up until E14.5 (Figure 1B). After E14.5, there was a step-like increase in the half-peak duration in cells with humped APs from 1.81±0.09 to 2.62±0.13 ms (P<0.001, Mann–Whitney, n=24–36) (Figure 1B). No further changes occurred in the AP width of nociceptors between E15.5 and E16.5. Beginning at E18.5, the AP-duration increased further until values similar to those of adult nociceptors were reached at P0–P1 (3.04±0.12 ms, Figure 1B) (Stucky and Lewin, 1999).
The late increase in AP-width in nociceptors coincided with changes in the firing behaviour of these neurons. A standard 100-ms duration current injection never evoked more than one AP in nociceptors from embryos younger than E16.5, but at E18.5 and P0 increasing numbers of cells showed repetitive firing to current injection (Figure 1D). Repetitively firing neurons also had significantly wider APs than those firing a single AP (Supplementary Figure S1C). This finding is in good agreement with in vivo studies showing that late embryonic primary afferents first show sluggish firing to natural stimuli, which become more robust as development proceeds (Fitzgerald, 1987a, 1987b).
Development of mechanosensitivity
There are three types of mechanically activated currents in adult mouse DRG neurons. These currents can be readily distinguished by their inactivation kinetics (Figure 2A) and are classified into RA-type, IA-type and SA-type currents (McCarter et al, 1999; Drew et al, 2002; Hu and Lewin, 2006; McCarter and Levine, 2006). We recorded mechanosensitive currents by stimulating the soma with very small (⩾350 nm) and rapidly applied (3.5 μm/ms) displacement stimuli. At E11.5 and E12.5, none of the cells tested responded with inward current to mechanical stimulation (Figure 2B). Just 1 day later, at E13.5, 59% (42/71 tested neurons) exhibited mechanically activated currents (Figure 2B). Interestingly, at this stage, mechanosensitivity was largely restricted to a subpopulation of large diameter neurons (Figure 2C and D), which had also just appeared at E13.5 (Figure 2C, for size frequency plots see Supplementary Figure S2A); neurons smaller than 14 μm did not possess a mechanosensitive current at this stage (Figure 2D). Almost all neurons with a mechanosensitive current exhibited RA-currents (88.1%), whereas IA- and SA-currents were found in only 7.2 and 4.7% of the recorded cells, respectively (Figure 2B). The incidence and distribution of mechanosensitive currents at E14.5 was virtually the same as at E13.5. Many of the neurons recorded at E13.5 possessed a humped AP, but the AP width was still narrow compared with more mature nociceptors (Figure 1B and C). We, thus, carried out single-cell PCR experiments to characterize neurotrophin receptor expression in functionally characterized neurons with an RA-type current at E13.5. Of 15 large diameter neurons recorded with a mechanosensitive current (mean cell diameter 17.6±0.9 μm), all expressed either TrkC (5 cells), TrkB (5 cells) or both (5 cells) (Figure 2E). Importantly, TrkA transcripts could not be detected in any of the 15 mechanosensitive neurons tested. The control experiments demonstrated that the primers used to detect TrkA transcripts were equally efficient in amplifying TrkA as the TrkB and TrkC primers used (Supplementary Figure S3). Thus, the first neurons to acquire mechanosensitivity have the molecular make-up predicted for low threshold mechanoreceptors (Figure 2E).
Figure 2.
Sequential acquisition of mechanosensitivity in sensory neurons. (A) Example traces of mechanosensitive currents recorded from embryonic sensory neurons. Mechanical stimuli were applied (blue trace), and based on their inactivation time constant currents were classified as RA-, IA- and SA-currents. (B) Stacked histogram showing proportions of sensory neurons with mechanically activated currents at different developmental stages. Significant changes in proportions occur between E12.5 and E13.5 and between E18 and P0, respectively (***P<0.001; *P<0.05, χ2 test). (C) Phase-contrast photomicrographs showing acutely dissociated E12.5 and E13.5 cultures, large neurons are only present at E13.5 (red arrows). (D) RA-current proportions are plotted separately for large neurons (>14 μm, dark blue) and small neurons (<14 μm, pale blue). (E) Single-cell real-time PCR from neurons with an RA-current. Functionality of primers was confirmed using plasmids containing trk-receptor cDNAs and cDNA from whole E13.5 DRGs (Supplementary Figure S3).
No small neurons (<14 μm) with a mechanosensitive current were found until E15.5 when 44% possessed mechanosensitive currents (8/18 tested neurons), and this increased to 83% by E18.5 (10/12 tested neurons) (Figure 2B and C). All neurons with a cell diameter <14 μm had a humped and broad AP characteristic of a nociceptor. The acquisition of mechanosensitivity coincided with significant morphological changes, so that the median cell diameter of the smallest neurons increased rapidly between E15.5 and birth (Supplementary Figure S2A, red dashed line). At E18.5, >80% of neurons (44/51) exhibited mechanically activated currents, but the proportions of RA-, IA- and SA-currents among these cells (83.7% RAs, 6.9% IAs and 9.4% SAs) was little changed from earlier embryonic stages (Figure 2B). Before birth, the proportion of nociceptors with SA-like mechanosensitive currents was very low. Interestingly, immediately after birth, at P0–P1, the proportion of cells with SA-currents had increased dramatically (Figure 2B) to 32% (16/50 tested neurons), which was not significantly different from that observed in adult DRG neurons using the same methodology (P=0.19, χ2 test, data for adult neurons not shown).
Molecular identity of the first mechanosensitive neurons
Large sensory neurons with the electrophysiological properties of early born mechanoreceptors acquire mechanosensitivity during target innervation at E13.5 (Figure 2A). Using in situ hybridization, we showed that the distinctive population of neurons with large cell diameters that emerge at E13.5 are almost all TrkC+ or TrkB+, whereas small diameter neurons do not express TrkC or TrkB (Figure 3B–D). In contrast, almost all small sensory neurons express TrkA, but the TrkA receptor message was not detected in larger mechanosensitive neurons (Figure 3). Single-cell PCR experiments confirmed these results (Figure 2E).
Figure 3.
Correlation between Trk-receptor expression and cell size. (A) Photomicrograph and size frequency distribution of acutely dissociated E13.5 DRGs in culture. Note, only large neurons are mechanosensitive at this stage (blue arrows). (B–D) TrkA, TrkB and TrkC receptor expression in E13.5 whole mount DRGs was examined using in situ hybridization. Size frequency distribution of TrkC, TrkB and trkA-expressing neurons reveals that the subpopulation of large cells is almost exclusively TrkB and/or TrkC-positive (blue bars), whereas TrkA expression is primarily found in small cells (red bars).
Three waves of mechanosensitivity acquisition
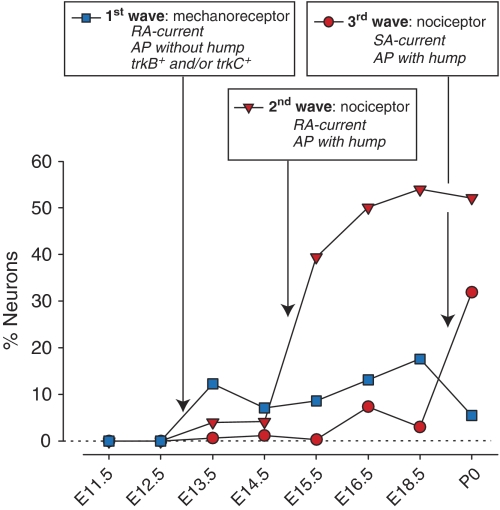
The proportion of cells with a mechanosensitive current (shown in Figure 2B) was calculated from the recorded population. However, the cell size distribution of the recorded cells, especially at early stages, was not identical to that of the total population measured morphologically. Therefore, a weighted analysis was made to estimate the true proportion of cells with mechanosensitive currents in cultures from different embryonic stages (Figure 4), for a detailed description of the calculation see Supplementary Figure S2). This analysis shows more quantitatively the three waves of mechanosensitive current acquisition in the different neuronal populations. First, very early differentiating neurons already present at E13.5 with large cell bodies characteristic of mechanoreceptors acquire an RA-current, this population stays relatively constant at approximately 15% of the total through to birth. In fact, at every developmental stage after E13.5 virtually all neurons with a narrow AP possess an RA-type current (76.5% at E13.5 to 87.5% at E18.5). Second, a later differentiating wave consisting of nociceptors with a humped AP acquire an RA-current in large numbers at E15.5, and this population makes up around 50% of all cells by E18.5. However, not all nociceptive neurons have acquired mechanosensitivity by birth. Only after birth do a substantial number of the remaining nociceptors acquire an SA-current (Figure 4). The SA-current is pharmacologically and biophysically distinct from the RA-type current, and at birth, the distribution of mechanosensitive currents in mechanoreceptors and nociceptors largely reflects that observed in the adult (Hu and Lewin, 2006).
Figure 4.
Three waves of mechanosensitivity acquisition. The proportion of neurons that acquire mechanosensitivity in each of the three waves is calculated after correcting for cell size sampling (see Materials and methods). For each of the three waves, the key characteristics of the neurons that acquire mechanosensitivity is indicated (text boxes).
Mechanosensitive currents showed no systematic change in their kinetic properties as a function of development (Supplementary Table S1). The biophysical properties of the major embryonic mechanosensitive current (RA-type) were examined, and the kinetic characteristics were indistinguishable from RA-currents measured in adult neurons (Supplementary Table S1). The RA-current is primarily permeable to sodium ions (Hu and Lewin, 2006) and consistent with this embryonic RA-currents (E14.5) reversed at positive potentials (+37 mV, Supplementary Figure S4A) and at a holding potential of -60 mV were reduced by 76.3±11.1% when extracellular sodium was replaced by the nonpermeant cation NMDG+. Embryonic RA-currents (E14.5) were also potentiated by 71.7±18.6% in Ca2+/Mg2+-free solutions (Supplementary Figure S4B) and were blocked by Gadolinium (Gd3+) with an EC50 of 16.1 μM (Supplementary Figure S4C). These results indicate that the RA-current already exists in its mature form from the time it appears in embryonic sensory neurons.
In vitro differentiation of sensory neuron mechanosensitivity
To test whether time in culture and neurotrophins might contribute to the acquisition of mechanosensitivity, we cultured sensory neurons from non-mechanosensitive stages (E10.5 to E12.5) for 24 h. Culture media were supplemented with NGF, BDNF and NT-3 (10 ng/ml). After 24 h in culture, neurons from E10.5 embryos did not acquire mechanosensitive currents and those from E11.5 only rarely (13%, 3/23 tested neurons). In contrast, the majority (77%, 17/22 tested neurons) of E12.5 neurons acquired mechanosensitive currents after 24 h in culture (Figure 5A). We cultured neurons from E12.5 embryos with single neurotrophins (Figure 5B). Incubation with BDNF had no inductive effect on mechanosensitivity, but in these cultures only few cells survived up to 24 h. We found that 24 h after incubation with NGF or NT-3 many cells possessed a mechanosensitive current; 73% (27/37 tested neurons) and 49% (18/37 tested neurons) when treated with NGF and NT-3, respectively. Between E10.5 and E12.5 many DRG neurons are TrkC+ (Farinas et al, 1996), but mechanosensitive currents were only observed after NT-3 was added to E12.5 DRG cultures. Thus, we wondered whether mechanosensitive currents appear as a function of developmental age and to this end we incubated neurons from E11.5 embryos for 24 or 48 h in NT-3 alone. Interestingly, only after 48 h in culture did a significant number of sensory neurons (71%, 12/17 tested neurons) acquire mechanosensitive currents (Figure 5C and E). These data are suggestive of an intrinsic program that leads to the acquisition of a mechanosensitive current starting from E13.5 in mechanoreceptors. It is known that NT-3/TrkC signalling acts very early in gangliogenesis to control neuronal number, and it is, therefore, conceivable that NT-3 signals are required to initiate a mechanotransduction program in mechanoreceptors. We tested this idea directly by isolating sensory neurons from NT-3−/− mutant embryos at E13.5 and asked whether such neurons possess a mechanosensitive RA-current. We found that the number of large neurons with narrow APs that possess an RA-current at this stage is indistinguishable from controls (Figure 5D). Thus, NT-3 is not obligatory for the mechanosensory development of the first mechanoreceptor population (Figure 5D and E).
Figure 5.
In vitro acquisition of mechanosensitivity. (A) Recordings were made from E10.5, E11.5 and E12.5 cultures treated with NGF, BDNF and NT-3 for 24 h. The cell diameter of each patched neuron is plotted, and the symbol colour indicates the type of current exhibited by a particular cell (see inset). (B, C) Stacked histograms showing the proportions of neurons with RA- or IA-mechanosensitive currents, the neurotrophin treatment is indicated for each bar, the number of cells recorded is indicated above each column. (C) Note that neurons from E11.5 require 48 h in culture before mechanosensitive currents appear in large neurons. (D) The proportion of large diameter neurons with an RA-current recorded in acutely dissociated DRG cultures from wild-type and NT-3−/− mice. (E) Schema summarizing the key findings in A–D.
We noted several critical differences in the effects of NGF and NT-3 on neurons isolated from E12.5 embryos. After NGF treatment, 73% (27/37 tested neurons) had a mechanosensitive current and of these 52% exhibited RA-currents and 48% exhibited an IA-current. In contrast, after NT-3 treatment, a mechanosensitive current was only observed in 49% of the cells, and 95% (17/18 tested neurons) possessed an RA-type current (Figure 5B). The effects of NT-3 and NGF also differed in another important aspect: NGF-induced mechanosensitive currents in both small and large cells (Supplementary Figure S5B), but after NT-3 treatment only neurons larger than 14 μm responded to mechanical stimulation (80%, 18/23 cells tested) (Supplementary Figure S5A). It might be argued that these differences are due to a selective survival of neuronal subpopulations in NGF or NT-3. However, in both cases, a substantial number of small and large neurons survived in culture despite an overall loss of around 30% during the 24-h culture period (Supplementary Figure S5C). Even in the complete absence of any neurotrophin, a significant proportion of E12.5 neurons (14%, 2/14 cells tested) acquired a mechanosensitive current after 24 h in culture (Figure 5B), and these cells were larger than 14 μm.
Interestingly, neurons kept in culture with NT-3 from E12.5 until the in vitro equivalent of E13.5 had very similar characteristics to neurons taken out at embryonic day E13.5 (compare Figure 2B and D, Supplementary Figure S2A with Supplementary Figure S5A). In contrast, incubation with NGF-induced mechanosensitive currents prematurely in small neurons (Supplementary Figure S5A) that do not normally acquire a mechanosensitive current until E15.5 in vivo (Figure 2B and D). This effect could not be attributed to a survival enhancing effect of NGF as in the absence of NGF many small E12.5 neurons survive and are electrically excitable after 24 h in culture; 12/12 cells lacking a mechanosensitive current exhibited a mature AP (data not shown). It may be the case that the surviving small cells are kept alive by NT-3 or other factors in the culture, nevertheless they did not possess a mechanosensitive current. Thus, we cultured E12.5 neurons for 24 h in NT-3 alone and then added NGF for another 24 h (Figure 6A). If NGF responsive neurons are present in such cultures then NGF may also be capable of inducing a mechanosensitive current. We found that the proportion of small neurons (<14 μm) with a mechanosensitive current was very similar in the NT-3 plus NGF experiment (77%, 10/13 tested neurons) as with 24-h incubation with NGF alone (67%, 14/21 tested neurons). However, 48-h incubation with NT-3 alone did not lead to an appreciable induction of mechanosensitive current in small cells (12%) (Figure 6A). In each experiment, we verified that small cells (<14-μm diameter) were nociceptors with humped APs (data not shown).
Figure 6.
NGF induces mechanosensitivity in nociceptors. (A) Schema of the experiment and the stacked bars showing the proportions of mechanically activated currents in small diameter neurons (<14 μm) under the different culture conditions (B) Photomicrograph showing DRG neurons from BAX−/− mice, note that neurites are almost completely lacking in anti-NGF-treated cultures (right panel). (C) Proportions of small neurons (<14 μm) with an RA-current recorded in BAX−/− DRG cultures. Note, blocking NGF signalling with an anti-NGF antibody completely prevents RA-current acquisition.
To study mechanosensitivity acquisition without the confounding effects of neuronal apoptosis we used BAX−/− embryos in which the neurons survive in culture without added neurotrophins (Knudson et al, 1995; Deckwerth et al, 1996). Sensory neurons from E12.5 BAX−/− mice survive even in the presence of anti-NGF function blocking antibodies (Supplementary Figure S6). After 24 h in culture, neurite outgrowth was observed in the absence of added neurotrophins, suggesting that nonneuronal cells in the culture secrete growth factors. We found that after 24 h >25% of the small neurons in these cultures possess an RA-mechanosensitive current. However, in cultures treated with anti-NGF, no mechanosensitive currents were observed in any of the small neurons recorded, neurite outgrowth in these cultures was also virtually absent (Figure 6B and C). Thus NGF, presumably produced by nonneuronal cells in the culture, is necessary and sufficient to induce RA-mechanosensitive currents in small diameter sensory neurons in vitro.
The IA-mechanosensitive current was found in nearly half of the neurons in NGF-treated cultures, but this current is found rarely in the adult DRG (Hu and Lewin, 2006) and was also rare in embryonic sensory neurons cultured for a few hours (Figure 2B). All cells with an IA-current could also be shown to possess an RA-current, thus a rapid prodding stimulus invariably evoked an RA-current that was kinetically indistinguishable from the RA-currents in other cells (Figure 7A) (McCarter and Levine, 2006). Our data suggested that NGF can induce an IA-current in embryonic sensory neurons. This effect was seemingly independent of developmental stage as incubation of postnatal sensory neurons with NGF for 24 h also increased the proportion of neurons with an IA-current (Figure 7B). Taken together, our data suggest that target-derived NGF is an inductive factor for mechanosensitive currents. Consistent with this idea, we find that exposure of neurons to NGF before target innervation is sufficient to prematurely induce mechanosensitive currents in nociceptive sensory neurons.
Figure 7.
(A) Example traces showing the RA- and IA-currents with different mechanical stimuli in the same cell. Neurons with an IA-current in response to the standard stimulus (sustained, left trace, τ=11.9±3.4 ms, n=9) exhibit an RA-type current (τ=1.2±0.3 ms, n=9) with rapid poking stimuli. (B) Stacked histograms showing the proportions of P0–P1 neurons with different mechanically gated currents cultured for 24 h in the absence (control) and in the presence of NGF, respectively.
Candidate ion channel expression during mechanosensory acquisition
If an ion channel is alone necessary for the emergence of the mechanosensitive current, the mRNA level for the channel might be low at non-mechanosensitive stages (E11.5) and dramatically elevated at mechanosensitive stages (E14.5). We tested this hypothesis using quantitative real-time PCR for a panel of all known members (33 in total) of the transient receptor potential (TRP) family of ion channels. The members of this channel family have been implicated in mechanosensory transduction in several species (Lin and Corey, 2005; Lumpkin and Caterina, 2007). We also tested the expression of 10 members of the Degenerin/ENaC sodium channel super family, the Caenorhabditis elegans members of this family MEC-4 and MEC-10 are necessary for normal body touch transduction (Ernstrom and Chalfie, 2002; Goodman et al, 2004; O'Hagan et al, 2005). Of the 43 candidate genes tested (for complete list see Supplementary Table S2), only 8 genes fulfilled the ideal expression pattern in that mRNA was barely detectable at E11.5 but was significantly higher (>2-fold) in the E14.5 and adult DRG (Figure 8A). This group of ion channel genes includes Acid Sensitive Ion Channels (ASIC2b and ASIC3) that have been implicated in mechanosensitivity (Price et al, 2000, 2001), but also a number of TRP channels (Figure 8A). We took E12.5 DRG cultures incubated with NT-3 and asked whether the expression of the eight candidate channels is up-regulated coincident with the appearance of mechanosensitive current. The expression of each mRNA was compared between 4 and 24 h in culture, and the expression level of each gene was normalized to a panel of neuron-specific genes. Of all the tested genes, only the mRNA for ASIC2b, ASIC3, TRPV1, TRPM8 and TRPC4 were up-regulated under these conditions (Figure 8B). Several members of the TRP family of channels can be activated by membrane stretch (Clapham et al, 2005) and direct gating of channels by membrane stretch has been proposed as a possible mechanism for mechanotransduction in sensory neurons (Cho et al, 2002, 2006; Kung, 2005). However, the expression of stretch activated TRP channels was not consistent with such a model as high expression levels for these genes were already found at non-mechanosensitive stages (Figure 8C). The increased expression of ASIC2b and ASIC3 mRNA at mechanosensitive stages led us to test whether the RA-type mechanosensitive current is altered or absent in E13.5 sensory neurons taken from ASIC2−/− or ASIC3−/− mice. The incidence, amplitude and inactivation kinetics of the RA-type current were, however, found to be unchanged compared with wild-type controls (Figure 8D and E; Supplementary Table S3). The TRPC4 gene also fulfilled our expression criteria and has not been implicated earlier in sensory mechanotransduction. We asked whether TRPC4 transcripts are enriched in larger sensory neurons cultured from E13.5 embryos. To do this, we collected single neurons larger or smaller than 14 μm in diameter using a glass micropipette (500 small neurons and 500 large neurons, n=3), cDNA was synthesized for the two populations and real-time PCR used to detect TRPC4 mRNA. In 6/6 reactions on pools of large neurons, the TRPC4 transcript was always detected. However, from pools of small neurons, TRPC4 was only detectable in 2/6 experiments.
Figure 8.
Developmental expression of TRP channels and Deg/ENaC channels. (A) Expression levels of TRP- and Deg/ENaC family members that exhibit a >2-fold increase in expression between E11.5 and E14.5 and between E11.5 and adult. mRNA was extracted from whole DRGs and expression levels were normalized to the housekeeping gene HPRT. (B) x-fold increase of channel mRNA expression in culture prepared from E12.5 DRGs after 24 h NT-3 treatment. In such cultures, the majority of large neurons acquire RA-currents (see Figure 5b). Channel mRNAs that are up-regulated are highlighted in red. (C) Relative expression levels of known stretch-activated TRP channels. (D, E) RA-currents were measured in ASIC2- and ASIC3-deficient mice, no significant differences were found with respect to maximal RA-current amplitude (D) and inactivation time constant (E).
Discussion
Here, we describe the sequential emergence of electrical excitability and mechanosensitivity in sensory neuron subpopulations during embryonic development (Figure 9). We show that sensory neurons acquire a subtype specific AP soon after differentiation from dividing precursors. Subsequently and coincident with peripheral target innervation, the first mechanosensitive currents appear in sensory neurons. The emergence of mechanosensitive currents in embryonic sensory neurons proceeds in three primary waves that correspond to the well-described epochs in the development of the sensory ganglia. We show that mechanoreceptors acquire mechanosensitivity independent of NT-3. In contrast, NGF appears to be a critical target-derived factor required for an early developing wave of nociceptors to acquire mechanosensitivity. A second substantial wave of nociceptors first acquires mechanosensitivity in the form of an SA-type current after birth. The highly choreographed appearance of mechanosensitive currents in vivo and in vitro has allowed us to screen for known ion channel genes whose expression is regulated coincident with the appearance of mechanosensitive currents.
Figure 9.
Model summarizing the three waves of mechanosensitivity acquisition in different sensory neuron subtypes. Developmental stage is depicted from top to bottom for mechanoreceptors (blue) and nociceptors (red). Cartoons (right) depict the innervation of the limb with the known distribution of the neurotrophins NT-3 and NGF.
Subtype specific APs are acquired shortly after sensory neurons are born and are fine-tuned at later developmental stages
Earlier reports in the chick showed that in vitro differentiating sensory neurons exhibit voltage-gated sodium and calcium currents shortly after they are born (Gottmann et al, 1991). On the basis of morphological and molecular markers, it is thought that mechanoreceptors are born first between E10.5 and E11.5 (Lawson and Biscoe, 1979; Marmigere and Ernfors, 2007). Consistent with this we show here that sensory neurons with narrow APs characteristic of adult mechanoreceptors are already present at E11.5. Nociceptors are born later and accordingly APs typical for nociceptors are only observed in significant numbers from E13.5 onwards (Figure 1C). Interestingly, between E14.5 and E15.5, the major period of target innervation, APs in nociceptors undergo an abrupt increase in AP width (Figure 1B), which also coincides with the acquisition of mechanosensitive currents. NGF can increase AP width both in vitro and in vivo (Chalazonitis et al, 1987; Aguayo et al, 1991; Ritter and Mendell, 1992) and target innervation would presumably mark the first exposure to NGF for developing nociceptors. Starting from E16.5, we also observed a second period of AP widening in nociceptors (Figure 1B).
Three waves of mechanosensitive current acquisition
The first wave of mechanosensitivity starting at E13.5, measured by the presence of an RA-mechanosensitive current, is seen in presumptive mechanoreceptors born between E10 and E11. Using single-cell PCR and in situ hybridization, we could show that these sensory neurons possess TrkC or TrkB receptors, but not TrkA receptors (Figures 2E and 4). Interestingly, E13.5 is also the time point at which the first sensory axons have been observed to innervate the hindlimb skin (Berg and Farel, 2000). The development of transduction in the early born mechanoreceptors is completed very early in development as there is little evidence of newly added neurons after E14.5 and virtually all neurons with a narrow nonhumped AP have a mature RA-mechanosensitive current from E13.5 onwards (Figure 4; Supplementary Figure S4).
Nociceptors are born later, between E11 and E13 (Lawson and Biscoe, 1979) and consistent with this, we observed that humped APs characteristic of such neurons also appear later in development. Nociceptors also start to innervate the skin later as evidenced by the appearance of TrkA+ fibres in the skin starting around E15 in the mouse (White et al, 1996). Embryonic day 15.5 is also the time point at which we observe the appearance of the second wave of mechanosensitivity in small nociceptive neurons with humped APs (Figures 2D and 4). We also provide evidence that the small sensory neurons that acquire mechanosensitivity at E15.5 are probably TrkA positive nociceptors (Figure 3).
It is, however, not the case that all nociceptors have a mechanosensitive current by late embryonic development as at E16.5 nearly 40% of the neurons tested do not possess a current (Figures 2B and 4). A third wave of mechanosensitivity acquisition is then observed just after birth when the remaining nociceptors gain mechanosensitivity in the form of an SA-type mechanosensitive current. This third wave is very different from the first two waves as, first a different mechanosensitive current is acquired the SA-type current, which is pharmacologically distinct from the RA-type current (Hu and Lewin, 2006; Drew et al, 2007). Second, these neurons do not seem to acquire mechanosensitivity coincident with target contact as the innervation of the limb is largely complete before birth. By P0, the incidence of the SA-type current in nociceptors is essentially the same as that observed in the adult ganglia, where the SA-type current is restricted to around 40% of nociceptors (Hu and Lewin, 2006). The appearance of the SA-type current correlates with the start of a postnatal loss of NGF dependence in the immediate postnatal period (Molliver et al, 1997). The loss of NGF dependence coincides with an increase in TrkA− nociceptors that bind isolectin-B4 (IB4+ cells). However, we found no clear correlation between the presence of SA-current and IB4 binding (data not shown).
There is always a very small population of neurons observed throughout development that possess an IA- or an SA-type mechanosensitive current, which is between 3 and 6% at stages up (Figure 2B). Given the rare nature of these neurons, it is difficult to study precisely how these neurons arise. It is conceivable that this population of neurons represent an early born nociceptor population with a distinctive functional role.
Target-derived ‘mechanosensitivity' factor or genetic program?
Our data show that the acquisition of mechanosensitive currents by both mechanoreceptors and nociceptors is coincident with two distinct phases of target innervation by these two populations (Figure 9). This observation suggests that factors in the target may directly induce mechanosensitivity and functional mechanosensitive channels. A target-derived ‘mechanosensitivity' factor should be capable of prematurely inducing mechanosensitive currents in sensory neurons that have not yet reached their targets. We provide several lines of evidence suggesting that the classic target-derived survival factor NGF may be such a ‘mechanosensitivity' factor. Thus, RA-mechanosensitive currents are first observed in small diameter nociceptors in vivo at E15.5 (Figure 2D) at the time these neurons start to innervate NGF-rich peripheral targets (Figure 9). Small diameter neurons taken from E12.5 embryos prematurely acquire an RA-mechanosensitive current when cultured with NGF (Figure 5A and B). Small diameter sensory neurons in cultures from E12.5 embryos normally require NGF for survival, and so it is difficult to ask whether mechanosensitive currents can develop in the complete absence of NGF. To address this issue, we took advantage of BAX−/− embryos where neurons survive in the absence of added growth factors (Knudson et al, 1995; Deckwerth et al, 1996). We recorded from small diameter sensory neurons cultured for 24 h from E12.5 embryos and found that, even without added growth factors, >25% had acquired an RA-mechanosensitive current. In parallel cultures treated with blocking antibodies to NGF none of the recorded neurons possessed a mechanosensitive current (Figure 6B and C). Thus NGF, presumably synthesized by nonneuronal cells in the culture, appears to be necessary and sufficient to induce mechanosensitivity in vitro. Our experiment also shows that NGF in the culture also promotes neurite outgrowth; however, the presence or absence of neurites is unrelated to mechanosensitivity as evidenced by the fact that mechanosensitive currents are routinely measured from acutely dissociated neurons lacking neurites (Figure 2C).
In contrast, NT-3 had no obvious mechanosensitivity inducing effect in large diameter sensory neurons in which its cognate receptor TrkC is expressed (Figure 5). NT-3 is expressed in close proximity to the developing DRG as early as E11.5 (Figure 9) and is biologically relevant by that time as demonstrated by the early loss of the TrkC+ neurons in NT-3-deficient mice (Ernfors et al, 1994; Farinas et al, 1994; White et al, 1996). However, mechanosensitivity in large diameter mechanoreceptors is not observed before E13.5 (Figure 2B). Sensory neurons taken from E11.5 embryos, the majority of which are TrkC+ (Farinas et al, 1998), did not prematurely acquire RA-currents 24 h after NT-3 treatment. Instead such neurons only become mechanosensitive 48 h later, which is the in vitro equivalent to E13.5 (Figure 5C). We were also able to isolate large diameter sensory neurons from E13.5 NT-3−/− embryos and the incidence of RA-mechanosensitive current in these cells was not significantly different from wild type (Figure 5D). We presume that the surviving neurons in NT-3−/− embryos were TrkB+/TrkC+ or TrkB+ at earlier embryonic stages (Tessarollo et al, 1994; Farinas et al, 1996). Thus, NT-3 does not appear capable of directly inducing a mechanosensitive current in sensory neurons. However, it is possible that early exposure to other soluble factors initiates a time-dependent mechanosensitivity induction program in early born neurons.
NGF can induce IA mechanosensitive currents
The IA-current is rare (Figure 2B), and it is not clear whether it is mediated through a different channel than the RA-current. We show here that a powerful factor for inducing IA-mechanosensitive currents in sensory neurons is NGF, both in embryonic and postnatal neurons (Figures 5B and 7B). This finding provides a potential explanation for discrepancies found in the inactivation kinetics of mechanosensitive currents measured in different laboratories. For instance, many groups routinely add NGF to the culture medium of dissociated sensory neurons when measuring mechanosensitive currents (McCarter et al, 1999; Drew et al, 2002, 2004; Coste et al, 2007), although these neurons do not require this factor for survival (Lindsay, 1988). However, we observe IA-type currents very rarely in acutely dissociated embryonic (Figure 2B) and adult neurons (Hu and Lewin, 2006). However, after addition of NGF, we observed a clear increase in the incidence of IA-currents in our cultures (Figure 7B). NGF is normally available in only limited quantities in embryonic and adult tissues and, therefore, we hypothesize that as well as regulating target innervation density (Patel et al, 2000), it may also limit the number of neurons with an IA-current and may even be crucial to initiate mechanosensitivity in nociceptors.
Molecular nature of the mechanotransducer
The highly regulated appearance of mechanosensitive currents in vivo and in vitro during development allowed us to screen candidate ion channels whose expression profile matches mechanosensitive current appearance (Figure 8). The mRNA expression profile for the majority of the candidate channels did not correlate with mechanosensitivity, so these channels are unlikely to play a functional role in mechanosensitivity. Interestingly, two of the ion channels genes whose expression pattern is coincident with mechanosensitive current acquisition, ASIC2b and ASIC3, have already been implicated in mechanotransduction (Price et al, 2000, 2001). However, there is no evidence that these genes are required for mechanosensitive currents in adult neurons (Drew et al, 2004). We re-examined this issue by recording mechanosensitive currents from E13.5 sensory neurons taken from ASIC2−/− and ASIC3−/− embryos. We were not able to find any difference in the kinetics, amplitude or incidence of the RA-mechanosensitive current in neurons lacking ASIC2 or ASIC3 ion channel subunits (Figure 8D and E). Thus, although the in vivo mechanosensitivity of sensory neurons is altered in ASIC mutant mice (Price et al, 2000, 2001), this is not underpinned by dramatic changes in mechanosensitive currents measured in vitro. Two of the TRP genes identified, TRPM8 and TRPV1, have been implicated in thermosensation and there is little evidence that they are involved in mechanosensory mechanisms (Caterina et al, 2000; Bautista et al, 2007). The canonical TRP channel family member TRPC4 was the last remaining ion channel, with an expression pattern coincident with mechanosensitivity acquisition (Figure 8B), and real-time PCR experiments showed that at early embryonic stages, the mRNA is enriched in large mechanosensitive neurons. This channel has recently been implicated in regulating neuronal growth in DRG neurons (Wu et al, 2008); however, further experiments will be required to determine whether TRPC4 is functionally indispensible for mechanotransduction. So far, analysis of TRPC4 mutant mice has not indicated a strong sensory phenotype (Freichel et al, 2005). In summary, we have screened the expression of virtually all known channel genes that have been discussed in the context of mechanotransduction. Our data does not strongly support the idea that these genes alone are necessary for mechanosensitivity competence. We conclude that it is most likely that a hitherto unknown or uncharacterized membrane protein constitutes the ion channel that underlies mechanosensitive currents in sensory neurons. The recent identification of TMEM16A and family members as constituting a calcium-activated chloride current shows that new ion channel proteins may still be identified (Schroeder et al, 2008; Yang et al, 2008).
Conclusion
Our study represents the first systematic examination of the functional properties of sensory neurons starting from neuronal differentiation, peripheral target innervation and subsequent maturation. Our study not only shows that different subtypes of sensory neurons acquire mechanosensitivity at different times but that different developmental mechanisms are used to coordinate this process.
Materials and methods
Cell culture
Timed pregnant C57BL/6N mice were killed in a 100% CO2 chamber. The morning of the vaginal plug was considered as embryonic day 0.5 (E0.5). Fourteen-day-old embryos were staged as E14.5, E15 embryos as E15.5, etc. Early embryos (between E10.5 and E13.5) were staged by a combination of somite number and maturity of limb development (Kaufman, 1994). DRGs from all spinal segments from at least two embryos were dissected, collected in Ca2+ and Mg2+-free PBS and treated with trypsin (0.05%, Invitrogen, Karlsruhe, Germany,) for 12–20 min at 37°C. DRGs taken from E16.5 and older embryos were additionally treated with collagenase IV (1 mg/ml, Sigma-Aldrich) for 15 min at 37°C, before trypsin-treatment. Digested DRGs were washed twice with medium [DMEM-F12 (Invitrogen) supplemented with L-glutamine (2 μM, Sigma-Aldrich), Glucose (8 mg/ml, Sigma-Aldrich), Penicilin (200 U/ml)–Streptomycin (200 μg/ml) 5% fetal horse serum], triturated using fire-polished Pasteur pipettes and plated in a droplet of growth medium on a glass coverslip precoated with poly-L-lysin (20 μg/cm2, Sigma-Aldrich) and laminin (4 μg/cm2, Invitrogen). Coverslips were kept for 3–4 h at 37°C in a humidified 5% incubator before being used for patch-clamp experiments. For overnight cultures, fresh medium was added after 4 h. Neurotrophic factors were added at the time of plating.
Neuronal survival was assessed by comparing the number of neurons present after 24 h with that after 3 h. Cells were plated in triplicates, and neurons counted in 10 nonoverlapping microscopic fields from each culture. Soma diameters were calculated from the mean of the longest and the shortest diameters of each cell measured with MetaFluor Imaging software (Molecular Devices), which was calibrated with a stage micrometer (Zeiss).
Electrophysiology
Whole-cell patch clamp recordings were made at room temperature (20–24°C) from cultures prepared as described above. Patch pipettes were pulled from borosilicate glass capillaries (Hilgenberg, Malsfeld, Germany), filled with a solution consisting of (mM) KCl (110), NaCl (10), MgCl2 (1), EGTA (1) and HEPES (10), adjusted to pH 7.3 with KOH and had tip resistances of 6–8 MΩ. The bathing solution contained (mM) NaCl (140), KCl (4), CaCl2 (2), MgCl2 (1), glucose (4), HEPES (10), adjusted to pH 7.4 with NaOH. Drugs were applied with a gravity driven multi-barrel perfusion system (WAS-02) (Dittert et al, 1998). Recordings were made using an EPC-10 amplifier (HEKA, Lambrecht, Germany) with Patchmaster© and Fitmaster® software (HEKA). Pipette and membrane capacitance were compensated using the auto function of Patchmaster and series resistance was compensated by 70% to minimize voltage errors.
Mechanically activated currents were recorded as described earlier (Hu and Lewin, 2006; Wetzel et al, 2007). Briefly, neurons were clamped to −60 mV, stimulated mechanically with a fire-polished glass pipette (tip diameter 2–3 μm) that was driven by a nanomotor® (MM3A, Kleindiek Nanotechnik, Reutlingen, Germany) the evoked whole cell currents were recorded with a sampling frequency of 200 kHz. The stimulation probe was positioned at an angle of 45° to the surface of the dish and moved with a velocity of 3.5 μm/ms. In contrast to our earlier work (Hu and Lewin, 2006; Wetzel et al, 2007), here only the cell soma was stimulated. Stimulation of neurites was not possible, as embryonic neurons have few and thin neurites. Currents traces were fit with single exponential functions and classified as RA-, IA- and SA-type currents according to their inactivation time constant (Hu and Lewin, 2006).
In situ hybridization
In situ hybridization were performed on whole mount adult DRGs using a modified protocol from Shin et al (2003). DRGs were dissected in PBS, fixed in 4% PFA and dehydrated in PBT-EtOH. Digoxigenin (DIG)-labelled probes were amplified with gene specific sets of PCR primers and cDNA templates prepared from E14.5 mouse DRG and hybridized at 70°C for 4 h. After washing, DIG-labelled probes were visualized using sheep anti-DIG-AP antibody and staining with NBT/BCIP solution.
Real-time PCR and single-cell PCR
Dissected DRGs were collected in RNAlater (Qiagen). Total RNA was extracted and DNA digested using the RNeasy Mini Kit (Qiagen). RNA was quantified using an Ultrospec 1000 Spectrophotometer (Pharmacia Biotech). A measure of 2 μg of RNA per reaction were reverse transcribed using the SuperscriptII Reverse Transciptase (Invitrogen) and random hexamers. With this cDNA 40 qPCR reactions were performed on an Abi Prism 7000 Sequence Detection System (Applied Biosysytems). Probes were from the Universal Probe Library (Roche) and primers designed using the Universal Probe Library Assay Design Center. The TaqMan Universal PCR Master Mix (Applied Biosysytems) was used in 20-μl reactions. All PCR reactions were done in duplicate. mRNA levels in whole DRGs were normalized to the mRNA levels of the housekeeping gene HPRT1. mRNA levels from culture extracts were normalized to the mean mRNA levels of the neuronal markers neuron-specific enolase and microtubule-associated protein-2.
Single-cell PCR: Individual mechanosensitive neurons were aspirated into a large-diameter glass electrode filled with lysis buffer (50 mM Tris–Cl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 5 U ml–1 RNasin (Promega)) and flash frozen in liquid nitrogen. cDNA was prepared using SuperscriptII reverse transcriptase. Each cDNA sample was split up into three aliquots, which were used for TrkA, B and C real-time PCR on an Abi Prism 7000 Sequence Detection System (Applied Biosysytems) as described above.
Weighted analysis of subtype proportions
The cell size distributions of the recorded cells diverged from the true cell size distribution of the culture. A weighted analysis was made to estimate the true proportions of mechanosensitive neurons at each embryonic stage (Supplementary Figure S2A). Neurons were divided into small (<15 μm), medium (15–20 μm) and large (>20 μm), and the proportion of mechanosensitive neurons in each size range calculated on the basis of the experimental data (Supplementary Figure S3B; Psmall, Pmedium, Plarge). The weighted proportions (Supplementary Figure S2D; PRA, PIA, etc.) were calculated according to the following equation: PRA=PS−RA × Psmall+PM−RA × Pmedium+PL−RA × Plarge. All errors are expressed as standard error of the mean (s.e.m.).
Supplementary Material
Supplementary Figures
Supplementary Information
Acknowledgments
SGL and GRL designed and planned the experiments. SGL and RW acquired electrophysiological data. HF performed ISH and RT–PCR. SGL analyzed the data. SGL and GRL wrote the manuscript. This work was funded by a collaborative research centre grant from the Deutsche Forschungsgemeinschaft (DFG), SFB665 to GRL. Anja Wegner provided excellent technical support. We thank Ines Ibanez-Tallon, Kate Poole and Ewan St John Smith for critically reading the MS. The authors declare no financial conflicts of interest.
References
- Aguayo LG, Weight FF, White G (1991) TTX-sensitive action potentials and excitability of adult rat sensory neurons cultured in serum- and exogenous nerve growth factor-free medium. Neurosci Lett 121: 88–92 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208 [DOI] [PubMed] [Google Scholar]
- Berg JS, Farel PB (2000) Developmental regulation of sensory neuron number and limb innervation in the mouse. Brain Res Dev Brain Res 125: 21–30 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313 [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Peterson ER, Crain SM (1987) Nerve growth factor regulates the action potential duration of mature sensory neurons. Proc Natl Acad Sci USA 84: 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q (2006) Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron 49: 365–377 [DOI] [PubMed] [Google Scholar]
- Cho H, Koo JY, Kim S, Park SP, Yang Y, Oh U (2006) A novel mechanosensitive channel identified in sensory neurons. Eur J Neurosci 23: 2543–2550 [DOI] [PubMed] [Google Scholar]
- Cho H, Shin J, Shin CY, Lee SY, Oh U (2002) Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J Neurosci 22: 1238–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G (2005) International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 57: 427–450 [DOI] [PubMed] [Google Scholar]
- Coste B, Crest M, Delmas P (2007) Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. J Gen Physiol 129: 57–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson EM Jr, Snider WD, Korsmeyer SJ (1996) BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17: 401–411 [DOI] [PubMed] [Google Scholar]
- Dittert I, Vlachova V, Knotkova H, Vitaskova Z, Vyklicky L, Kress M, Reeh PW (1998) A technique for fast application of heated solutions of different composition to cultured neurones. J Neurosci Methods 82: 195–201 [DOI] [PubMed] [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN (1998) Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. J Physiol 513 (Part 3): 857–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN (2004) Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol 556: 691–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rugiero F, Cesare P, Gale JE, Abrahamsen B, Bowden S, Heinzmann S, Robinson M, Brust A, Colless B, Lewis RJ, Wood JN (2007) High-threshold mechanosensitive ion channels blocked by a novel conopeptide mediate pressure-evoked pain. PLoS ONE 2: e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Wood JN, Cesare P (2002) Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J Neurosci 22: RC228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R (1994) Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell 77: 503–512 [DOI] [PubMed] [Google Scholar]
- Ernstrom GG, Chalfie M (2002) Genetics of sensory mechanotransduction. Annu Rev Genet 36: 411–453 [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF (1994) Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature 369: 658–661 [DOI] [PubMed] [Google Scholar]
- Farinas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A (1998) Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo. Neuron 21: 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Yoshida CK, Backus C, Reichardt LF (1996) Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron 17: 1065–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M (1987a) Cutaneous primary afferent properties in the hind limb of the neonatal rat. J Physiol 383: 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M (1987b) Spontaneous and evoked activity of fetal primary afferents in vivo. Nature 326: 603–605 [DOI] [PubMed] [Google Scholar]
- Freichel M, Vennekens R, Olausson J, Stolz S, Philipp SE, Weissgerber P, Flockerzi V (2005) Functional role of TRPC proteins in native systems: implications from knockout and knock-down studies. J Physiol 567: 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Lumpkin EA, Ricci A, Tracey WD, Kernan M, Nicolson T (2004) Molecules and mechanisms of mechanotransduction. J Neurosci 24: 9220–9222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K, Rohrer H, Lux HD (1991) Distribution of Ca2+ and Na+ conductances during neuronal differentiation of chick DRG precursor cells. J Neurosci 11: 3371–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M (2007) Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci 27: 2435–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Lewin GR (2006) Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol 577: 815–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Milenkovic N, Lewin GR (2006) The high threshold mechanotransducer: a status report. Pain 120: 3–7 [DOI] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D (2003) Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol 13: 487–492 [DOI] [PubMed] [Google Scholar]
- Kaufman MH (1994) The Atlas of Mouse Development. London: Academic Press pp 515–525 [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ (1995) Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270: 96–99 [DOI] [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM (1988) Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol 60: 1584–1596 [DOI] [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S (2006) A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron 49: 379–393 [DOI] [PubMed] [Google Scholar]
- Kung C (2005) A possible unifying principle for mechanosensation. Nature 436: 647–654 [DOI] [PubMed] [Google Scholar]
- Lawson SN (2002) Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol 87: 239–244 [PubMed] [Google Scholar]
- Lawson SN, Biscoe TJ (1979) Development of mouse dorsal root ganglia: an autoradiographic and quantitative study. J Neurocytol 8: 265–274 [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA (1996) Physiology of the neurotrophins. Annu Rev Neurosci 19: 289–317 [DOI] [PubMed] [Google Scholar]
- Lewin GR, Moshourab R (2004) Mechanosensation and pain. J Neurobiol 61: 30–44 [DOI] [PubMed] [Google Scholar]
- Lin SY, Corey DP (2005) TRP channels in mechanosensation. Curr Opin Neurobiol 15: 350–357 [DOI] [PubMed] [Google Scholar]
- Lindsay RM (1988) Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci 8: 2394–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ (2007) Mechanisms of sensory transduction in the skin. Nature 445: 858–865 [DOI] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD (2007) A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron 54: 739–754 [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ (1999) Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev 13: 1717–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P (2007) Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci 8: 114–127 [DOI] [PubMed] [Google Scholar]
- McCarter GC, Levine JD (2006) Ionic basis of a mechanotransduction current in adult rat dorsal root ganglion neurons. Mol Pain 2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter GC, Reichling DB, Levine JD (1999) Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci Lett 273: 179–182 [DOI] [PubMed] [Google Scholar]
- Mirnics K, Koerber HR (1997) Properties of individual embryonic primary afferents and their spinal projections in the rat. J Neurophysiol 78: 1590–1600 [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD (1997) IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19: 849–861 [DOI] [PubMed] [Google Scholar]
- O'Hagan R, Chalfie M, Goodman MB (2005) The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 8: 43–50 [DOI] [PubMed] [Google Scholar]
- Patel TD, Jackman A, Rice FL, Kucera J, Snider WD (2000) Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 25: 345–357 [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ (2000) The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407: 1007–1011 [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ (2001) The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083 [DOI] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM (1992) Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol 68: 2033–2041 [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA (1982) The development of the segmental pattern of skin sensory innervation in embryonic chick hind limb. J Physiol 330: 203–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JB, Martinez-Salgado C, Heppenstall PA, Lewin GR (2003) A T-type calcium channel required for normal function of a mammalian mechanoreceptor. Nat Neurosci 6: 724–730 [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR (1999) Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci 19: 6497–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Vogel KS, Palko ME, Reid SW, Parada LF (1994) Targeted mutation in the neurotrophin-3 gene results in loss of muscle sensory neurons. Proc Natl Acad Sci USA 91: 11844–11848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, Erdmann B, Machelska H, Heppenstall PA, Lewin GR (2007) A stomatin-domain protein essential for touch sensation in the mouse. Nature 445: 206–209 [DOI] [PubMed] [Google Scholar]
- White FA, Silos-Santiago I, Molliver DC, Nishimura M, Phillips H, Barbacid M, Snider WD (1996) Synchronous onset of NGF and TrkA survival dependence in developing dorsal root ganglia. J Neurosci 16: 4662–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Huang W, Richardson PM, Priestley JV, Liu M (2008) TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J Biol Chem 283: 416–426 [DOI] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Supplementary Information