Abstract
Vesicular trafficking such as macropinocytosis is a dynamic process that requires coordinated interactions between specialized proteins and lipids. A recent report suggests the involvement of CtBP1/BARS in epidermal growth factor (EGF)-induced macropinocytosis. Detailed mechanisms as to how lipid remodelling is regulated during macropinocytosis are still undefined. Here, we show that CtBP1/BARS is a physiological activator of PLD1 required in agonist-induced macropinocytosis. EGF-induced macropinocytosis was specifically blocked by 1-butanol but not by 2-butanol. In addition, stimulation of cells by serum or EGF resulted in the association of CtBP1/BARS with PLD1. Finally, CtBP1/BARS activated PLD1 in a synergistic manner with other PLD activators, including ADP-ribosylation factors as demonstrated by in vitro and intact cell systems. The present results shed light on the molecular basis of how the ‘fission protein' CtBP1/BARS controls vesicular trafficking events including macropinocytosis.
Keywords: CtBP1/BARS, macropinocytosis, phospholipase D
Introduction
Endocytosis is a vital process required for many cellular activities, including extracellular nutrient uptake, membrane recycling and signal transduction. Although the pathway initiated by clathrin-coated pits has received the most attention because of its importance in receptor-mediated capture of external ligands (Conner and Schmid, 2003), it has recently become clear that other endocytic pathways exist, which serve additional physiological functions. One of these pathways is macropinocytosis (Conner and Schmid, 2003; Parton and Richards, 2003).
Macropinocytosis refers to the formation of large (0.2–5 μm) endocytic vesicles, called macropinosomes, by the closure of lamellipodia generated at ruffling membrane domains (for review, see Swanson and Watts, 1995). Macropinocytosis provides an efficient route for taking up extracellular macromolecules and nutrients. In the process of macropinocytosis, the formation of macropinosomes from cell surface ruffles is highly dependent on the actin cytoskeleton reorganization, which is spatiotemporally coordinated by the Rho family GTPases and phosphoinositide signalling (Araki et al, 1996, 2000, 2003; Anton et al, 2003). Although macropinocytosis is constitutive in specialized antigen-presenting cells such as macrophages and dendritic cells in which formed macropinosomes gradually mature and merge with the lysosomal degradative pathway (Steinman and Swanson, 1995), it can also be rapidly and synchronously induced by growth factors in other cell types in which the fluid content of macropinosomes is not delivered to the degradation pathway, but is instead extracellularly regurgitated by recycling pathways (Hewlett et al, 1994; Hamasaki et al, 2004). The molecular mechanism underlying the formation and subsequent maturation of macropinosomes is poorly understood.
It is also known that membrane trafficking is an energy-dependent event and occurs under strict control of specialized proteins such as docking and fusion proteins (Weber et al, 1998) in concert with membrane lipid remodelling. One of the enzymes known to be involved in membrane lipid remodelling during vesicular trafficking is phospholipase D (PLD) (Mettlen et al, 2006). PLD generates phosphatidic acid (PtdOH), a multifunctional lipid with many roles. It has been proposed to alter membrane curvature, serve as a protein attachment site and activate selected enzymes. PtdOH also represents the starting material for the production of additional signalling lipids, particularly in the context of membrane vesicle trafficking and cytoskeletal dynamics (for review, see Morris, 2007). Subsequently, CtBP1/BARS has been identified as an important factor in the fission of the macropinosome neck during epidermal growth factor (EGF)-mediated macropinocytosis (Liberali et al, 2008). These findings prompted us to study the mutual relationships between PLD and CtBP1/BARS during agonist-induced macropinocytosis.
CtBP1/BARS (short splice variant of the CtBP1 gene) was originally identified as a 50-kDa protein based on its ADP ribosylation in the presence of the toxin brefeldin A (Spanò et al, 1999). CtBP1/BARS belongs to a dual-function protein family that is known to be involved in both membrane fission and gene transcription (Chinnadurai, 2003; Corda et al, 2006). As a nuclear transcription factor, CtBP1/BARS regulates numerous cellular functions, including epithelial differentiation, tumorigenesis and apoptosis (Chinnadurai, 2002; Grooteclaes et al, 2003). In the cytoplasm, CtBP1/BARS controls the fission machinery that is involved in the formation of post-Golgi carriers, endocytic fluid-phase carriers (Bonazzi et al, 2005) and COPI-coated vesicles (Yang et al, 2005). CtBP1/BARS is also involved in mitotic Golgi partitioning (Hidalgo Carcedo et al, 2004; Colanzi et al, 2007). Precise molecular mechanisms underlying fission control by CtBP1/BARS are unclear.
In this study we show that CtBP1/BARS is a physiological activator of PLD1 during agonist-induced macropinocytosis. CtBP1/BARS activates PLD1 in a manner synergistic with other PLD activators including ADP ribosylation factor (ARF). The molecular mechanism underlying PLD and CtBP1/BARS-regulated agonist-stimulated macropinosome formation is discussed. The relationship between CtBP1/BARS and previously reported PLD activators is also elucidated herein.
Results
PLD is required for EGF-stimulated macropinocytosis
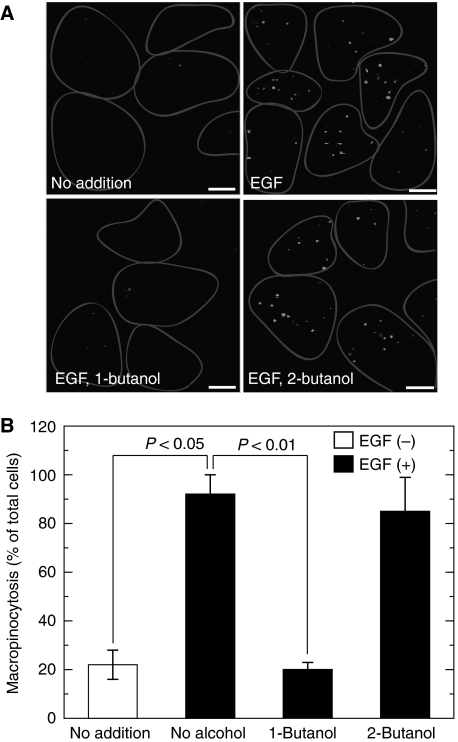
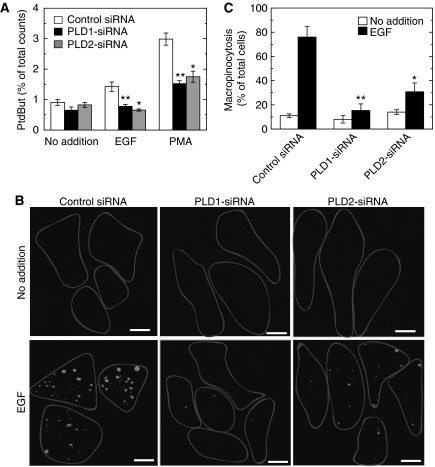
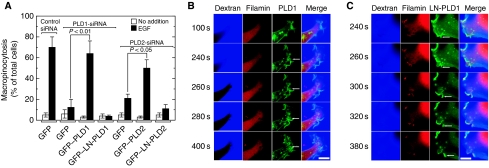
Fluid-phase macropinocytosis was analysed in COS7 cells. Macropinocytosis was inactive under basal conditions and was markedly enhanced upon stimulation by EGF (Figure 1A and B) as reported earlier (Hewlett et al, 1994). As membrane lipid remodelling is essential for dynamic vesicular trafficking including macropinocytosis, we hypothesized that membrane lipid-metabolizing enzyme(s) must be involved during EGF-induced macropinocytosis. Among various candidates for such enzymes, we focused on PLD because this enzyme is known to be involved in various vesicular trafficking steps and to be activated by various growth factors, including EGF (Billah and Anthes, 1990). To assess the possibility of PLD involvement in EGF-induced macropinocytosis, the effect of 1-butanol, which is known to inhibit PLD-mediated processes by facilitating the PLD-specific transphosphatidylation reaction at the expense of PtdOH production was studied. Importantly, EGF-induced macropinocytosis was completely blocked by 1-butanol (Figure 1A and B). However, 2-butanol, which is unable to facilitate transphosphatidylation by PLD, had no effect on macropinocytosis, indicating that the 1-butanol effect was a PLD-specific and non-cytotoxic one and that PLD-catalysed PtdOH formation may be necessary for EGF-induced macropinocytosis. To assess further the physiological importance of PLD in macropinocytosis, endogenous PLD was specifically downregulated by RNA interference and then the agonist-induced macropinocytosis was evaluated. Both PLD1 and PLD2 isoforms are expressed in COS7 cells with a higher ratio of PLD1/PLD2 when compared with other cell lines (Mitchell et al, 2003). Phorbol 12-myristate 13-acetate-induced PLD activity was downregulated by almost 50% in COS7 cells transfected either with PLD1- or PLD2-siRNA (small interfering RNA) compared with control siRNA-treated cells (Figure 2A), confirming that both siRNAs worked properly in this system as reported earlier (Du et al, 2004; Sonoda et al, 2007). These PLD isoform-downregulated cells showed lower macropinocytotic activity compared with cells treated with control siRNA, whereas PLD1-downregulated cells exhibited stronger inhibition than PLD2-downregulated cells (Figure 2B and C). The inhibition of EGF-induced macropinocytosis caused either by PLD1 or PLD2 knockdown was rescued by the co-expression of the respective rat PLD isoforms, whereas the expression of a lipase inactive mutant, lipase-negative (LN)-PLD1 or LN-PLD2, did not restore macropinocytotic activity (Figure 3A). In these rescue experiments, actin dynamics in living COS7 cells were monitored using the actin-binding domain of filamin fused to RFP (ABD-filamin–RFP) (Figure 3B and C). This marker enabled visualization of early stages of EGF-induced macropinocytosis. GFP–PLD1 accumulated in the membrane-ruffled area of the cells that invaginated inward to form cup-shaped structures (Figure 3B, see arrows). It is interesting to note that transfected LN-PLD1 also accumulated in the membrane-ruffled area but failed in subsequent closure of invaginated and cup-shaped structures (Figure 3C, see arrows), suggesting that PLD1 activity is necessary for early stages of macropinocytosis. Expression of PLD2 also rescued the EGF-induced macropinocytosis in PLD2-knockdown cells. In contrast to PLD1, however, PLD2 accumulation surrounding macropinosomes was observed at relatively later stages of macropinocytosis (20 min after EGF treatment) in COS7 cells transiently expressing GFP–PLD2 (see Supplementary Figure 1).
Figure 1.
Requirement of PLD for EGF-induced macropinocytosis. (A) COS7 cells were serum-starved for 1 h and stimulated with 100 ng/ml EGF in the presence of tetramethylrhodamine-labelled dextran for 8 min in the absence or presence of 0.2% 1-butanol or 2-butanol, washed, fixed and analysed for macropinocytosis by confocal microscopy. One representative experiment from at least three separate experiments is shown here. Cells are outlined. Bars, 10 μm. (B) Quantification of macropinocytosing cells treated as in (A). Data are means±s.e. from at least four independent experiments carried out in triplicate.
Figure 2.
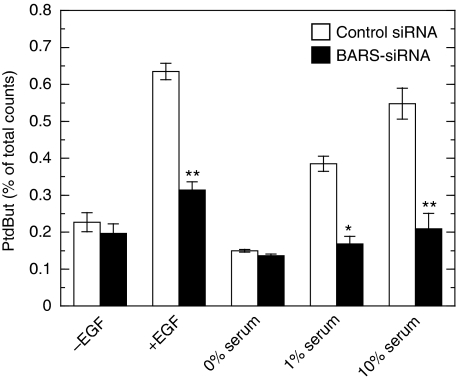
Effect of PLD downregulation by subtype-specific siRNAs on EGF-induced macropinocytosis. (A) COS7 cells were transfected with control or PLD subtype-specific siRNAs and cultured for 30 h. Cells were then metabolically labelled with radioactive lysophosphatidylcholine for 18 h and assayed for PLD in the presence of 0.3% 1-butanol. PtdBut, phosphatidylbutanol. (B) Transfected cells as in (A) were serum-starved for 1 h and stimulated with 100 ng/ml EGF in the presence of tetramethylrhodamine-labelled dextran for 8 min, washed, fixed and analysed for macropinocytosis by confocal microscopy. Bars, 10 μm. (C) Quantification of macropinocytosing cells treated as in (B). Data are means±s.e. from at least four independent experiments carried out in triplicate. *P<0.05 and **P<0.01 compared with control siRNA.
Figure 3.
Rescue of PLD subtype-specific downregulation-induced inhibition of macropinocytosis by the expression of individual subtypes. (A) COS7 cells were transfected with control or PLD subtype-specific siRNAs. After 6 h, cells were transfected with expression vectors encoding both RFP–ABD-filamin and GFP, GFP–PLD1 or GFP–PLD2 and cultured for 30 h. Cells were serum-starved for 1 h and stimulated with 100 ng/ml EGF in the presence of Alexa Fluor 647-labelled dextran for 8 min, washed and analysed by confocal microscopy. Macropinocytosing cells were quantitated. Data are the means±s.e. of five independent experiments carried out in triplicate. (B, C) Both RFP–ABD-filamin and GFP-fused PLD subtypes were expressed in PLD subtype siRNA-treated COS7 cells as in (A). Representative frames of time-lapse images for GFP–PLD1 or GFP–LN-PLD1 (green), RFP–ABD-filamin (red) and Alexa Fluor 647-labelled dextra (blue) and merged signals are shown. Arrows indicate newly forming macropinosomes. Bars, 10 μm.
Both CtBP1/BARS and PLD are required for EGF-stimulated macropinocytosis
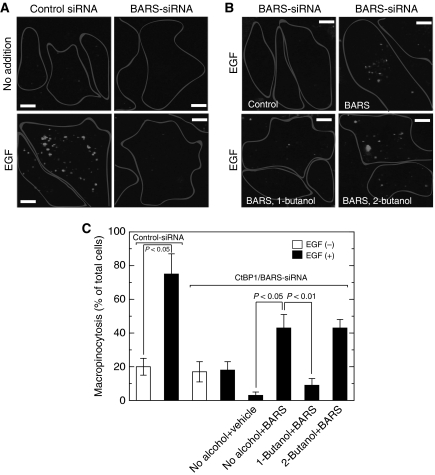
As it has recently been reported that a ‘fission protein' CtBP1/BARS is required for EGF-induced macropinocytosis (Liberali et al, 2008), the involvement of both PLD and CtBP1/BARS in EGF-induced macropinocytosis was assessed. Consistently, EGF-induced macropinocytosis was markedly diminished in cells where endogenous CtBP1/BARS was downregulated by treatment with CtBP1/BARS-siRNA (Figure 4A and B), confirming an important role of this protein in this phenomenon. Next, we introduced purified FLAG–CtBP1/BARS into CtBP1/BARS-downregulated COS7 cells using a BioPORTER reagent (see Materials and methods) and macropinocytosis was analysed. Even though this reagent itself had inhibitory effects on macropinocytosis, introduced CtBP1/BARS restored the inhibitory effects on macropinocytosis caused by CtBP1/BARS-siRNA to some extent (Figure 4C). Importantly, this CtBP1/BARS-supported macropinocytosis in EGF-treated cells was potently suppressed by treatment with 1-butanol but not with 2-butanol, suggesting strongly that PLD- and CtBP1/BARS-mediated signals are converging into a common pathway and not acting separately.
Figure 4.
CtBP1/BARS and PLD are required for EGF-stimulated macropinocytosis. (A) COS7 cells were transfected with control or CtBP1/BARS-siRNA and cultured for 48 h. Cells were serum-starved for 1 h and stimulated with 100 ng/ml EGF in the presence of tetramethylrhodamine-labelled dextran for 8 min, washed, fixed and analysed for macropinocytosis by confocal microscopy. (B) CtBP1/BARS-siRNA-transfected cells were administrated with either control vehicle or purified recombinant CtBP1/BARS (200 nM) with the BioPORTER reagent. Macropinocytosis assay was carried out as above in the presence or absence of 0.2% 1-butanol or 2-butanol. One representative experiment from at least three separate experiments is shown here. Bars, 10 μm. (C) Quantification of macropinocytosing cells treated as in (B). Data are means±s.e. from at least three independent experiments carried out in triplicate.
Requirement of CtBP1/BARS for agonist-induced PLD activation in intact cells
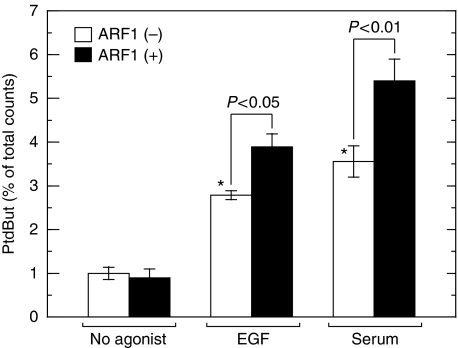
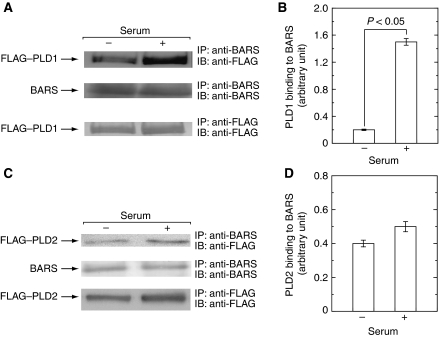
It is well known that PLD is activated by a variety of growth factors and cytokines, including EGF and serum, in various types of mammalian cells. The observation that both CtBP1/BARS and PLD were required for EGF-induced macropinocytosis prompted us to assess the causal relationship between the two proteins. First, we studied the effect of CtBP1/BARS on agonist-stimulated PLD activation. When COS7 cells were stimulated with EGF, PLD was activated as assessed by the accumulation of PtdBut in the presence of 1-butanol in culture medium (Figure 5). Serum also induced PLD activation as reported earlier (Billah and Anthes, 1990). Importantly, when cellular CtBP1/BARS was downregulated by CtBP1/BARS-targeted gene silencing, EGF- or serum-induced activation of PLD was strongly attenuated, suggesting that CtBP1/BARS is required for the agonist-induced PLD activation. To gain further insight into the molecular mechanisms underlying the involvement of CtBP1/BARS in agonist-induced PLD activation, we analysed the interaction between CtBP1/BARS and PLD before and after agonist stimulation. Endogenous CtBP1/BARS was immunoprecipitated from COS7 cell lysates prepared from the cells treated with or without EGF or serum and immunoprecipitated beads were subjected to PLD assay. EGF or serum stimulation of COS7 cells caused increase in PLD activity pulled down with CtBP1/BARS-containing beads (Figure 6), indicating that EGF or serum stimulation of cells facilitated the association of the two endogenous proteins in intact cells. In mammals, there are two PLD isoforms, PLD1 and PLD2. To identify the PLD isoform involved in this interaction, COS7 cells were transiently transfected with either PLD1 or PLD2 and endogenous CtBP1/BARS was immunoprecipitated using anti-CtBP1/BARS antibody and associated PLD isoform was identified by immunoblot analysis. In the cells expressing PLD1, CtBP1/BARS was associated with this isoform in a serum-dependent manner (Figure 7A and B). This association was diminished again under low serum conditions (data not shown). These results suggest that PLD1 has the capacity to associate with CtBP1/BARS in a manner reversibly regulated by serum. On the other hand, PLD2 showed relatively higher association with CtBP1/BARS under the basal conditions and the association changed little even under high serum conditions (Figure 7C and D). These results suggest that agonists such as serum may induce CtBP1/BARS association with PLD1 under physiological conditions as seen in Figure 6. To support this notion in an endogenous cell system, PLD activity pulled down by anti-CtBP1/BARS antibody in cell lysates prepared from serum-stimulated cells was stimulated by the small G-protein ARF1 (Figure 6), an activity characteristic of PLD1 (Exton, 1999).
Figure 5.
Requirement of CtBP1/BARS for agonist-induced PLD activation. COS7 cells were transfected either with control or with CtBP1/BARS-siRNA and cultured for 30 h. Cells were then metabolically labelled with radioactive lysophosphatidylcholine for 18 h in the absence of serum. After stimulation of cells with 100 ng/ml EGF for 8 min or with various concentrations of fetal calf serum for 30 min in the presence of 0.3% 1-butanol, accumulation of PtdBut in the cells was measured and expressed as a percentage of total radioactivity. Data are the means±s.e. of three independent experiments carried out in triplicate. PtdBut, phosphatidylbutanol. *P<0.05 and **P<0.01 compared with control siRNA.
Figure 6.
Serum induces association of CtBP1/BARS with native PLD. After serum starvation for 16 h, COS7 cells were stimulated with 100 ng/ml EGF or 10% fetal calf serum for 30 min. Cells were lysed and immunoprecipitated with anti-CtBP1/BARS antibody. Pulled down PLD-associated with CtBP1/BARS was assayed for PtdBut production in the absence or presence of 50 nM ARF. Data presented are means±s.e. of six independent experiments carried out in triplicate. *P<0.05 compared with no addition of agonists.
Figure 7.
Serum induces CtBP1/BARS association with recombinant PLD1. COS7 cells were transiently transfected with expression vectors encoding either FLAG–PLD1 (A, B) or FLAG–PLD2 (C, D) and cultured for 2 days. After serum starvation for 12 h, cells were stimulated with 10% fetal calf serum, lysed, immunoprecipitated with anti-CtBP1/BARS antibody and subjected to immunoblot analysis (A, C). The amounts of FLAG–PLD1 (B) or FLAG–PLD2 (D) pulled down with CtBP1/BARS in immunoblots (A, C) were quantitated. One representative experiment from at least three separate experiments is shown here. It is noted that serum treatment results in increased association of CtBP1/BARS with PLD1.
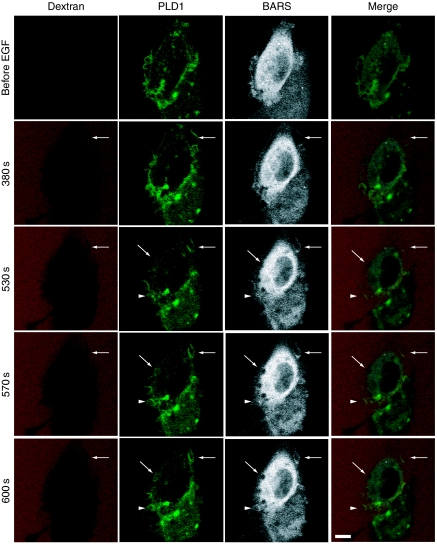
To demonstrate more clearly that EGF-induced association of PLD1 and CtBP1/BARS is implicated in the formation of macropinosomes, we monitored the dynamics of these proteins during macropinocytosis in living cells. COS7 cells transiently expressing both GFP–PLD1 and CFP–CtBP1/BARS were stimulated with EGF and the dynamics of these fluorescent proteins during macropinocytosis were visualized by time-lapse confocal microscopy (Figure 8). GFP–PLD1 distributed predominantly in the cytoplasm in a reticular pattern, whereas CFP–CtBP1/BARS was localized in the cytoplasm in a diffuse pattern. On stimulation with EGF, both proteins gathered around newly macropinocytosing areas in a concurrent manner, that is, these two proteins gathered almost simultaneously around macropinocytosing cups (Figure 8, see arrows and arrowheads). These results suggest functional roles for both proteins in the induction of macropinocytosis.
Figure 8.
Involvement of both PLD1 and CtBP1/BARS in EGF-induced macropinocytosis. COS7 cells were transiently co-transfected with expression vectors encoding GFP–PLD1 or CFP–CtBP1/BARS and cultured for 2 days. Cells were serum-starved for 1 h and stimulated with 100 ng/ml EGF in the presence of tetramethylrhodamine-labelled dextran and analysed for macropinocytosis by confocal microscopy. Representative frames of time-lapse images for GFP–PLD1 (green), CFP–CtBP1/BARS (grey) and tetramethylrhodamine-labelled dextran (red) and merged signals are shown. Arrows and arrowheads indicate newly forming macropinosomes. Bars, 10 μm.
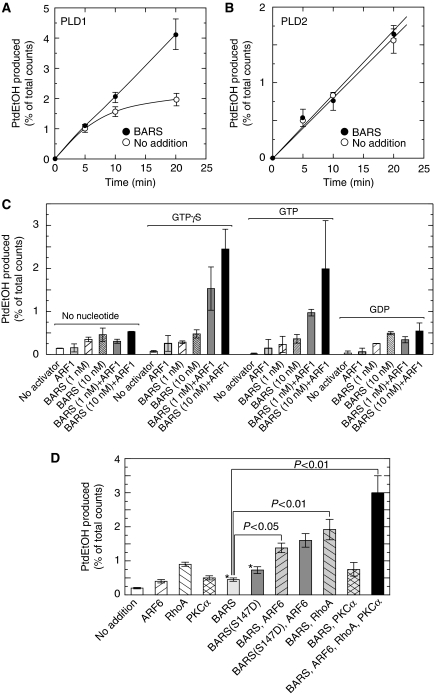
CtBP1/BARS activates PLD1 but not PLD2 in vitro
Next, the ability of CtBP1/BARS to activate PLD was tested in a purified in vitro system. In contrast to bacterial and plant PLDs, mammalian enzyme is latent in its activity in a purified system and requires several cofactors such as phosphatidylinositol 4,5-bisphosphate (PtdIns 4,5-P2) (Brown et al, 1993) and phosphatidylethanolamine (Nakamura et al, 1996) to express high activity. For this reason, the effect of CtBP1/BARS on PLD activity was measured in mixed phospholipid vesicles (Brown et al, 1993). Importantly, CtBP1/BARS doubled PLD1 activity (Figure 9A). PLD2 activity was unaffected by CtBP1/BARS (Figure 9B). These results are consistent with the observation that association of CtBP1/BARS with PLD1 but not PLD2 increased after serum stimulation of COS7 cells (Figure 7). The activation of PLD1 by CtBP1/BARS was further characterized by using various combinations of cofactors. ARF1, a small G protein implicated in macropinocytosis (Cohen et al, 2007) and a well-characterized subtype known to activate PLD, was also tested for its ability to stimulate PLD in concert with CtBP1/BARS. When both CtBP1/BARS and ARF1 were added in the PLD assay, synergistic activation was observed in a manner dependent on GTPγS or GTP but not on GDP (Figure 9C), suggesting that CtBP1/BARS synergizes with the active form of ARF1. As ARF6 is also considered to be an important regulator for macropinocytosis (Donaldson et al, 2009) along with the observation of ARF6 accumulation surrounding the newly formed macropinosomes (see Supplementary Figure 2), the ability of this ARF subtype was also assessed for PLD activation with other known PLD activators, RhoA (Bowman et al, 1993; Malcolm et al, 1994) and protein kinase Cα (PKCα) (Conricode et al, 1992; Singer et al, 1996). ARF6- and RhoA-supported PLD activity was further stimulated by CtBP1/BARS to 2.8- and 2.1-fold, respectively, although further stimulation of PKCα-supported PLD activity was not further enhanced by CtBP1/BARS (Figure 9D). Interestingly, simultaneous addition of all these proteins caused a robust activation of PLD1, suggesting that CtBP1/BARS causes a synergistic activation of PLD1 with other activators and that CtBP1/BARS is acting on a site of PLD1 distinct from the sites for previously reported PLD activators. Furthermore, it has recently been reported that during EGF-induced macropinocytosis, CtBP1/BARS undergoes p21-activated kinase-dependent phosphorylation at Ser-147, which is important for translocation to the macropinocytic cup and its surrounding membranes and subsequent fission of the macropinocytic cup (Liberali et al, 2008). To explain these phenomena through PLD-mediated signalling, we prepared the phosphorylation mimicking mutant of CtBP1/BARS, CtBP1/BARS (S147D) and tested its ability to stimulate PLD activity. As expected, this mutant showed about two-fold higher capacity to stimulate PLD1 as compared with wild-type CtBP1/BARS (Figure 9D).
Figure 9.
Activation of PLD1 by CtBP1/BARS in a purified recombinant protein system. PLD1 (A) and PLD2 (B) activities were measured as a function of time in the absence or presence of 10 nM CtBP1/BARS using mixed phospholipid vesicles. PLD1 activity was reconstituted with various combinations of 1 nM CtBP1/BARS and 50 nM ARF1 in the absence or presence of 100 μM guanine nucleotides (C) or with various combinations of 1 nM CtBP1/BARS, 1 nM CtBP1/BARS(S147D), 4 nM ARF6, 300 nM RhoA and 100 nM PKCα (D). Enzymatic reactions proceeded for 20 min and PtdEtOH formation was measured. Data presented are means±s.e. of at least six independent experiments carried out in triplicate. *P<0.05 compared with no addition of activators. PtdEtOH, phosphatidylethanol.
Discussion
CtBP1/BARS was originally identified as an ADP ribosylation substrate in the presence of the toxin brefeldin A (Spanò et al, 1999) and later implicated in dynamin-independent endocytosis and fission of the Golgi membranes (Corda et al, 2006). CtBP1/BARS was originally thought to possess intrinsic lysophosphatidic acid acyltransferase activity inducing membrane fission (Weigert et al, 1999). Weigert et al also suggested that CtBP1/BARS was unable to activate PLD: under conditions where PtdOH synthesis by Golgi membranes was stimulated by CtBP1/BARS in the presence of radioactive palmitoyl-CoA, the labelling pattern of other lipids was unchanged. This was a useful model to explain the mechanism underlying the generation of PtdOH during CtBP1/BARS-induced membrane fission. However, the enzymatic activity of various mutants of CtBP1/BARS does not correlate with the membrane fission activity (Hidalgo Carcedo et al, 2004). More recently, observations have revealed that CtBP1/BARS does not possess intrinsic acyltransferase activity and that the activity associated with CtBP1/BARS was a co-purification artefact (Gallop et al, 2005).
In this study, we have shown that CtBP1/BARS is involved in PLD1 activation and that this PLD activation is required for EGF-induced macropinocytosis. CtBP1/BARS activation of PLD was verified using several approaches. First, serum or EGF stimulation of PLD required CtBP1/BARS (Figure 5). Second, serum stimulation resulted in the association of CtBP1/BARS with PLD1 as assessed by an endogenous (Figure 6) or recombinant protein system (Figure 7). Finally, CtBP1/BARS activated PLD1 in vitro (Figure 9). Conversely, the present results showed that not only PLD1-siRNA treatment but PLD2 downregulation also inhibited EGF-induced macropinocytosis, which was rescued by the co-expression of wild-type PLD2 but not by catalytically inactive mutant LN-PLD2 (Figure 3).
The spatiotemporal involvement of PLD subtypes in macropinocytosis was further assessed during EGF-induced macropinocytosis using time-lapse video monitoring. PLD1 was recruited at earlier stages of macropinocytosis especially from the beginning to closure steps in a manner concurrent with CtBP1/BARS (Figures 3 and 8). On the other hand, PLD2 but not LN-PLD2 surrounded macropinosomes at relatively later stages of macropinocytosis (Supplementary Figure 1). These results strongly suggest that PLD1 and PLD2 participate in macropinocytosis differently, that is, PLD1 regulates macropinocytosis at the earlier steps presumably at the formation and the closure of macropinocytic cups with the aid of CtBP1/BARS (Liberali et al, 2008), whereas PLD2 may participate in subsequent steps of macropinosome trafficking with mechanisms of regulation currently unknown. In terms of enzyme regulation, PLD1 differs from PLD2, that is, PLD1 is regulated by various protein and lipid factors (see below), whereas PLD2 is insensitive to most of these protein factors but is regulated strongly, at least in vitro, by PtdIns 4,5-P2. It may be interesting to surmise that PLD1 is mainly implicated in the phenomena that are controlled by key proteins such as ARF, PKC and CtBP1/BARS, whereas PLD2 may participate generally in the phenomena involving membrane lipid dynamics during vesicular trafficking.
In contrast to bacterial and plant PLDs, mammalian PLD is latent in its basal activity and is activated upon stimulation of cells by a wide variety of agonists including growth factors and cytokines (Billah and Anthes, 1990). Subsequently, various activators of PLD, especially PLD1 have been identified in an in vitro system using mixed phospholipid vesicles for example, the 50-kDa protein (Bourgoin et al, 1995; Lambeth et al, 1995), ARF (Brown et al, 1993; Cockcroft et al, 1994), PKCα (Conricode et al, 1992; Singer et al, 1996), GM2 activator (Nakamura et al, 1998), PtdIns 4,5-P2 (Brown et al, 1993; Liscovitch et al, 1994) and phosphatidylethanolamine (Nakamura et al, 1996). Although the 50-kDa protein was described in earlier studies using neutrophils, the nature of this protein remains undefined. It is possible that CtBP1/BARS and the 50-kDa PLD activator are identical or closely related, as both proteins are cytoplasmic proteins with a molecular mass of 50-kDa that activate PLD1 synergistically with ARF. In fact, treatment of the cytosolic fractions obtained from various tissues with anti-CtBP1/BARS antibody resulted in the depletion of PLD stimulatory activities in the fractions around 50-kDa as estimated by molecular-sieve column chromatography (data not shown). As information on the 50-kDa PLD activator is available only for granulocytes and HL60 cells, further studies are necessary to elucidate the relationship between CtBP1/BARS and the 50-kDa PLD activator in greater detail.
It has been reported that CtBP1/BARS controls the fission events in the formation of post-Golgi carriers (Bonazzi et al, 2005), COPI-coated vesicles (Yang et al, 2005) and macropinosomes (Liberali et al, 2008). In this study, we have demonstrated that PLD activation is required for agonist-induced macropinocytosis. PtdOH generated by PLD may recruit and activate downstream effectors, or change the biophysical properties of the membrane and directly induce membrane bending and/or destabilization. As the local generation of PtdOH has a key function in the regulation of vesicular trafficking including formation of post-Golgi carriers (Sweeney et al, 2002) and COPI-coated vesicles (Abousalham et al, 2002), it is important to clarify the role of CtBP1/BARS in the regulation of PLD in these phenomena.
Materials and methods
Cell cultures
COS7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C.
Plasmid construction
The rat PLD1 cDNA was a generous gift from Dr JH Exton (Howard Hughes Medical Institute, Vanderbilt University). The rat PLD2 cDNA was isolated by reverse transcription–PCR from total RNAs of rat brain as reported earlier (Sarkar et al, 2001). The rat ARF6 cDNA (DDBJ/EMBL/GenBank accession number NM024152) was isolated from total RNAs of rat brain by PCR (sense primer, 5′-TGAAGCTTGCCACCATGGGGAAGGTGCTATCC-3′ and antisense primer, 5′-TGAAGCTTGGATTTGTAGTTAGAGGT-3′). The human actin-binding domain of filaminB (ABD-filamin) cDNA (DDBJ/EMBL/GenBank accession number NM001457) was amplified from a human liver cDNA library by PCR (sense primer, 5′-TGAAGCTTTGGAAGAAGATCCAGCAG-3′ and antisense primer, 5′-TGAAGCTTGAACTGGGACAGGTAAGT-3′) using KOD-PLUS polymerase (Toyobo, Tokyo). Site-directed mutagenesis of PLD isoforms was performed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) to prepare LN mutants. The primers used were as follows: LN-PLD1 (K860R), 5′-GCTCATCTATGTCCACAGCAGGTTGTTAATTGCTGATG-3′ and 5′-CATCAGCAATTAACAACCTGCTGTGGACATAGATGAGC-3′; LN-PLD2 (K758R), 5′-CATCTATATCCACAGCAGGTTGCTCATTGCAGATGAC-3′ and 5′-GTCATCTGCAATGAGCAACCTGCTGTGGATATAGATG-3′. The full-length cDNAs encoding PLD1, LN-PLD1, PLD2, LN-PLD2 or ARF6 were subcloned into the mammalian expression vectors pCMV5, pEGFP-C1 (for PLD constructs), or pEGFP-N1 (for ARF6) to express FLAG–PLD1, FLAG–PLD2, ARF6–FLAG, GFP-PLD1, GFP-PLD2, or ARF6-GFP, respectively. ABD-filamin was subcloned into the mammalian expression vector pDsRed-Monomer C1 to express N-terminal red fluorescent protein-fused protein (RFP–ABD-filamin).
The rat CtBP1/BARS (rCtBP1/BARS) cDNA (DDBJ/EMBL/GenBank accession number NM019291) was amplified from a rat brain cDNA library by PCR (sense primer, 5′-TGGAATCCAATGTCAGGCGTCCGACCTCC-3′ and antisense primer, 5′-TGGAATCCCTACAACTGGTCAGTCGTAT-3′) using ExTaq polymerase (Takara, Otsu, Japan). The full-length CtBP1/BARS was subcloned into the mammalian expression vector pCMV5 or pECFP-C1 to express N-terminal FLAG-tagged or CFP fusion proteins, respectively. Site-directed mutagenesis of rCtBP1/BARS was performed using the QuikChange site-directed mutagenesis kit to prepare FLAG-tagged rCtBP1/BARS(S147D). The primers used were as follows: sense primer, 5′-GCACTCGGGTCCAGGATGTAGAGCAGATCC-3′ and antisense primer, 5′-GGATCTGCTCTACATCCTGGACCCGAGTGC-3′.
All constructs were verified by DNA sequencing.
siRNA for CtBP1/BARS
siRNAs for human CtBP1/BARS (5′-CCGUCAAGCAGAUGAGACAdTdT-3′ and 5′-GAGCAGGCAUCCAUCGAGAdTdT-3′, 5′-GCUCGCACUUGCUCAACAAdTdT-3′ and 5′-GGAUAGAGACCACGCCAGUdTdT-3′; dT is deoxyribosylthymine throughout) human PLD1 (5′-GUUAAGAGGAAAUUCAAGCdTdT-3′ and 5′-GCUUGAAUUUCCUCUUAACdTdT-3′), human PLD2 (5′-GACACAAAGUCUUGAUGAGdTdT-3′ and 5′-CUCAUCAAGACUUUGUGUCdTdT-3′) and the control siRNA (5′-UUCUCCGAACGUGUCACGUdTdT-3′ and 5′-ACGUGACACGUUCGGAGAAdTdT-3′) were synthesized at Japan Bio Services Saitama, Japan. COS7 cells were transfected with the siRNAs using Oligofectamine according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). After transfection, the intracellular CtBP1/BARS levels were assessed by immunoblotting and the cells were further processed according to the various experimental procedures as necessary.
Immunoprecipitation
CtBP1/BARS-specific polyclonal antibody was raised in rabbits against a full-length CtBP1/BARS fused with glutathione S-transferase. This antibody can be used for immunoblotting and immunoprecipitation.
For immunoprecipitation, COS7 cells were lysed with the lysis buffer (20 mM HEPES/NaOH, pH 7.4, 2 mM MgCl2, 1 mM EGTA, 1% octylglucoside, 1 mM DTT, 20 μg/ml leupeptin and 0.1% ovalbumin) for 30 min on ice. The lysates were clarified by centrifugation for 15 min at 15 000 g and incubated with anti-CtBP1/BARS antibody adsorbed protein A-Sepharose beads at 4°C for 16 h. The immunoprecipitates were subjected to immunoblot analysis or PLD assay.
Purification of recombinant proteins
Recombinant PLD1, PLD2 and CtBP1/BARS were purified from COS7 cells transiently expressing FLAG–PLD1, FLAG–PLD2 or tag-free CtBP1/BARS using either anti-FLAG antibody M2 beads (Sigma) or anti-CtBP1/BARS antibody-adsorbed protein A-Sepharose beads and finally eluted with FLAG peptide (Sigma) or 0.1 M glycine (pH 3.5), respectively, and used for in vitro assay. Recombinant N-myristoylated human ARF1 was prepared from Escherichia coli expressing recombinant human ARF1 and human myristoyltransferase (kind gifts from Dr RA Kahn, Emory University, Atlanta) and purified to near homogeneity (more than 90% purity) by sequential column chromatography on DEAE Sephacel and Superdex 75 as described earlier (Randazzo and Kahn, 1995).
Introduction of recombinant CtBP1/BARS into cells
Purified recombinant FLAG–CtBP1/BARS was delivered into COS7 cells using the BioPORTER reagent (Sigma) according to the manufacturer's instructions. Briefly, purified recombinant FLAG–CtBP1/BARS (2.5 μg) was diluted in PBS, added to BioPORTER dry film and mixed by pipetting, then mixed with 250 μl of serum-free medium and added to cells cultured in Lab-Tec Permanox four-chamber slides (Nunc) for 4 h. Cells were subsequently used for macropinocytosis assays.
PLD assay
In vitro PLD activity was determined with PtdIns 4,5-P2-containing mixed lipid vesicles essentially as described earlier (Brown et al, 1993). Under the standard assay conditions, the reaction mixture (100 μl) contained 5 μM [14C]PtdCho (55 000 d.p.m./nmol), 80 μM PtdEtn, 7 μM PtdIns 4,5-P2, 20 mM HEPES/NaOH, pH 7.4, 2% ethanol, 1 mM MgCl2, purified recombinant FLAG–CtBP1/BARS, PLD1 or PLD2. In some experiments, various combinations of myristoylated ARF1, FLAG-tagged ARF6, hexahistidine-tagged RhoA (Cytoskeleton, Denver) and hexahistidine-tagged PKCα (Jena Bioscience, Jena, Germany) were added in the reaction. After various times of incubation at 37°C, reactions were stopped by the addition of 1 ml of ice-cold chloroform–methanol–HCl (1:1:0.006, by volume). Lipids were extracted and analysed as described earlier (Nakamura et al, 1995).
For in vivo PLD assays, cells were metabolically labelled with [14C]lysophosphatidylcholine (0.5 μCi/1 × 107 cells; GE Healthcare, Buckinghamshire, UK) for 18 h in the absence of serum. After washing with PBS, PLD reaction was initiated by adding DMEM containing 0.3% 1-butanol in the absence or presence of 100 ng/ml EGF or fetal calf serum. Termination of the reactions, lipid extraction and lipid separation were carried out as above. PLD activity was expressed as a percentage of [14C]PtdBut in the total radioactivity found in all spots in one lane.
Macropinocytosis assays
Macropinocytosis assays were carried out essentially as described earlier (Liberali et al, 2008). COS7 cells were cultured on Lab-Tec Permanox 4-chamber slides (Nunc). Cells were incubated for 1 h in serum-free Ringer's buffer (155 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM Na2HPO4, 10 mM glucose, 10 mM HEPES/NaOH, pH 7.2 and 0.5 mg/ml BSA). The serum-starved cells were stimulated with 100 ng/ml EGF for 8 min in the presence of 0.5 mg/ml tetramethylrhodamine-labelled 10 kDa dextran (Molecular Probes) or Alexa Fluor 647-labelled dextran (Invitrogen), as probes of fluid-phase macropinocytosis in Ringer's buffer. The cells were then washed to remove the unbound dextran, fixed with 4% paraformaldehyde and analysed for macropinocytosis by confocal microscopy. Cells showing 10 and more dextran-positive structures were considered to be macropinocytosing and were scored positive for macropinocytosis. The levels of macropinocytosis are given as percentages of the total cells in this scoring category.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Acknowledgments
We thank Dr Stephen T Safrany (Department of Pharmacy, University of Wolverhampton, UK) for his valuable comments on this manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research (C) and a Grant–in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan, the Bilateral Exchange Program between Japan Society for the Promotion of Science and Polish Academy of Sciences, and the Osaka Medical Research Foundation for Incurable Diseases.
References
- Abousalham A, Hobman TC, Dewald J, Garbutt M, Brindley DN (2002) Cell-permeable ceramides preferentially inhibit coated vesicle formation and exocytosis in Chinese hamster ovary compared with Madin–Darby canine kidney cells by preventing the membrane association of ADP-ribosylation factor. Biochem J 361: 653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton IM, Saville SP, Byrne MJ, Curcio C, Ramesh N, Hartwig JH, Geha RS (2003) WIP participates in actin reorganization and ruffle formation induced by PDGF. J Cell Sci 116: 2443–2451 [DOI] [PubMed] [Google Scholar]
- Araki N, Hatae T, Furukawa A, Swanson JA (2003) Phosphoinositide-3-kinase-independent contractile activities associated with Fcγ-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci 116: 247–257 [DOI] [PubMed] [Google Scholar]
- Araki N, Hatae T, Yamada T, Hirohashi S (2000) Actinin-4 is preferentially involved in circular ruffling and macropinocytosis in mouse macrophages: analysis by fluorescence ratio imaging. J Cell Sci 113: 3329–3340 [DOI] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA (1996) A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol 135: 1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah MM, Anthes JC (1990) The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J 269: 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi M, Spano S, Turacchio G, Cericola C, Valente C, Colanzi A, Kweon HS, Hsu VW, Polishchuck EV, Polishchuck RS, Sallese M, Pulvirenti T, Corda D, Luini A (2005) CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat Cell Biol 7: 570–580 [DOI] [PubMed] [Google Scholar]
- Bourgoin S, Harbour D, Desmarais Y, Takai Y, Beaulieu A (1995) Low molecular weight GTP-binding proteins in HL-60 granulocytes: assessment of the role of ARF and of a 50-kDa cytosolic protein in phospholipase D activation. J Biol Chem 270: 3172–3178 [DOI] [PubMed] [Google Scholar]
- Bowman EP, Uhlinger DJ, Lambeth JD (1993) Neutrophil phospholipase D is activated by a membrane-associated Rho family small molecular weight GTP-binding protein. J Biol Chem 268: 21509–21512 [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC (1993) ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell 75: 1137–1144 [DOI] [PubMed] [Google Scholar]
- Chinnadurai G (2002) CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol Cell 9: 213–224 [DOI] [PubMed] [Google Scholar]
- Chinnadurai G (2003) CtBP family proteins: more than transcriptional corepressors. BioEssays 25: 9–12 [DOI] [PubMed] [Google Scholar]
- Cockcroft S, Thomas GMH, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty NF, Truong O, Hsuan JJ (1994) Phospholipase D: a downstream effector of ARF in granulocytes. Science 263: 523–526 [DOI] [PubMed] [Google Scholar]
- Cohen LA, Honda A, Varnai P, Brown FD, Balla T, Donaldson JG (2007) Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol Biol Cell 18: 2244–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colanzi A, Carcedo CH, Persico A, Cericola C, Turacchio G, Bonazzi M, Luini A, Corda D (2007) The Golgi mitotic checkpoint is controlled by BARS-dependent fission of the Golgi ribbon into separate stacks in G2. EMBO J 26: 2465–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422: 37–44 [DOI] [PubMed] [Google Scholar]
- Conricode KM, Brewer KA, Exton JH (1992) Activation of phospholipase D by protein kinase C. Evidence for a phosphorylation-independent mechanism. J Biol Chem 267: 7199–7202 [PubMed] [Google Scholar]
- Corda D, Colanzi A, Luini A (2006) The multiple activities of CtBP/BARS proteins: the Golgi view. Trends Cell Biol 16: 167–173 [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Porat-Shliom N, Cohen LA (2009) Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal 21: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Huang P, Liang BT, Frohman MA (2004) Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell 15: 1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton JH (1999) Regulation of phospholipase D. Biochim Biophys Acta 1439: 121–133 [DOI] [PubMed] [Google Scholar]
- Gallop JL, Butler PJ, McMahon HT (2005) Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature 438: 675–678 [DOI] [PubMed] [Google Scholar]
- Grooteclaes M, Deveraux Q, Hildebrand J, Zhang Q, Goodman RH, Frisch SM (2003) C-terminal-binding protein corepresses epithelial and proapoptotic gene expression programs. Proc Natl Acad Sci USA 100: 4568–4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki M, Araki N, Hatae T (2004) Association of early endosomal autoantigen 1 with macropinocytosis in EGF-stimulated A431 cells. Anat Rec 277: 298–306 [DOI] [PubMed] [Google Scholar]
- Hewlett LJ, Prescott AR, Watts C (1994) The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol 124: 689–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo Carcedo C, Bonazzi M, Spano S, Turacchio G, Colanzi A, Luini A, Corda D (2004) Mitotic Golgi partitioning is driven by the membrane-fissioning protein CtBP3/BARS. Science 305: 93–96 [DOI] [PubMed] [Google Scholar]
- Lambeth JD, Kwak J-Y, Bowman EP, Perry D, Uhlinger DJ, Lopez I (1995) ADP-ribosylation factor functions synergistically with a 50-kDa cytosolic factor in cell-free activation of human neutrophil phospholipase D. J Biol Chem 270: 2431–2434 [DOI] [PubMed] [Google Scholar]
- Liberali P, Kakkonen E, Turacchio G, Valente C, Spaar A, Perinetti G, Böckmann RA, Corda D, Colanzi A, Marjomaki V, Luini A (2008) The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J 27: 970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M, Chalifa V, Pertile P, Chen C-S, Cantley LC (1994) Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem 269: 21403–21406 [PubMed] [Google Scholar]
- Malcolm KC, Ross AH, Qiu R-G, Symons M, Exton JH (1994) Activation of rat liver phospholipase D by the small GTP-binding protein RhoA. J Biol Chem 269: 25951–25954 [PubMed] [Google Scholar]
- Mettlen M, Platek A, Van Der Smissen P, Carpentier S, Amyere M, Lanzetti L, de Diesbach P, Tyteca D, Courtoy PJ (2006) Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic 7: 589–603 [DOI] [PubMed] [Google Scholar]
- Mitchell R, Robertson DN, Holland PJ, Collins D, Lutz EM, Johnson MS (2003) ADP-ribosylation factor-dependent phospholipase D activation by the M3 muscarinic receptor. J Biol Chem 278: 33818–33830 [DOI] [PubMed] [Google Scholar]
- Morris AJ (2007) Regulation of phospholipase D activity, membrane targeting and intracellular trafficking by phosphoinositides. Biochem Soc Symp 74: 247–257 [DOI] [PubMed] [Google Scholar]
- Nakamura S, Akisue T, Jinnai H, Hitomi T, Sarkar S, Miwa N, Okada T, Yoshida K, Kuroda S, Kikkawa U, Nishizuka Y (1998) Requirement of GM2 ganglioside activator for phospholipase D activation. Proc Natl Acad Sci USA 95: 12249–12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Kiyohara Y, Jinnai H, Hitomi T, Ogino C, Yoshida K, Nishizuka Y (1996) Mammalian phospholipase D: phosphatidylethanolamine as an essential component. Proc Natl Acad Sci USA 93: 4300–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Shimooku K, Akisue T, Jinnai H, Hitomi T, Kiyohara Y, Ogino C, Yoshida K, Nishizuka Y (1995) Mammalian phospholipase D: activation by ammonium sulfate and nucleotides. Proc Natl Acad Sci USA 92: 12319–12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Richards AA (2003) Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4: 724–738 [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Kahn RA (1995) Myristoylation and ADP-ribosylation factor function. Methods Enzymol 250: 394–405 [DOI] [PubMed] [Google Scholar]
- Sarkar S, Miwa N, Kominami H, Igarashi N, Hayashi S, Okada T, Jahangeer S, Nakamura S (2001) Regulation of mammalian phospholipase D2: interaction with and stimulation by G(M2) activator. Biochem J 359: 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer WD, Brown HA, Jiang X, Sternweis PC (1996) Regulation of phospholipase D by protein kinase C is synergistic with ADP-ribosylation factor and independent of protein kinase activity. J Biol Chem 271: 4504–4510 [DOI] [PubMed] [Google Scholar]
- Sonoda H, Okada T, Jahangeer S, Nakamura S (2007) Requirement of phospholipase D for ilimaquinone-induced Golgi membrane fragmentation. J Biol Chem 282: 34085–34092 [DOI] [PubMed] [Google Scholar]
- Spanò S, Silletta MG, Colanzi A, Alberti S, Fiucci G, Valente C, Fusella A, Salmona M, Mironov A, Luini A, Corda D (1999) Molecular cloning and functional characterization of brefeldin A-ADP-ribosylated substrate. A novel protein involved in the maintenance of the Golgi structure. J Biol Chem 274: 17705–17710 [DOI] [PubMed] [Google Scholar]
- Steinman RM, Swanson J (1995) The endocytic activity of dendritic cells. J Exp Med 182: 283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JA, Watts C (1995) Macropinocytosis. Trends Cell Biol 5: 424–428 [DOI] [PubMed] [Google Scholar]
- Sweeney DA, Siddhanta A, Shields D (2002) Fragmentation and re-assembly of the Golgi apparatus in vitro. A requirement for phosphatidic acid and phosphatidylinositol 4,5-bisphosphate synthesis. J Biol Chem 277: 3030–3039 [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE (1998) SNAREpins: minimal machinery for membrane fusion. Cell 92: 759–772 [DOI] [PubMed] [Google Scholar]
- Weigert R, Silletta MG, Spanò S, Turacchio G, Cericola C, Colanzi A, Senatore S, Mancini R, Polishchuk EV, Salmona M, Facchiano F, Burger KNJ, Mironov A, Luini A, Corda D (1999) CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 402: 429–433 [DOI] [PubMed] [Google Scholar]
- Yang JS, Lee SY, Spano S, Gad H, Zhang L, Nie Z, Bonazzi M, Corda D, Luini A, Hsu VW (2005) A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J 24: 4133–4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2