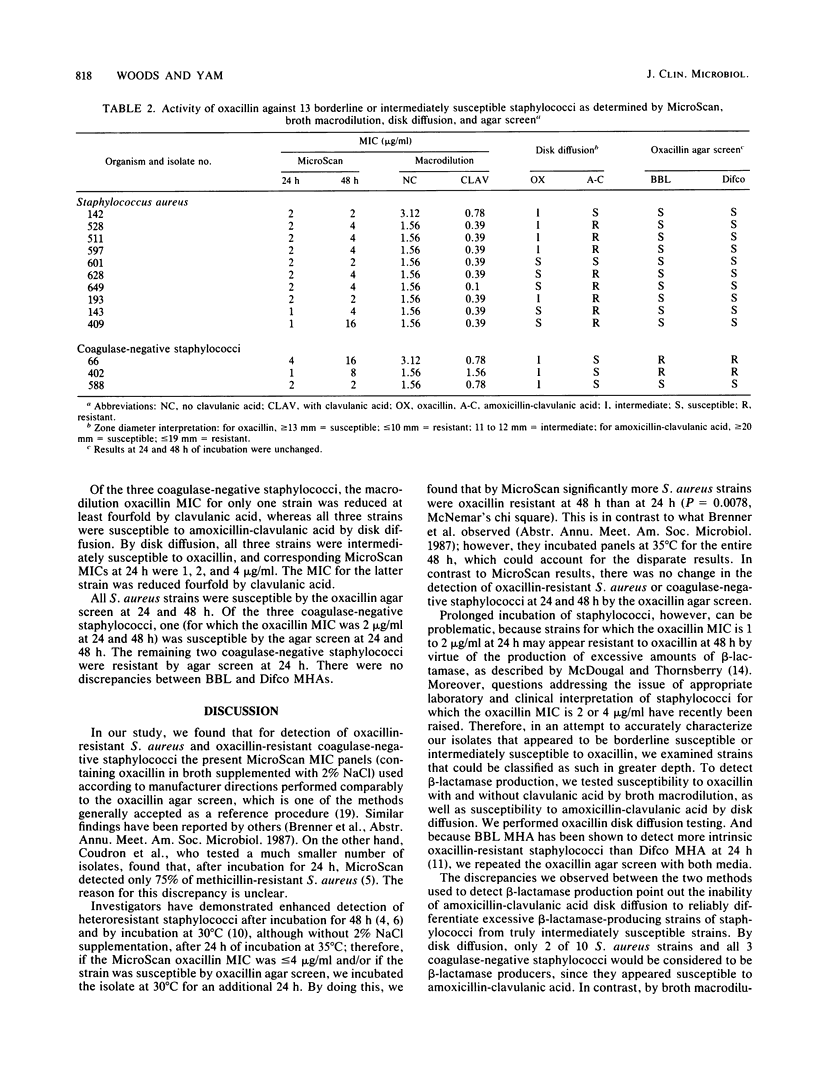
Abstract
Clinical isolates of staphylococci (420 Staphylococcus aureus isolates and 248 coagulase-negative staphylococci) were tested by both MicroScan MIC panels (MicroScan, West Sacramento, Calif.) and an oxacillin agar screen (Mueller-Hinton agar [Difco Laboratories, Detroit, Mich.] containing 6 micrograms of oxacillin per ml and 4% NaCl) to evaluate the ability of MicroScan to detect oxacillin-resistant strains. MicroScan panels and oxacillin agar screen plates were incubated at 35 degrees C for 24 h and at 30 degrees C for an additional 24 h. Endpoints were recorded at 24 and 48 h. By MicroScan, 23 (5.5%) and 30 (7%) S. aureus isolates and 161 (65%) and 162 (65%) coagulase-negative staphylococci were oxacillin resistant at 24 and 48 h, respectively. At both 24 and 48 h, 23 (5.5%) S. aureus isolates and 162 (65%) coagulase-negative staphylococci were resistant by the oxacillin agar screen. Five strains for which the oxacillin MIC was 2 or 4 micrograms/ml and eight strains resistant to oxacillin only at 48 h were further evaluated by broth macrodilution testing for oxacillin with and without clavulanic acid, by oxacillin and amoxicillin-clavulanic acid disk diffusion, and by oxacillin agar screen comparing Mueller-Hinton agars purchased from Difco and BBL Microbiology Systems, Cockeysville, Md. By this additional testing, all 10 S. aureus isolates and 1 of 3 coagulase-negative staphylococci examined produced increased amounts of beta-lactamase. One coagulase-negative staphylococcus appeared to be truly intermediately oxacillin susceptible. There was no significant difference in the rate of detection of oxacillin resistance between MicroScan and the agar screen. MicroScan panels should be incubated for 24 h only, because prolonged incubation caused strains producing excessive amounts of beta-lactamase to appear to be falsely oxacillin resistant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Janney A., Sanders C. V., Marier R. L. Interlaboratory variation of antibiograms of methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains with conventional and commercial testing systems. J Clin Microbiol. 1983 Nov;18(5):1226–1236. doi: 10.1128/jcm.18.5.1226-1236.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L. Antimicrobial susceptibility and selection of resistance among Staphylococcus epidermidis isolates recovered from patients with infections of indwelling foreign devices. Antimicrob Agents Chemother. 1978 Sep;14(3):353–359. doi: 10.1128/aac.14.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. M., Lytle L. S., Walsh D. A. Detection of methicillin-resistant Staphylococcus aureus by microdilution and disk elution susceptibility systems. J Clin Microbiol. 1984 Dec;20(6):1068–1075. doi: 10.1128/jcm.20.6.1068-1075.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger R. J. A methicillin-resistant strain of Staphylococcus aureus. Clinical and laboratory experience. Ann Intern Med. 1967 Jul;67(1):81–89. doi: 10.7326/0003-4819-67-1-81. [DOI] [PubMed] [Google Scholar]
- Coudron P. E., Jones D. L., Dalton H. P., Archer G. L. Evaluation of laboratory tests for detection of methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. J Clin Microbiol. 1986 Nov;24(5):764–769. doi: 10.1128/jcm.24.5.764-769.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L. E., Brown A. E., Miller D. R., Armstrong D. Staphylococcus epidermidis septicemia in children with leukemia and lymphoma. Am J Dis Child. 1984 Aug;138(8):715–719. doi: 10.1001/archpedi.1984.02140460007005. [DOI] [PubMed] [Google Scholar]
- Haley R. W., Hightower A. W., Khabbaz R. F., Thornsberry C., Martone W. J., Allen J. R., Hughes J. M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982 Sep;97(3):297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- Hansen S. L., Freedy P. K. Variation in the abilities of automated, commercial, and reference methods to detect methicillin-resistant (heteroresistant) Staphylococcus aureus. J Clin Microbiol. 1984 Sep;20(3):494–499. doi: 10.1128/jcm.20.3.494-499.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J. H., Coe A. W., Parker M. T. The detection of methicillin resistance in Staphylococcus aureus. J Med Microbiol. 1969 Nov 4;2(4):443–456. doi: 10.1099/00222615-2-4-443. [DOI] [PubMed] [Google Scholar]
- Hindler J. A., Inderlied C. B. Effect of the source of Mueller-Hinton agar and resistance frequency on the detection of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985 Feb;21(2):205–210. doi: 10.1128/jcm.21.2.205-210.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchmer A. W., Archer G. L., Dismukes W. E. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med. 1983 Apr;98(4):447–455. doi: 10.7326/0003-4819-98-4-447. [DOI] [PubMed] [Google Scholar]
- Klimek J. J., Marsik F. J., Bartlett R. C., Weir B., Shea P., Quintiliani R. Clinical, epidemiologic and bacteriologic observations of an outbreak of methicillin-resistant Staphylococcus aureus at a large community hospital. Am J Med. 1976 Sep;61(3):340–345. doi: 10.1016/0002-9343(76)90370-3. [DOI] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986 May;23(5):832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Mortensen J. E., Griffith S. B. Susceptibility testing of methicillin-resistant Staphylococcus aureus with three commercial microdilution systems. Diagn Microbiol Infect Dis. 1986 Sep;5(3):245–253. doi: 10.1016/0732-8893(86)90008-8. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983 Nov;18(5):1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. C., Schimpff S. C., Newman K. A., Wiernik P. H. Staphylococcus epidermidis: an increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982 Oct;97(4):503–508. doi: 10.7326/0003-4819-97-4-503. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Dudnick D. V., Chapin M., Ho W. G., Gale R. P., Martin W. J. Coagulase-negative staphylococcal bacteremia in patients receiving immunosuppressive therapy. Arch Intern Med. 1983 Jan;143(1):32–36. [PubMed] [Google Scholar]
- Woods G. L., Hall G. S., Rutherford I., Pratt K. J., Knapp C. C. Detection of methicillin-resistant Staphylococcus epidermidis. J Clin Microbiol. 1986 Sep;24(3):349–352. doi: 10.1128/jcm.24.3.349-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


