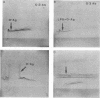
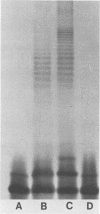
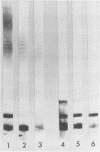
Abstract
Lipopolysaccharide (LPS) was extracted and purified from three Pseudomonas aeruginosa strains isolated from the infected lungs of patients with cystic fibrosis. Two of the strains could be typed by O-specific antibody (O:3 and O:9), and the third was polyagglutinable (O:3/9). The separated LPS was characterized by chemical and serological methods. The main neutral sugar constituents (glucose, rhamnose, and heptose) were found in various proportions in the three strains, whereas the amounts of glucosamine, galactosamine, ketodeoxyoctonate, and phosphate were more constant. Ester-bound C12, C16, 3-OH-C10, and 2-OH-C12, together with amide-bound 3-OH-C12, fatty acids were present in equimolar proportions in all three strains. Considerable amounts of LPS were liberated in the culture supernatant of the O:3 bacteria but not in those from the other two strains. This free LPS was shown to be immunologically identical to the cell-bound LPS and the extracted LPS. By sodium dodecyl sulfate-polyacrylamide gel electrophoresis, O:3 and O:9 LPS showed a ladder pattern characteristic of smooth LPS, while O:3/9 LPS appeared rough. Rabbit antisera used for O-typing were found by enzyme-linked immunosorbent assay to contain anti-LPS antibodies that reacted strongly with homologous LPS, moderately with O:3/9 LPS, and slightly with heterologous LPS. Immunoblotting showed that common antigenic determinants in the core-lipid A part were involved in the observed cross-reaction. The polyagglutinability of P. aeruginosa may be explained by the antibodies to these common determinants that arose from the partial absence of O polysaccharides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade H., Galanos C. Isolation, purification, and chemical analysis of the lipopolysaccharide and lipid A of Acinetobacter calcoaceticus NCTC 10305. Eur J Biochem. 1982 Feb;122(2):233–237. doi: 10.1111/j.1432-1033.1982.tb05871.x. [DOI] [PubMed] [Google Scholar]
- Cadieux J. E., Kuzio J., Milazzo F. H., Kropinski A. M. Spontaneous release of lipopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1983 Aug;155(2):817–825. doi: 10.1128/jb.155.2.817-825.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L. A., Ahlstedt S., Fasth A., Jodal U., Kaijser B., Larsson P., Lindberg U., Olling S., Sohl-Akerlund A., Svanborg-Edén C. Antigens of Escherichia coli, human immune response, and the pathogenesis of urinary tract infections. J Infect Dis. 1977 Aug;136 (Suppl):S144–S149. doi: 10.1093/infdis/136.supplement.s144. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J. Aberrant migration of lipopolysaccharide in sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Eur J Biochem. 1983 Jul 1;133(3):685–688. doi: 10.1111/j.1432-1033.1983.tb07517.x. [DOI] [PubMed] [Google Scholar]
- Hoiby N., Axelsen N. H. Identification and quantitation of precipitins against Pseudomonas aeruginosa in patients with cystic fibrosis by means of crossed immunoelectrophoresis with intermediate gel. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Jun;81(3):298–308. doi: 10.1111/j.1699-0463.1973.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B., McDonald I. J., Russel R. R. Cellular and free lipopolysaccharides of some species of Neisseria. Can J Microbiol. 1975 Dec;21(12):1969–1980. doi: 10.1139/m75-285. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry R. R., Tinsley I. J. A simple, sensitive method for lipid phosphorus. Lipids. 1974 Jul;9(7):491–492. doi: 10.1007/BF02534277. [DOI] [PubMed] [Google Scholar]
- Meadow P. M., Rowe P. S., Wells P. L. Characterization of polyagglutinating and surface antigens in Pseudomonas aeruginosa. J Gen Microbiol. 1984 Mar;130(3):631–644. doi: 10.1099/00221287-130-3-631. [DOI] [PubMed] [Google Scholar]
- Mikkelsen O. S. Serotyping of Pseudomonas aerunginosa. I. Studies on the production of anti O sera. Acta Pathol Microbiol Scand. 1968;73(3):373–390. [PubMed] [Google Scholar]
- Morgan W. T., Elson L. A. A colorimetric method for the determination of N-acetylglucosamine and N-acetylchrondrosamine. Biochem J. 1934;28(3):988–995. doi: 10.1042/bj0280988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeniyi B., Baek L., Høiby N. Polyagglutinability due to loss of O-antigenic determinants in Pseudomonas aeruginosa strains isolated from cystic fibrosis patients. Acta Pathol Microbiol Immunol Scand B. 1985 Feb;93(1):7–13. doi: 10.1111/j.1699-0463.1985.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Ojeniyi B., Rosdahl V. T., Høiby N. Changes in serotype caused by cell to cell contact between different Pseudomonas aeruginosa strains from cystic fibrosis patients. Acta Pathol Microbiol Immunol Scand B. 1987 Feb;95(1):23–27. doi: 10.1111/j.1699-0463.1987.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Pitt T. L., Erdman Y. J. The specificity of agglutination reactions of Pseudomonas aeruginosa with O antisera. J Med Microbiol. 1978 Feb;11(1):15–23. doi: 10.1099/00222615-11-1-15. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G. Composition and structure of lipopolysaccharides from Pseudomonas aeruginosa. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S941–S949. doi: 10.1093/clinids/5.supplement_5.s941. [DOI] [PubMed] [Google Scholar]